Abstract
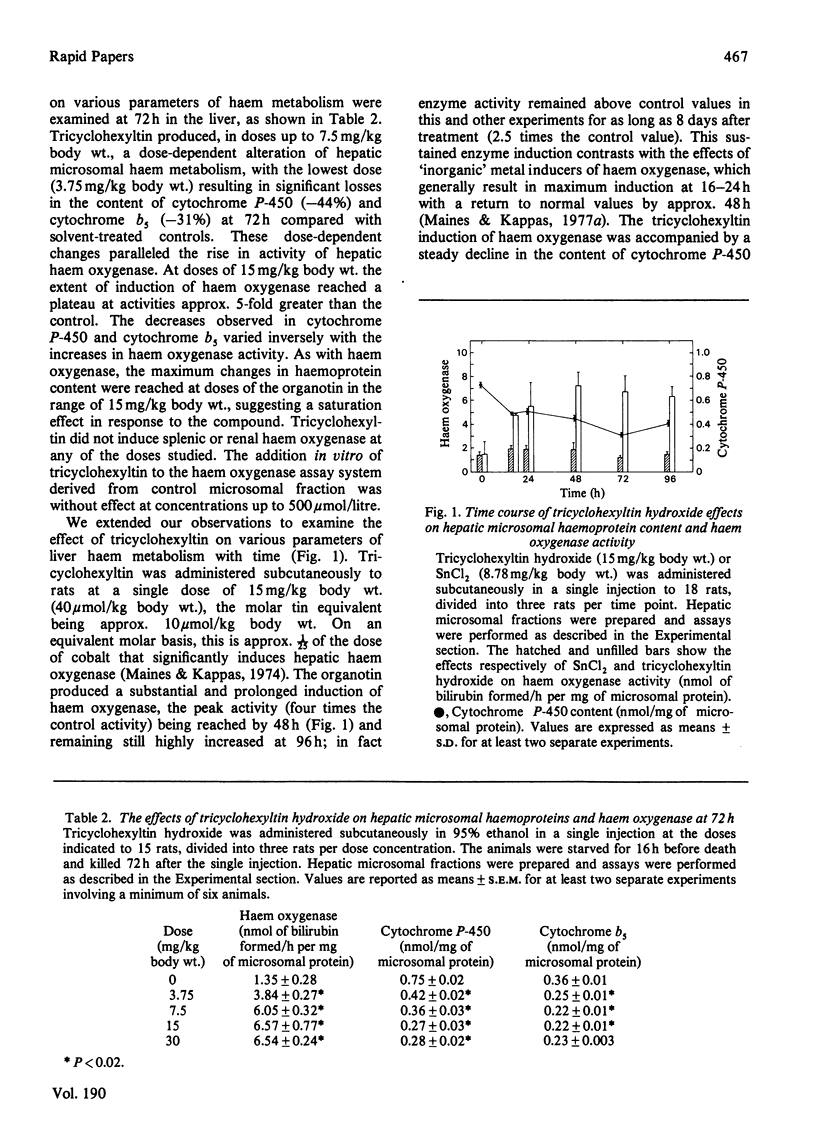
The administration of organotin compounds to rats in single doses causes a significant and prolonged induction of haem oxygenase and a sustained decrease in haemoprotein content in the liver. The extent of induction of hepatic haem oxygenase varied between 3 and 5-fold at 72h after a single injection of water-insoluble organotins of differing structure. The alterations in haem metabolism produced by tricyclohexyltin hydroxide were studied in detail. The effects were dose-dependent, with doses as low as 3.75 mg/kg body wt. resulting in significant induction of haem oxygenase and a decrease in cytochrome P-450 and cytochrome b5 contents at 72h in the liver. The effects with time of a single dose of tricyclohexyltin on various parameters of liver haem metabolism were also examined. The organotin produced a substantial and very prolonged induction of haem oxygenase accompanied by a steady decline in cytochrome P-450 content for periods up to 8 days. The long duration of action of these organotins with respect to induction of haem oxygenase and depletion of cellular haemoprotein content provides a highly sensitive metabolic system with which to define further the toxic potential of organometals as well as to study the adaptive responses in liver to long-term perturbations of haem metabolism by foreign chemicals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge W. N., Casida J. E., Fish R. H., Kimmel E. C., Street B. W. Action on mitochondria and toxicity of metabolites of tri-n-butyltin derivatives. Biochem Pharmacol. 1977 Nov 1;26(21):1997–2000. doi: 10.1016/0006-2952(77)90008-9. [DOI] [PubMed] [Google Scholar]
- BARNES J. M., STONER H. B. The toxicology of tin compounds. Pharmacol Rev. 1959 Jun;11(2 Pt 1):211–231. [PubMed] [Google Scholar]
- Bock K. W., Siekevitz P. Turnover of heme and protein moieties of rat liver microsomal cytochrome b5. Biochem Biophys Res Commun. 1970 Oct 23;41(2):374–380. doi: 10.1016/0006-291x(70)90514-0. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Manganese and zinc blockade of enzyme induction: studies with microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5331–5335. doi: 10.1073/pnas.76.10.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Maines M. D. Tin: a potent inducer of heme oxygenase in kidney. Science. 1976 Apr 2;192(4234):60–62. doi: 10.1126/science.1257757. [DOI] [PubMed] [Google Scholar]
- Kimbrough R. D. Toxicity and health effects of selected organotin compounds: a review. Environ Health Perspect. 1976 Apr;14:51–56. doi: 10.1289/ehp.761451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel E. C., Fish R. H., Casida J. E. Bioorganotin chemistry. Metabolism of organotin compounds in microsomal monooxygenase systems and in mammals. J Agric Food Chem. 1976 Jan-Feb;25(1):1–9. doi: 10.1021/jf60209a002. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Enzymes of heme metabolism in the kidney: regulation by trace metals which do not form heme complexes. J Exp Med. 1977 Nov 1;146(5):1286–1293. doi: 10.1084/jem.146.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Studies on the mechanism of induction of haem oxygenase by cobalt and other metal ions. Biochem J. 1976 Jan 15;154(1):125–131. doi: 10.1042/bj1540125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollister D. D., Schober A. E. Assessing toxicological properties of organotin Compounds. Environ Qual Saf. 1975;4:80–95. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Rose M. S., Aldridge W. N. The interaction of triethyltin with components of animal tissues. Biochem J. 1968 Feb;106(4):821–828. doi: 10.1042/bj1060821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Kappas A., Bernstein S. E., Alvares A. P. Heme biosynthesis and drug metabolism in mice with hereditary hemolytic anemia. Heme oxygenase induction as an adaptive response for maintaining cytochrome P-450 in chronic hemolysis. J Biol Chem. 1979 Feb 10;254(3):729–735. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]


