Abstract
Purpose
Coronavirus disease 2019 (COVID-19) and non-COVID-19 community-acquired pneumonia (NC-CAP) often result in hospitalization with considerable risks of mortality, ICU treatment, and long-term morbidity. A comparative analysis of clinical outcomes in COVID-19 CAP (C-CAP) and NC-CAP may improve clinical management.
Methods
Using prospectively collected CAPNETZ study data (January 2017 to June 2021, 35 study centers), we conducted a comprehensive analysis of clinical outcomes including in-hospital death, ICU treatment, length of hospital stay (LOHS), 180-day survival, and post-discharge re-hospitalization rate. Logistic regression models were used to examine group differences between C-CAP and NC-CAP patients and associations with patient demography, recruitment period, comorbidity, and treatment.
Results
Among 1368 patients (C-CAP: n = 344; NC-CAP: n = 1024), C-CAP showed elevated adjusted probabilities for in-hospital death (aOR 4.48 [95% CI 2.38–8.53]) and ICU treatment (aOR 8.08 [95% CI 5.31–12.52]) compared to NC-CAP. C-CAP patients were at increased risk of LOHS over seven days (aOR 1.88 [95% CI 1.47–2.42]). Although ICU patients had similar in-hospital mortality risk, C-CAP was associated with length of ICU stay over seven days (aOR 3.59 [95% CI 1.65–8.38]). Recruitment period influenced outcomes in C-CAP but not in NC-CAP. During follow-up, C-CAP was linked to a reduced risk of re-hospitalization and mortality post-discharge (aOR 0.43 [95% CI 0.27–0.70]).
Conclusion
Distinct clinical trajectories of C-CAP and NC-CAP underscore the need for adapted management to avoid acute and long-term morbidity and mortality amid the evolving landscape of CAP pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-024-02292-z.
Keywords: COVID-19, Community-acquired pneumonia, SARS-CoV-2, Observational cohort study
Introduction
Community-acquired pneumonia (CAP) is among the most frequent causes of hospitalization worldwide and the associated mortality remains high [1, 2]. Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread worldwide, increasing morbidity and mortality in most populations. The clinical spectrum of COVID-19 ranges from asymptomatic carriers to severe illness. Moderate and severe COVID-19 cases require hospitalization and are characterized by pneumonia as leading clinical feature [3]. The expansion of the SARS-CoV-2 pandemic to Central Europe in 2020 and the subsequent implementation of non-pharmacological interventions altered the spectrum of predominant pathogens causing CAP, placing SARS-CoV-2 among the primary pathogens of community-acquired pneumonia [4].
The outstanding characteristics of COVID-19 community-acquired pneumonia (C-CAP) compared to the non-COVID-19 CAP (NC-CAP) group are manifold: The pathophysiology of COVID-19, involving vascular activation [5], hypercoagulability [6], and the fibroproliferative activation of pulmonary macrophages [7], is one domain highlighting the disease’s peculiarity. Also on the clinical level, COVID-19 pneumonia is distinct from how CAP has been observed so far, e. g. considering the trajectory of severe disease: While severe bacterial CAP is often linked to a rapidly evolving respiratory failure and septic shock [8], respiratory failure in COVID-19 pneumonia develops more gradually [9]. In severe disease, both diagnoses are typically accompanied by complications resulting from intensive care unit (ICU) treatment, such as ventilator-associated pneumonia or catheter-associated bloodstream infections [10]. In the acute and convalescence phase of both COVID-19 and NC-CAP, an increase in cardiovascular events and cardiovascular mortality is observed [11, 12].
Contextualizing C-CAP outcomes with NC-CAP might help estimating the excess risk linked to C-CAP, e.g. in an emergency room or ICU setting. As for policymaking and resource allocation during pandemics, the expected length of hospital stay (LOHS) is a parameter of critical importance in estimating in-patient health-care demands [13]. This study presents data from two prospective observational cohorts of C-CAP and NC-CAP patients recruited following the same study protocol. We compare the risk for unfavorable hospitalization outcomes such as in-hospital mortality, ICU treatment, invasive mechanical ventilation (MV), vasopressor use, LOHS and length of ICU stay (ICULOS) between C-CAP and NC-CAP patients. Using data from a 180-days follow-up, we moreover compare risk for post-discharge mortality and morbidity (represented by the re-hospitalization rate) in the two groups. In congruence with experiences from now four years of COVID-19, we hypothesized that increased risk for in-hospital death and severe disease was associated with C-CAP. We furthermore suspected that C-CAP leads to prolonged LOHS and thus higher resource demand than NC-CAP. Considering follow-up outcomes, we conclusively assumed that post-discharge mortality and morbidity in C-CAP exceed those of NC-CAP patients.
Methods
Dataset
We analyzed data collected by the multi-national network CAPNETZ (Competence network community-acquired pneumonia) in the framework of the eponymous multinational prospective cohort study conducted in 35 Central European clinical centers (34 hospitals and one outpatient clinic), of which 30 were in Germany, two in Switzerland, and one each in Austria, Italy, and the Netherlands (https://capnetz.de/infrastruktur/). Recruitment and data collection followed the study protocols CAPNETZ 2.0 (January 2017 until June 2021) or CAPNETZ-PROVID (October 2020 until June 2021). CAPNETZ 2.0 is an updated version of the CAPNETZ study protocol [14]. CAPNETZ-PROVID is an amendment to CAPNETZ, affiliated with the consortium PROVID (Clinical, Molecular and Functional Biomarkers for Prognosis, Pathomechanisms and Treatment Strategies of COVID-19), which was established in 2020 as a response to the SARS-CoV-2 pandemic. Inclusion criteria for participation in CAPNETZ-PROVID were age of 18 years or older and a positive SARS-CoV-2 polymerase chain reaction (PCR) at screening visit. CAPNETZ-PROVID recruitment took place in 12 of the CAPNETZ study centers. CAPNETZ 2.0 and CAPNETZ-PROVID were approved by the Ethics Committee of Hannover Medical School (301–2008) and are registered at ClinicalTrials.gov (NCT02139163, NCT04952337). All participants or their legal guardian provided written informed consent for study participation.
Study design
From both the CAPNETZ 2.0 and the CAPNETZ-PROVID datasets, patients analyzed in this study were required to have a diagnosis of COVID-19 CAP or NC-CAP, defined by the CAPNETZ 2.0 inclusion criteria (CAP criteria): (i) Presentation of at least one clinical sign or symptom of pneumonia (fever, cough, purulent sputum, or rales/crackles in pulmonary auscultation) at study enrollment, (ii) Pulmonary infiltrations found in chest imaging, and (iii) Exclusion of hospital-acquired pneumonia (assumed if the patients were not hospitalized during the last 28 days and if diagnosis of pneumonia was made within 48 h after hospitalization). Patients with severe immunosuppression (recent chemotherapy, neutropenia, recent systemic steroid therapy or history of solid organ or stem cell transplant) were excluded. To facilitate appropriate group assignment (C-CAP vs. NC-CAP), we excluded patients from the analysis if no SARS-CoV-2 PCR test was performed at study enrollment in patients after the pandemic onset or if SARS-CoV-2 ribonucleic acid detection first occurred during the hospitalization. According to the SARS-CoV-2 PCR result from screening visit, we assigned participants to the C-CAP or NC-CAP group. Baseline demographics, comorbidities, and administration of key medications (antibiotics, remdesivir, dexamethasone) during the hospital phase were documented. We only considered participants who were hospitalized at study inclusion and not transferred to another hospital. Patients who withdrew their written study participation during follow-up and whose datasets were incomplete regarding our study participation criteria or outcomes were excluded. The recruitment date was classified as pre-pandemic, first, second, and third wave according to the classification of pandemic phases proposed by Tolksdorf et al. [15]. Participants or their legal representatives were contacted 180 days after inclusion for follow-up. If no information on post-discharge vital status was obtained, participants were considered lost to follow-up.
Outcomes and subgroups
Outcomes of the hospitalization phase were in-hospital death, ICU treatment, use of invasive mechanical ventilation (MV), vasopressor treatment, LOHS over seven days, and patient status on day 28 (discharged, hospitalized, or death during hospitalization). For patients treated on an ICU, we analyzed ICULOS, time from hospitalization to first ICU admission, length of invasive MV, and time to intubation. LOHS was analyzed in subgroups defined by level of care and survival status. Post-discharge follow-up outcomes were death and, in participants who completed the 180 days post-hospital admission follow-up, additional hospitalizations.
Statistical analysis
Continuous and discrete variables are presented as median with interquartile range (IQR). Group differences were assessed using Mann–Whitney-U test for continuous/discrete variables and Fisher’s exact (in observed frequencies under five in one of the groups) or Chi2 test for categorical variables. Kruskal–Wallis test compared ordinal variables among groups. Adjusted odds ratios with a 95% confidence interval, calculated based on multivariate logistic regression, compared risks for the different outcomes associated with C-CAP and NC-CAP, adjusting for sex, age, BMI, and the five most frequent comorbidities from both groups. Multivariate logistic regression analyzed factors associated with in-hospital mortality, LOHS over seven days, and ICU treatment, adjusting for age, sex, BMI, recruitment period, the five most common comorbidities within each diagnosis group and, in C-CAP, the use of remdesivir, dexamethasone, and antibiotics. Bar plots and Kaplan–Meier curves serve to illustrate the development of patient status and length of hospital stay. Right censoring was undertaken for LOHS over 28 days. Log-rank test was used to examine significant group differences in time-to-event analysis. Statistical significance was assumed for p < 0.05. Missing values are reported in sTable 1. Analyses and visualizations were performed using RStudio (Version 4.1.2) with the R packages ‘survival’, ‘survminer’ and ‘ggplot2’ [16, 17].
Results
Study participants
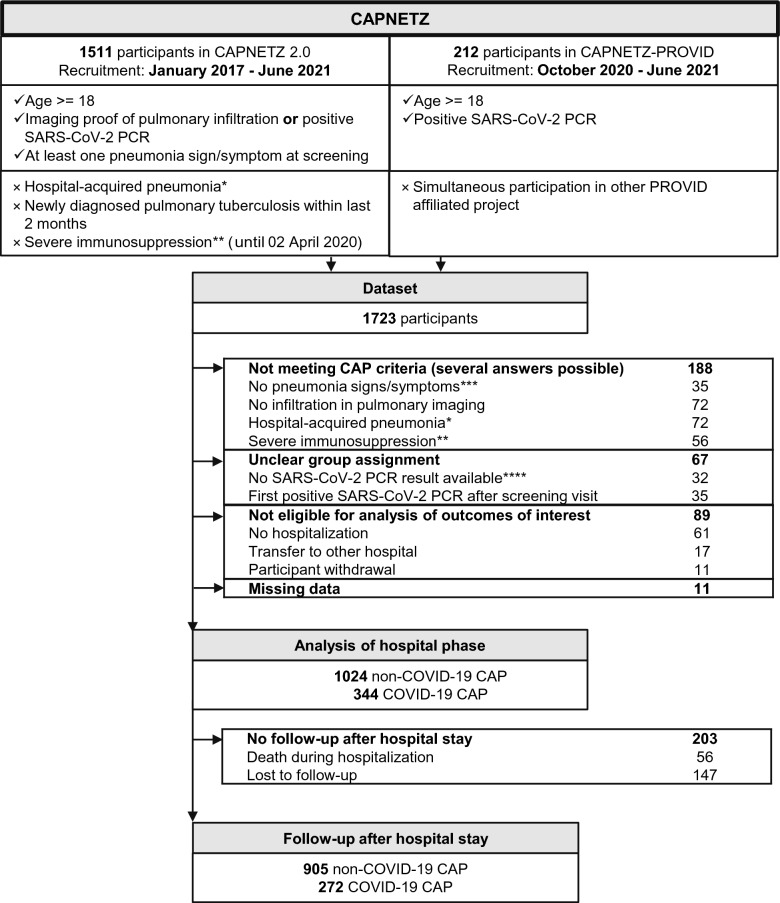
Figure 1 depicts the participant flowchart in accordance with STROBE (Strengthening the Reporting of Observational studies in Epidemiology) recommendations [18]. The dataset included 1723 participants (1511 in CAPNETZ, 212 in CAPNETZ-PROVID). Exclusions (n = 355) were made based on CAP criteria, unclear group assignment, non-eligibility, or missing data. The remaining study sample (n = 1368) comprised 344 C-CAP and 1024 NC-CAP patients. The follow-up cohort (n = 1177) comprised 191 fewer patients, as 56 died during the initial hospital stay and 147 were lost to follow-up.
Fig. 1.
Patient flowchart. Ticks (✓) indicate inclusion criteria, crosses ( × ) exclusion criteria for CAPNETZ (left) and CAPNETZ-PROVID participation. *) Pneumonia onset ≥ 48 h after hospitalization or hospitalization during the last 28 days. **) Recent chemotherapy, neutropenia, recent systemic steroid therapy or history of solid organ or stem cell transplant ***) Fever, cough, purulent sputum, or rales/crackles in pulmonary auscultation at screening visit. ****) Applies after pandemic onset. CAPNETZ: competence network community-acquired pneumonia, PROVID: Clinical, Molecular and Functional Biomarkers for Prognosis, Pathomechanisms and Treatment Strategies of COVID-19, SARS-CoV-2: severe acute respiratory syndrome coronavirus 2, PCR: polymerase chain reaction, NC-CAP: non-COVID-19 community-acquired pneumonia, C-CAP: COVID-19 community-acquired pneumonia
Patient characteristics
Table 1 summarizes patient characteristics. C-CAP patients had lower age (60 vs. 66 years) and higher median body mass index (BMI, 27.4 kg/m2 vs. 25.7 kg/m2) than NC-CAP patients. Most C-CAP patients were recruited during the second wave of the pandemic (44.2%), while most NC-CAP patients were recruited before the pandemic (80.6%). The most common comorbidities in both groups were arterial hypertension (39.5% vs. 47.9%), diabetes mellitus (21.5% vs. 18.5%), atrial fibrillation (7.6% vs. 15.1%) and malignant diseases (8.1% vs. 17.1%), complemented by asthma in C-CAP (7.3%) and chronic obstructive pulmonary disease in NC-CAP (22.6%). Antibiotics were used in about half of the C-CAP patients and in most NC-CAP patients (51.2% vs. 98.5%). Among C-CAP patients, 19.5% received antiviral treatment with remdesivir and 45.6% anti-inflammatory treatment with dexamethasone. sTable 2 summarizes patient characteristics during the pandemic waves according to Tolksdorf et al. [15] Regarding C-CAP treatment throughout the pandemic waves, the use of antibiotics decreased (60.5% vs. 46.7% vs. 44.1%, p = 0.0323) while use of dexamethasone increased (17.7% vs. 55.3% vs. 75.0%, p < 0.0001).
Table 1.
Demographics, comorbidities, and clinical characteristics of patients with COVID -19 disease community-acquired pneumonia (C-CAP) and non-COVID-19 community-acquired pneumonia (NC-CAP)
| NC-CAP | C-CAP | p-value | |
|---|---|---|---|
| Total (n) | 1024 | 344 | |
| Demography | |||
| Age (y) median (IQR) | 66 (53–77) | 60 (49–70) | < 0.0001 |
| Sex female (%) | 356 (34.8) | 127 (36.9) | 0.5107 |
| BMI (kg/m2) median (IQR)* | 25.7 (22.8–29.6) | 27.4 (24.9–31.8) | < 0.0001 |
| Recruitment phase | |||
| Pre-pandemic | 825 (80.6) | 0 (0.0) | < 0.0001 |
| First wave | 122 (11.9) | 124 (36.0) | |
| Second wave | 37 (3.6) | 152 (44.2) | |
| Third wave | 40 (3.9) | 68 (19.8) | |
| Comorbidities and lifestyle factors | |||
| Arterial hypertension (%) | 490 (47.9) | 136 (39.5) | 0.0089 |
| Atrial fibrillation (%) | 155 (15.1) | 26 (7.6) | 0.0005 |
| Pre-existing heart failure (%) | 63 (6.2) | 8 (2.3) | 0.0086 |
| Coronary heart disease (%) | 120 (11.7) | 18 (5.2) | 0.0008 |
| COPD (%) | 231 (22.6) | 13 (3.8) | < 0.0001 |
| Asthma (%) | 71 (6.9) | 25 (7.3) | 0.9301 |
| Diabetes mellitus (%) | 189 (18.5) | 74 (21.5) | 0.2441 |
| Hypercholesterinemia (%)* | 135 (13.2) | 21 (6.2) | 0.0007 |
| Malignant disease (%) | 175 (17.1) | 28 (8.1) | 0.0001 |
| Liver disease (%) | 32 (3.1) | 4 (1.2) | 0.0516 |
| Chronic kidney disease (%) | 132 (12.9) | 19 (5.5) | 0.0002 |
| Neurological disease (%) | 105 (10.3) | 21 (6.1) | 0.0282 |
| Autoimmune disease (%) | 65 (6.3) | 11 (3.2) | 0.0384 |
| HIV-positive (%) | 45 (4.4) | 4 (1.2) | 0.0038 |
| Smoking history (%)* | 666 (65.7) | 97 (30.5) | < 0.0001 |
| Laboratory parameters at hospital admission | |||
| WBC (count/nl) median (IQR)* | 11.3 (8.3–15.4) | 6.0 (4.7–8.8) | < 0.0001 |
| CRP (mg/l) median (IQR)* | 126.2 (55.0–218.2) | 62.1 (26.7–109.5) | < 0.0001 |
| PCT ≥ 0.5 ng/ml (%)* | 168 (41.0) | 26 (9.3) | < 0.0001 |
| Lactate ≥ 20 mg/dl (%)* | 34 (17.2) | 25 (13.6) | 0.4082 |
| LDH ≥ 250 U/l (%)* | 309 (47.6) | 234 (81.8) | < 0.0001 |
| Treatment during hospitalization | |||
| Antibiotic (%) | 1009 (98.5) | 176 (51.2) | < 0.0001 |
| Remdesivir (%) | 0 (0.0) | 67 (19.5) | < 0.0001 |
| Dexamethasone (%) | 6 (0.6) | 157 (45.6) | < 0.0001 |
Bold numbers indicate p-values < 0.05
Asterisks (*) mark items with missing values as reported in sTable 1.
IQR inter-quartile range, COPD chronic obstructive pulmonary disease, HIV human immunodeficiency virus, WBC white blood cells, CRP C-reactive protein, PCT procalcitonin, LDH lactate dehydrogenase
Hospitalization outcomes
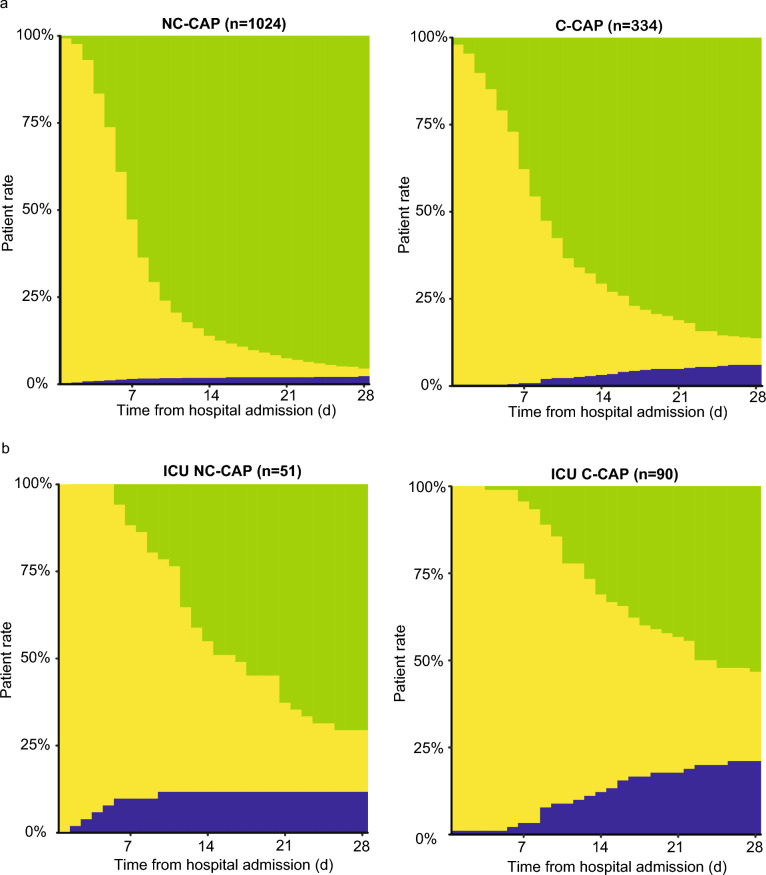
Table 2 details the hospitalization outcomes. The C-CAP group was at higher risk for in-hospital death than NC-CAP (7.6% vs. 2.9%, p = 0.0003, aOR 4.48 [95% CI 2.38–8.53]). ICU treatment risk was elevated in C-CAP compared to NC-CAP (26.2% vs. 5.0%, p < 0.0001, aOR 8.08 [95% CI 5.31–12.52]). C-CAP was linked to a higher rate of LOHS over seven days than NC-CAP (61.3% vs. 45.7%, p < 0.0001, aOR 2.21 [95% CI 1.67–2.92]). LOHS over 28 days was more prevalent in C-CAP (7.6% vs. 2.1%, p < 0.0001, aOR 4.56 [95% CI 2.35–8.97]). The 28-day trajectory of patient statuses (hospitalized vs. discharged alive vs. in-hospital death) is depicted in Fig. 2a. In C-CAP patients, in-hospital death occurred less frequently during the first wave than in the second and third wave (3.2% vs. 11.2% vs. 7.4%, p = 0.0451, sTable 3). Throughout all waves, rate of ICU treatment in C-CAP patients was comparable (22.6% vs. 25.7% vs. 33.8%, p = 0.2335). Median LOHS of C-CAP patients was highest during the first pandemic wave (11 d [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17] vs. 8 d [5, 6, 7, 8, 9, 10, 11, 12] vs. 9 d [6, 7, 8, 9, 10, 11, 12, 13], p = 0.0031), leading to a higher share of patients hospitalized longer than seven days (71.8% vs. 56.6% vs. 52.9%, p = 0.0102). No significant differences were observed in NC-CAP patients between periods before and during the pandemic (sTable 4).
Table 2.
Hospitalization outcomes of patients with COVID-19 community-acquired pneumonia (C-CAP) and non-COVID-19 community-acquired pneumonia (NC-CAP)
| NC-CAP | C-CAP | p-value | aOR (95% CI) | |
|---|---|---|---|---|
| Total (n) | 1024 | 344 | ||
| In-hospital death (%) | 30 (2.9) | 26 (7.6) | 0.0003 | 4.48 (2.38–8.53) |
| ICU treatment (%) | 51 (5.0) | 90 (26.2) | < 0.0001 | 8.08 (5.31–12.52) |
| Invasive MV (%) | 12 (1.2) | 28 (8.1) | < 0.0001 | 9.11 (4.26–20.89) |
| Vasopressor treatment (%) | 14 (1.4) | 33 (9.6) | < 0.0001 | 10.49 (5.17–22.65) |
| LOHS > 7 d (%) | 468 (45.7) | 211 (61.3) | < 0.0001 | 2.21 (1.67–2.92) |
| LOHS > 28 d (%) | 22 (2.1) | 26 (7.6) | < 0.0001 | 4.56 (2.35–8.97) |
| LOHS (d) median (IQR) | 7 (5–10) | 9 (6–15) | < 0.0001 |
Bold numbers indicate p-values < 0.05
Adjusted odds ratios were calculated using age, sex, and the most frequent five comorbidities of both groups as covariates
IQR inter-quartile range, aOR adjusted odds ratio, CI confidence interval, MV mechanical ventilation, LOHS length of hospital stay, ICU intensive care unit
Fig. 2.
Distribution of hospitalization status from admission until 28 days after hospitalization in all patients and ICU patients with C-CAP or NC-CAP. X-axes depict time (d) from hospitalization, Y-axes the rate of patients (%). Green bars represent the percentage of discharged patients, yellow bars the percentage of hospitalized patients, and blue bars the percentage of participants who died in the hospital. The right plots describe the trajectory of the C-CAP participants; the left plots of the NC-CAP participants. ICU: intensive care unit, NC-CAP: non-COVID-19 community-acquired pneumonia, C-CAP: COVID-19 community-acquired pneumonia
ICU patient outcomes
Hospitalization outcomes of ICU-treated patients are summarized in Table 3. Risk of in-hospital death was comparable between C-CAP and NC-CAP ICU patients (26.7% vs. 19.6%, p = 0.4613, aOR 3.08 [95% CI 0.83–12.86]). Risks of invasive MV (31.9% vs. 23.5%, p = 0.4442, aOR 1.42 [95% CI 0.53–4.11]) and vasopressor use (36.7% vs. 27.5%, p = 0.3526, aOR 1.90 [0.70–5.50]) were similar. C-CAP patients with ICU treatment were more likely to exceed seven days of LOHS (92.2% vs. 78.4%, aOR 6.01 [95% CI 1.47–28.83]) and had significantly higher risk for ICULOS over seven days (46.7% vs. 19.6%, aOR 3.36 [95% CI 1.28–9.64]). Median time until ICU admission (one day) and intubation (four days) were equal in C-CAP and NC-CAP. The 28-day trajectory of patient statuses is depicted in Fig. 2b.
Table 3.
Hospitalization outcomes of intensive care unit treated patients with COVID-19 community-acquired pneumonia (ICU C-CAP) and non-COVID-19 community-acquired pneumonia (ICU NC-CAP)
| NC-CAP | C-CAP | p-value | aOR (95% CI) | |
|---|---|---|---|---|
| Total (n) | 51 | 90 | . | . |
| In-hospital death (%) | 10 (19.6) | 24 (26.7) | 0.4613 | 3.08 (0.83–12.86) |
| Invasive MV (%) | 12 (23.5) | 28 (31.1) | 0.4442 | 1.42 (0.53–4.11) |
| Vasopressor treatment (%) | 14 (27.5) | 33 (36.7) | 0.3526 | 1.90 (0.70–5.50) |
| LOHS > 7 d (%) | 40 (78.4) | 83 (92.2) | 0.0361 | 6.01 (1.47–28.83) |
| ICULOS > 7 d (%) | 10 (19.6) | 42 (46.7) | 0.0025 | 3.36 (1.28–9.64) |
| LOHS (d) median (IQR) | 13 (9–22) | 17 (11–29) | 0.0475 | . |
| ICULOS (d) median (IQR) | 3 (1–7) | 7 (4–15) | < 0.0001 | . |
| Length of invasive mechanical ventilation (d) median (IQR) | 4 (1–8) | 15 (8–23) | 0.0115 | . |
| Length of vasopressor treatment (d) median (IQR) | 2 (1–8) | 10 (2–22) | 0.0127 | . |
| Time from hospital admission to ICU admission (d) median (IQR) | 1 (0–3) | 1 (0–3) | 0.9266 | . |
| Time from hospital admission to first intubation (d) median (IQR) | 4 (2–10) | 4 (3–7) | 0.9055 | . |
| Time from ICU admission to first intubation (d) median (IQR) | 1 (0–1) | 1 (0–1) | 0.4755 | . |
Bold numbers indicate p-values < 0.05
IQR inter-quartile range, CI confidence interval, ICU intensive care unit, MV mechanical ventilation, LOHS length of hospital stay, ICULOS length of ICU stay
Risk factor analysis
sTable 5 presents adjusted odds ratios from multivariate logistic regression with patient demography, recruitment period, comorbidity, and treatment as covariates for in-hospital death, LOHS over seven days, and ICU treatment. Female sex was associated with lower risk for ICU treatment in C-CAP (aOR 0.36 [95% CI 0.18–0.68]) but showed no association in NC-CAP (aOR 0.76 [95% CI 0.38–1.43]). Higher age was linked to elevated risks for in-hospital death and LOHS over seven days in both groups. In C-CAP, the second pandemic wave was linked to higher risk of in-hospital mortality (aOR 7.64 [95% CI 1.58–60.60]). Both the second and third pandemic wave were associated with lower risk of LOHS over seven days in C-CAP (second: aOR 0.28 [95% CI 0.13–0.58]; third: aOR 0.24 [95% CI 0.11–0.55]). In NC-CAP, recruitment period was not associated with the outcomes. Administration of antibiotics as well as dexamethasone were associated with LOHS over seven days and ICU treatment in C-CAP.
Subgroup analysis of LOHS
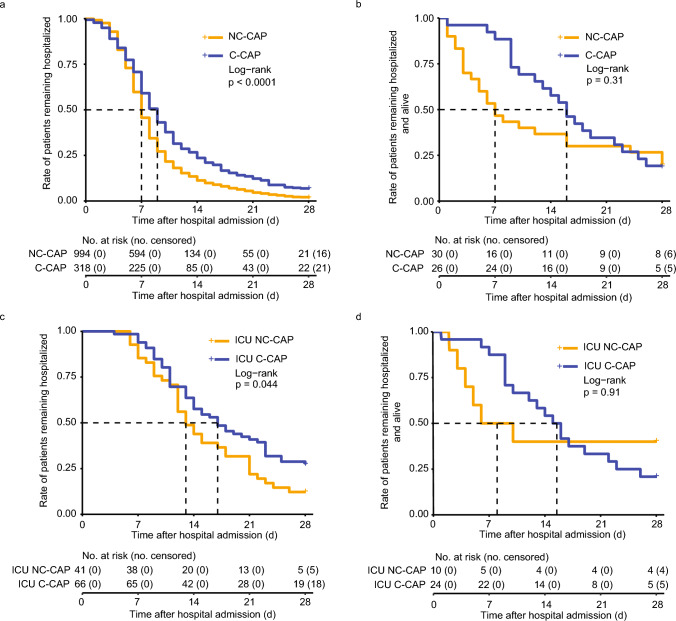
Figure 3 presents Kaplan–Meier curves of LOHS in C-CAP and NC-CAP. C-CAP patients who survived the hospitalization had a longer median LOHS than NC-CAP patients who were discharged alive (9 d vs. 7 d, p < 0.0001, Fig. 3a). In patients who deceased during hospitalization, median LOHS in C-CAP was longer than NC-CAP (16 d vs. 7 d), however remained below the assumed level of significance (p = 0.3054, Fig. 3b). In surviving ICU patients, median LOHS was longer in C-CAP than in NC-CAP (17 d vs. 13 d, p = 0.0441, Fig. 3c). In deceased ICU patients, median LOHS was longer in C-CAP than in NC-CAP (16 d vs. 8 d), however the difference remained under the assumed level of significance (p = 0.9056, Fig. 3d).
Fig. 3.
Time-to-event analysis of length of hospital stay. X-axes represent time (days) after hospital admission, Y-axes the rate of patients remaining hospitalized. Blue curves represent participants with COVID-19 CAP, orange curves non-COVID-19 CAP. Plus ( + ) sign indicates censoring (at day 28 after hospital admission). Dashed lines represent median length of hospital stay. a) Time from hospital admission to hospital discharge in survivors of hospitalization. b) Time from hospital admission to in-hospital death in participants who deceased during the hospital stay. c) Time from hospital admission to hospital discharge in ICU (intensive care unit) treated survivors of hospitalization. d) Time from admission to in-hospital death in ICU-treated non-survivors of hospitalization. NC-CAP: non-COVID-19 community-acquired pneumonia, C-CAP: COVID-19 community-acquired pneumonia. No: number
Follow-up
Outcomes from the 180-day follow-up are presented in sTable 6. 272 (79.1%) C-CAP patients and 905 (88.3%) NC-CAP patients were included in the 180 days post-hospitalization follow-up. Fewer C-CAP patients died after hospital discharge than NC-CAP patients (1.5% vs. 3.9%, p = 0.0540, aOR 0.65 [95% CI 0.11–1.99]). C-CAP patients were at a lower risk for re-hospitalization than NC-CAP patients (9.3% vs. 20.1%, p < 0.0001, aOR 0.43 [95% CI 0.27–0.70]).
Discussion
This study analyzed prospective cohort study data on hospitalized patients with C-CAP and NC-CAP, providing insights into clinical outcomes both during hospitalization and a subsequent follow-up. Spanning five countries and 35 clinical centers over 54 months, the findings underscore the enormous severity in COVID-19 pneumonia during the early stages of the pandemic. The results portray the temporal spectrum of COVID-19 pneumonia.
treatment, trajectory, and outcomes during the peak of a global health emergency, and put them into context with NC-CAP.
The analysis revealed a four-fold higher risk for in-hospital death, an eight-fold higher risk of ICU treatment, and two-fold higher risk of hospitalization exceeding seven days in C-CAP compared to NC-CAP patients. Underlining this, C-CAP patients’ median length of invasive MV exceeded NC-CAP by eleven days. In cases of in-hospital death, C-CAP patients’ treatment duration was more than double than in NC-CAP. This highlights an exceptional burden of severe illness and elevated healthcare demand in C-CAP compared to NC-CAP, particularly among ICU-treated patients, whose risk of remaining hospitalized over seven days was six-fold higher in C-CAP. We observed variability in C-CAP outcomes across pandemic phases, with longer hospital stays in the first wave, but higher risk of in-hospital death during the second and third wave. Having reported excess LOHS during the first wave compared to second and third wave resembling our results, investigators from Bologna assigned this to changing containment policies and improved clinical management during the later stages of the pandemic [19]. Additionally, Lampl et al. discussed caution regarding hospital admission of COVID-19 cases among both patients and clinics during the later stages of the pandemic, potentially leading to delayed hospitalizations and, consequently, to shorter hospital stays either through discharge or due to in-hospital mortality as in our study [20].
COVID-19 case fatality rates and mortality throughout the pandemic were shaped by the emergence of evolving virus variants, improved treatment guidelines, the demographic composition, including age structure and comorbidities among COVID-19 cases, as well as the rising rates of immunization [21, 22]. Supporting our results, Lampl et al. reported the highest COVID-19 case fatality rate in the Regensburg area during the second COVID-19 wave, attributing this to the spreading of the disease to an older-aged population during late 2020 and 2021, where a strained health-care system disposed over limited resources and effective SARS-CoV-2 vaccines were not yet broadly available [20]. Interestingly, there were no significant differences in NC-CAP outcomes comparing the recruitment phases before and during the pandemic in our study, emphasizing the distinct impact of the pandemic evolution on C-CAP outcomes.
Antibiotic treatment in C-CAP was associated with ICU treatment, in-hospital death, and excess LOHS in our cohort. Of note, though in our cohort the use of antibiotics in C-CAP decreased from the first to the third wave patients, mortality increased. We assume that this seemingly paradoxical relationship is caused by improved guidelines for antibiotic use in COVID-19 based on the observation that only less than 10% and especially ICU patients had bacterial superinfection during the early stages of the pandemic [23].
In the multivariate analyses of factors associated with in-hospital mortality, LOHS over seven days, and ICU treatment, we observed further differences between C-CAP and NC-CAP: Notably, female sex in C-CAP was independently associated with lower risk of ICU treatment, while sex had no significant impact on the outcomes in NC-CAP. The roles of sex and gender in COVID-19 have been extensively discussed: While gender-associated disparities in lifestyle, profession, and the resulting risk of SARS-CoV-2 transmission were crucial for the higher incidence and disease severity in male gender during the early stages of the pandemic [24], sex-determined differences are a major factor influencing a patient’s immune response to SARS-CoV-2 with different mechanisms of acute deterioration [25]. In contrast to our findings, a systematic review highlighted worse outcomes for men also in NC-CAP [26]. More research is needed to distinguish biological and social determinants for unfavorable outcomes in CAP.
These findings are an important contribution to existing results from retrospective and registry analyses attempting a contextualization of C-CAP among CAP. In line with our results, Cangemi et al. found in a prospective cohort study that COVID-19 was associated with a five-fold increase in the in-hospital mortality rate compared to NC-CAP [6]. In a retrospective analysis of hospitalized patients with COVID-19 or Influenza A from a nation-wide hospital network in Germany, Kodde et al. found that COVID-19 was associated with three-fold increased odds for in-hospital death than Influenza A patients [27]. Serrano Fernandez et al. observed rates of in-hospital death and invasive MV twice as high in COVID-19 pneumonia than in bacteremic pneumococcal CAP [28].
In our study, C-CAP was associated with a lower risk of recurrent hospitalization in a 180-days follow up than NC-CAP. This finding is in line with Novelli et al. [29], suggesting that long-term morbidity in COVID-19 depends less on the initial disease severity, but more on patients’ baseline morbidity. Nevertheless, it is important to note that post-discharge morbidity in COVID-19 can manifest in the diverse features of the post-COVID-19 syndrome, characterized by long-lasting fatigue, respiratory and neurocognitive symptoms, and pulmonary function impairment [30], and does not necessarily lead to re-hospitalization. As we did not evaluate the occurrence of long-lasting symptoms, we cannot draw conclusions regarding the post-COVID-19 syndrome and comparable features in NC-CAP. To improve our understanding of morbidity after hospitalization for community-acquired pneumonia, prospective studies like the German national pandemic cohort network NAPKON, evaluating symptoms, pathophysiology, and ideally interventional measures, are desperately needed [31].
Strengths of our analysis are the prospective, multi-national dataset and the harmonized definition of the study participation criteria, yielding a highly comparable sample of both C-CAP and NC-CAP patients examined under the same study protocol for in-hospital and post-discharge outcomes. This analysis provides a comprehensive report of hospitalization and follow-up outcomes comparing both disease groups, offering a retrospective contextualization of the pandemic’s impact on the spectrum of CAP patients. The data show the diverging disease trajectories in C-CAP and NC-CAP and how both treatment and outcomes in C-CAP changed chronologically with the progression of the pandemic.
This study has limitations. The total number of eligible patients is unknown, introducing potential selection bias. E. g., patients with severe C-CAP immediately intubated upon hospital arrival may not have been included, possibly impacting the results. However, time to intubation did not differ between C-CAP and NC-CAP ICU patients, supporting the data validity. Seventeen study participants were excluded, as they were transferred to another hospital. This could introduce a referral bias, particularly as cases with complex disease trajectories, such as those needing extracorporeal membrane oxygenation therapy or extended weaning from mechanical ventilation, are more likely to be referred to specialized hospitals. Furthermore, our analysis focused on in-hospital death, ICU treatment, and LOHS as indicators for disease severity. A more detailed classification of pneumonia outcomes, e. g. varying levels of respiratory support such as high flow nasal oxygen or extra-corporal membrane oxygenation, was not feasible in our data sample. Future large-scale prospective studies need to address CAP severity degrees in more detail.
Conclusion
Based on data from a multinational prospective cohort, this analysis shows the excess risk of C-CAP patients from the first three pandemic waves for in-hospital death, ICU treatment, and prolonged hospital and ICU stay compared to NC-CAP. Risk of re-hospitalization after discharge was elevated in NC-CAP, highlighting the role of CAP etiology in acute and chronic morbidity.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
CAPNETZ is a network to improve our understanding of diagnosis and treatment of patients with community-acquired pneumonia, comprising experts and contributors from various medical specialties. This work would not have been possible without the persistent support of the involved investigators, physicians, pneumologists, infectious diseases specialists, and study nurses from the local clinical centers, who deserve our sincerest gratitude. We further thank Grit Barten-Neiner, managing director of the CAPNETZ foundation, for her tremendous support. Finally, yet importantly, we thank all recruited patients for their study participation. Members of the CAPNETZ study group excluding the authors: A. Fuchs, M. Engelmann (III. Medical Clinic, University Hospital Augsburg); D. Stolz (Department of Pneumology, University Hospital Basel, Switzerland / Clinic of Pneumology, University Hospital Freiburg); W. Bauer, H. C. Mücke (Central Emergency Admission / Medical Admission Ward, Charité-Universitätsmedizin Berlin); S. Schmager (III. Medical Clinic, Carl-Thiem Hospital Cottbus); B. Schaaf, J. Kremling, D. Nickoleit-Bitzenberger, H. Azzaui, M. Hower, F. Hempel, K. Prebeg, K. Popkirova (Pneumology, Infectiology and Internal Intensive Care Medicine, Medical Clinic Nord, Dortmund); M. Kolditz (Medical Clinic I Department of Pneumology, University Hospital Dresden); C. Bellinghausen, A. Grünewaldt (Medical Clinic I—Pneumology/Allergology, University Hospital of Johann Wolfgang Goethe, Frankfurt), M. Panning (Institute of Virology, University Hospital Freiburg); T. Welte (Department of Pneumology, Hannover Medical School, Hannover); T. Fühner, M. van’t Klooster (Department of Pneumology, Intensive Care and Sleep Medicine, Siloah Hospital, Hannover), G. Barten-Neiner, W. Kröner, N. Adaskina, F. Eberherdt, C. Julius (CAPNETZ Office, Hannover); T. Illig, N. Klopp (Hannover Unified Biobank, Hannover Medical School); B. T. Schleenvoigt, C. Forstner, A. Moeser, J. Ankert (Institute for Infection Medicine and Hospital Hygiene (IIMK), University Hospital Jena); D. Drömann, P. Parschke, K. Franzen (Medical Clinic III, Pneumology, University Medical Center Schleswig-Holstein, Lübeck); N. Käding, F. Waldeck (Department of Infectious Diseases and Microbiology, University Hospital Schleswig-Holstein, Lübeck); C. Spinner, J. Erber, F. Voit, J. Schneider (Department of Internal Medicine II, University Hospital rechts der Isar, Technical University of Munich); D. Heigener, I. Hering (Department of Pneumology, Agaplesion Diakonieklinikum Rotenburg); W. Albrich, M. Seneghini, F. Rassouli, S. Baldesberger (Department of Infectiology and Hospital Hygiene, Kantonsspital St. Gallen, Switzerland); A. Essig, S. Stenger (Institute for Medical Microbiology and Hygiene, University Hospital Ulm), M. Wallner (2mt Software, Ulm); H. Burgmann, L. Traby, L. Schubert, R. Chen (University Clinic for Internal Medicine I, Medical University of Vienna); and all study nurses.
Author contributions
Study concept: H.-J. M., M. M., F. S. Data acquisition: CAPNETZ study group. Interpretation of data: H.-J. M., L. M., O. U., W. X., T. Z., M. M., F. S. Initial manuscript draft: H.-J. M. Final manuscript preparation: H.-J. M., S. B. , M. M.-P., G. R., M. W. P., J. R., N. S., M. W., T. Z., M. M., F.S. All authors have read and approved the definitive version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work received support from the German Federal Ministry of Education and Research (BMBF) via the studies PROVID (grant number 01KI20160A), CAPNETZ (01KI07145), PROGRESS (01KI07110 [Giessen], 01KI07111 [Jena], 01KI07113 [Leipzig], 01KI07114 [Berlin], 01KI1010I [Leipzig]), and for the SFB-TR84 (114933180). CAPNETZ has been associated member of the German Center for Lung Research since 2013 (82DZL002B4). HJM received a doctoral student scholarship from third party funding acquired by MW. The funding parties of the scholarship were Biotest AG and Vaxxilon Deutschland GmbH. MM is a fellow of the BIH-Charite Digital Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin, the Berlin Institute of Health, and the German Research Foundation (DFG).
Declarations
Conflict of interest
H.-J. M. reports a personal fee from Astra Zeneca for a lecture. G. R. reports personal fees from Astra Zeneca, Atriva, Boehringer Ingelheim, GSK, Insmed, MSD, Sanofi, Novartis, and Pfizer for consultancy during advisory board meetings and personal fees from Astra Zeneca, Berlin Chemie, BMS, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Sanofi, Solvay, Takeda, Novartis, Pfizer, and Vertex for lectures. M. W. received funding for research, lectures, or advisory from Alexion, Aptarion, Astra Zeneca, Bayer Health Care, Biotest, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Insmed, Novartis, Pantherna, Pfizer, Teva, and Vaxxilon. T. Z. received funding for research from Bundesministerium für Bildung und Forschung, Else Kröner Fresenius Stiftung and Gesellschaft für Internationale Zusammenarbeit.
Footnotes
Mirja Mittermaier and Fridolin Steinbeis have contributed equally.
Contributor Information
Fridolin Steinbeis, Email: fridolin.steinbeis@charite.de.
CAPNETZ study group:
A Fuchs, M Engelmann, D Stolz, W Bauer, H. C Mücke, S Schmager, B Schaaf, J Kremling, D Nickoleit-Bitzenberger, H Azzaui, M Hower, F Hempel, K Prebeg, K Popkirova, M Kolditz, C Bellinghausen, A Grünewaldt, M Panning, T Welte, T Fühner, M. van’t Klooster, G Barten-Neiner, W Kröner, N Adaskina, F Eberherdt, C Julius, T Illig, N Klopp, B. T Schleenvoigt, C Forstner, A Moeser, J Ankert, D Drömann, P Parschke, K Franzen, N Käding, F Waldeck, C Spinner, J Erber, F Voit, J Schneider, D Heigener, I Hering, W Albrich, M Seneghini, F Rassouli, S Baldesberger, A Essig, S Stenger, M Wallner, H Burgmann, L Traby, L Schubert, and R Chen
References
- 1.Quan TP, Fawcett NJ, Wrightson JM, Finney J, Wyllie D, Jeffery K, et al. Increasing burden of community-acquired pneumonia leading to hospitalization, 1998–2014. Thorax. 2016;71:535–42. 10.1136/thoraxjnl-2015-207688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S, Fullman N, Mosser J, Thompson RL, Reiner RC, Abajobir A. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Infect Dis. 2017;17:1133–61. 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montag K, Kampf G. Acute lower respiratory tract infections accounted for 56.2% of hospitalized COVID-19 cases in Germany during the first three waves. Int J Epidemiol. 2022;51:1032–3. 10.1093/ije/dyac059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dähne T, Bauer W, Essig A, Schaaf B, Spinner CD, Pletz MW, et al. The impact of the SARS-CoV-2 pandemic on the prevalence of respiratory tract pathogens in patients with community-acquired pneumonia in Germany. Emerg Microbes Infect. 2021;10:1515–8. 10.1080/22221751.2021.1957402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuurman AR, Reijnders TD, van Engelen TS, Léopold V, de Brabander J, van Linge C, Schinkel M, Pereverzeva L, Haak BW, Brands X, Kanglie MM. The host response in different aetiologies of community-acquired pneumonia. EBioMedicine. 2022;81:104082. 10.1016/j.ebiom.2022.104082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cangemi R, Calvieri C, Falcone M, Cipollone F, Ceccarelli G, Pignatelli P, et al. Comparison of thrombotic events and mortality in patients with community-acquired pneumonia and COVID-19: a multicenter observational study. Thromb Haemost. 2022;122:257–66. 10.1055/a-1692-9939. [DOI] [PubMed] [Google Scholar]
- 7.Wendisch D, Dietrich O, Mari T, von Stillfried S, Ibarra IL, Mittermaier M, et al. SARS-CoV-2 infection triggers profibrotic macrophage responses and lung fibrosis. Cell. 2021;184:6243-61.e27. 10.1016/j.cell.2021.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aliberti S, Brambilla AM, Chalmers JD, Cilloniz C, Ramirez J, Bignamini A, et al. Phenotyping community-acquired pneumonia according to the presence of acute respiratory failure and severe sepsis. Respir Res. 2014;15:27. 10.1186/1465-9921-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Roquetaillade C, Bredin S, Lascarrou J-B, Soumagne T, Cojocaru M, Chouserman BG, et al. Timing and causes of death in severe COVID-19 patients. Crit Care. 2021;25:224. 10.1186/s13054-021-03639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583–90. 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang C-CH, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–74. 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclerc QJ, Fuller NM, Keogh RH, Diaz-Ordaz K, Sekula R, Semple MG, et al. Importance of patient bed pathways and length of stay differences in predicting COVID-19 hospital bed occupancy in England. BMC Health Serv Res. 2021;21:566. 10.1186/s12913-021-06509-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welte T, Suttorp N, Marre R. CAPNETZ-community-acquired pneumonia competence network. Infection. 2004;32:234–8. 10.1007/s15010-004-3107-z. [DOI] [PubMed] [Google Scholar]
- 15.Tolksdorf K, Loenenbach A, Buda S. Dritte Aktualisierung der ‘Retrospektiven Phaseneinteilung der COVID-19-Pandemie in Deutschland.’ Epid Bull. 2022;38:3–6. 10.25646/10598. [Google Scholar]
- 16.Therneau TM, Lumley T, Elizabeth A, Cynthia C. survival: survival analysis. 2023; published online Jan 9. https://CRAN.R-project.org/package/survival. Accessed 27 Feb 2024.
- 17.Kassambara A, Kosinski M, Biecek P, Fabian S. survminer: drawing survival curves using ‘ggplot2’. 2021; published online March 9. https://CRAN.R-project.org/package=survminer. Accessed 27 Feb 2024.
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) stagement: guidelines for reporting observational studies. BMJ. 2007;335:806–8. 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeleke AJ, Moscato S, Miglio R, Chiari L. Length of stay analysis of COVID-19 hospitalizations using a count regression model and quantile regression: a study in Bologna, Italy. Int J Environ Res Public Health. 2022;19:2224. 10.3390/ijerph19042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lampl BMJ, Edenharter B, Leitzmann MF, Salzberger B. COVID-19-related deaths: a 2-year inter-wave comparison of mortality data from Germany. Infection. 2023;51:1147–52. 10.1007/s15010-023-01982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin D-Y, Gu Y, Wheeler B, Young H, Holloway S, Sunny SK, et al. Effectiveness of Covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386:933–41. 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sievers C, Zacher B, Ullrich A, Huska M, Fuchs S, Buda S, et al. SARS-CoV-2 Omicron variants BA.1 and BA.2 both show similarly reduced disease severity of COVID-19 compared to Delta, Germany, 2021 to 2022. Eurosurveillance. 2022;27:2200396. 10.2807/1560-7917.ES.2022.27.22.2200396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–75. 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA. Sex difference in coronavirus disease (COVID-19): a systematic review and meta-analysis. BMJ Open. 2020;10:e040129. 10.1136/bmjopen-2020-040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–20. 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corica B, Tartaglia F, D’Amico T, Romiti GF, Cangemi R. Sex and gender differences in community-acquired pneumonia. Intern Emerg Med. 2022;17:1575–88. 10.1007/s11739-022-02999-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodde C, Bonsignore M, Schöndube D, Bauer T, Hohenstein S, Bollmann A, et al. Mortality in cancer patients with SARS-CoV-2 or seasonal influenza: an observational cohort study from a German-wide hospital network. Infection. 2023;51:119–27. 10.1007/s15010-022-01852-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano Fernández L, Ruiz Iturriaga LA, España Yandiola PP, Méndez Ocaña R, Pérez Fernández S, Tabernero Huget E, et al. Bacteraemic pneumococcal pneumonia and SARS-CoV-2 pneumonia: differences and similarities. Int J Infect Dis. 2022;115:39–47. 10.1016/j.ijid.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novelli L, Raimondi F, Carioli G, Carobbio A, Pappacena S, Biza R, et al. One-year mortality in COVID-19 is associated with patients’ comorbidities rather than pneumonia severity. Respir Med Res. 2023;83:100976. 10.1016/j.resmer.2022.100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–15. 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schons M, Pilgram L, Reese J-P, Stecher M, Anton G, Appel KS, et al. The German National Pandemic Cohort Network (NAPKON): rationale, study design and baseline characteristics. Eur J Epidemiol. 2022;37:849–70. 10.1007/s10654-022-00896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.