Abstract
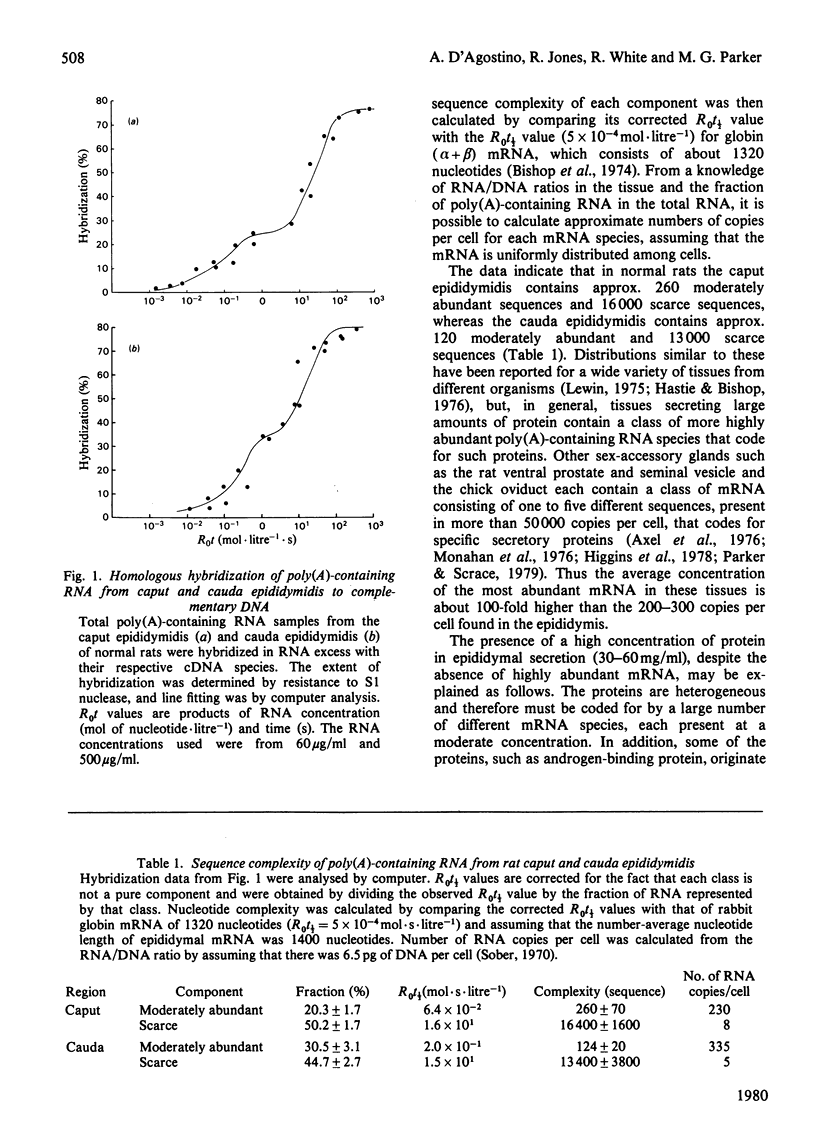
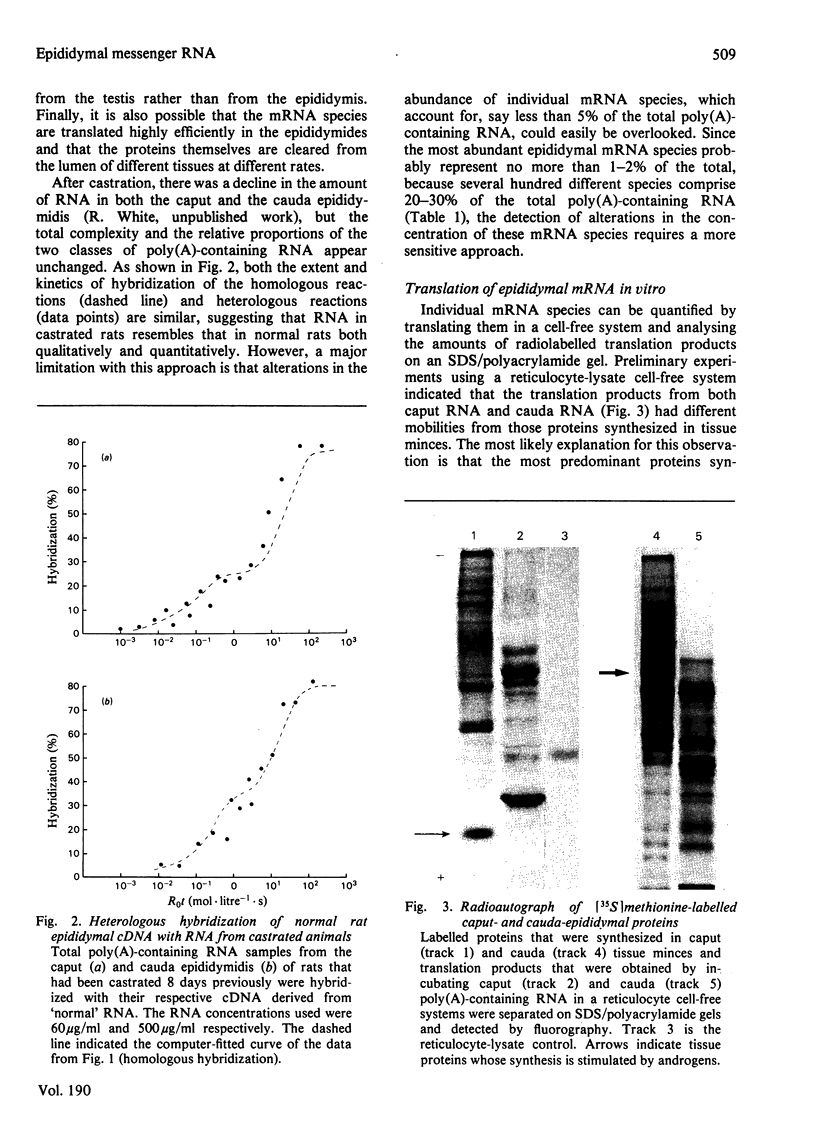
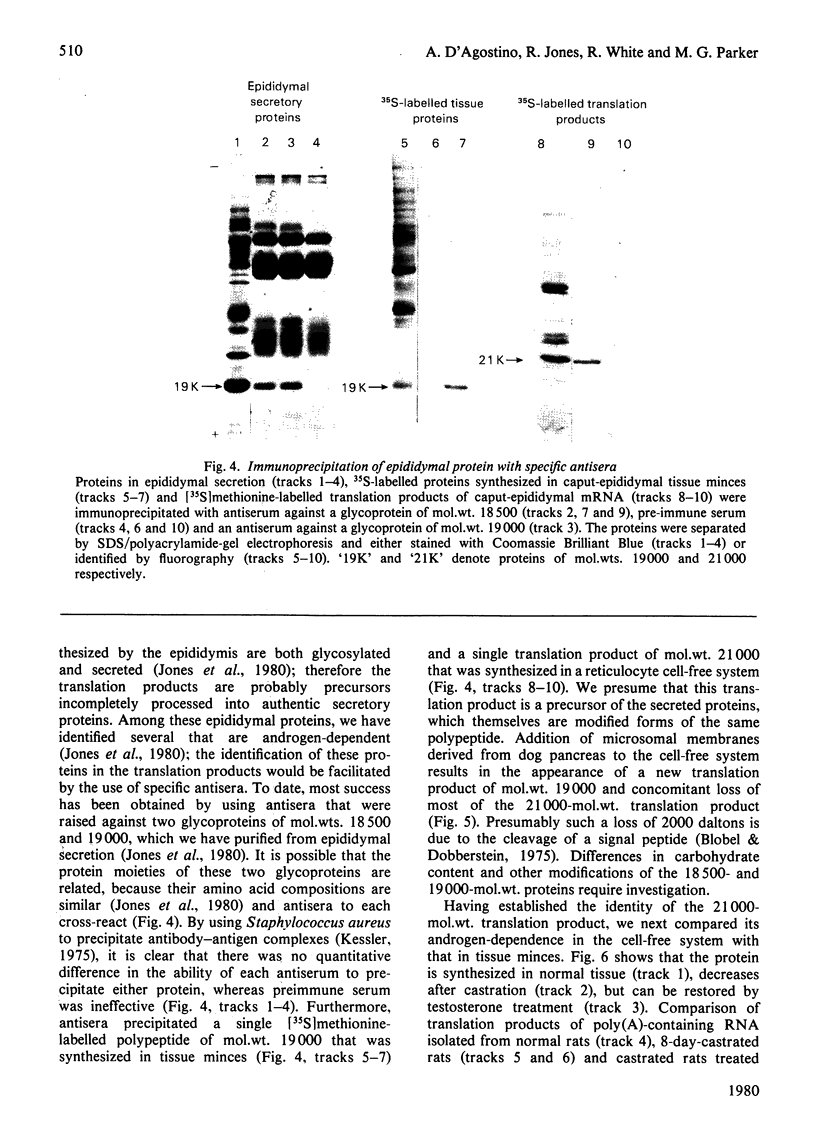
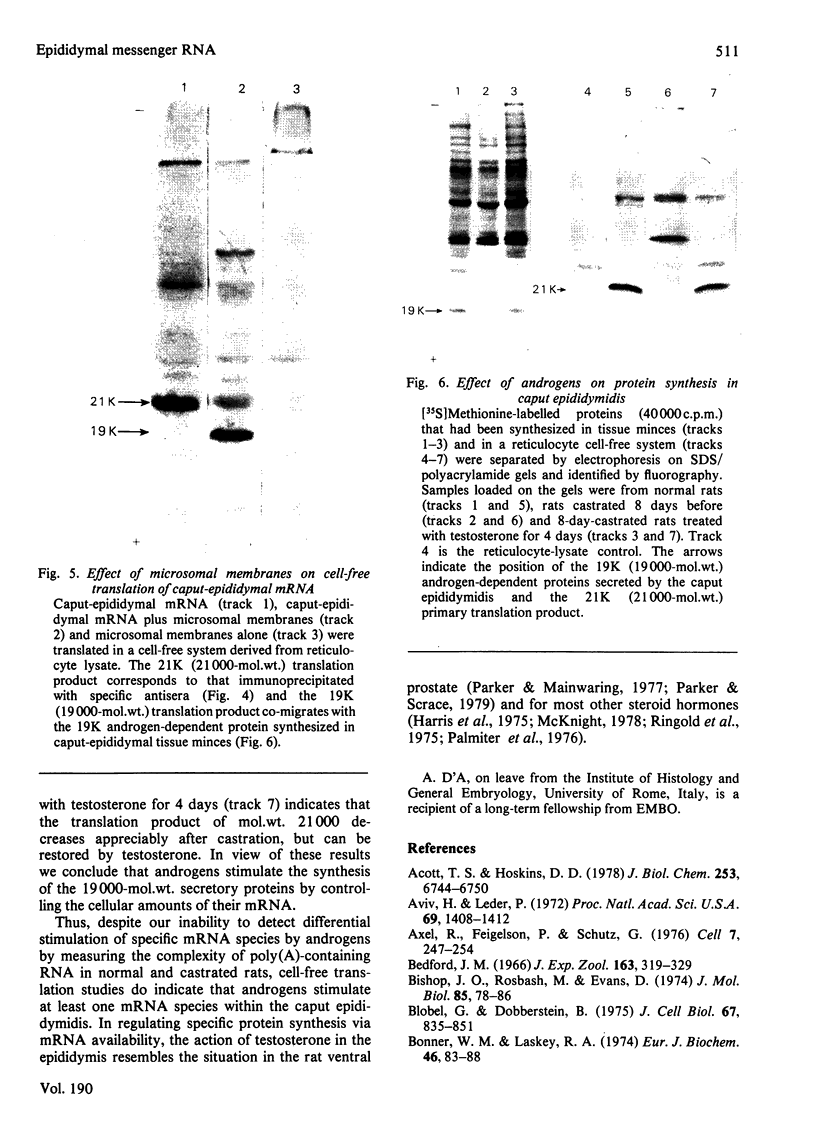
1. The regulation by testosterone of mRNA complexity and mRNA activity was investigated in rat caput and cauda epididymidis. 2. The sequence complexity of cytoplasmic poly(A)-containing RNA from normal rats was determined by homologous hybridization with radiolabelled complementary DNA probes by using RNA in excess. Computer analysis of results suggested that hybridization could best be described by curves composed of two components distinguished by their relative abundance. Thus caput-epididymidal RNA consists of approx. 260 moderately abundant and 16400 scarce sequences, whereas cauda-epididymidal RNA consists of approx. 124 moderately abundant and 13400 scarce sequences. Judging by heterologous-hybridization reactions, castration did not result in appreciable alterations in either sequence complexity or the relative abundance of the two classes of poly(A)-containing RNA. 3. To investigate if individual mRNA sequences were regulated by androgens, mRNA was translated in a cell-free system derived from reticulocyte lysate. Since most of the translation products had a different mobility on sodium dodecyl sulphate/polyacrylamide gels from the authentic proteins synthesized in tissue minces, antibodies were used to identify specific translation products. Antibodies to the two related major proteins (mol.wt. 18500 and 19000) secreted by the caput epididymidis and whose synthesis is stimulated by testosterone both precipitated a single translation product of mol.wt. 21000. That this polypeptide was a precursor to the secreted proteins was suggested by the fact that the addition of microsomal membranes isolated from dog pancreas resulted in the appearance of a polypeptide of mol. wt. 19000. 4. Translation of RNA from the caput epididymidis of rats of different hormonal status showed that mRNA activity for the 21000-dalton polypeptide declined after castration, but could be restored by treating rats with testosterone. 5. It is concluded that testosterone stimulates the synthesis of a major protein secreted by the caput epididymidis by regulating its mRNA activity.
Full text
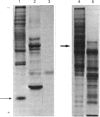
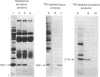
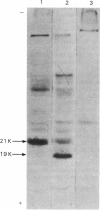
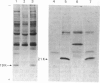
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acott T. S., Hoskins D. D. Bovine sperm forward motility protein. Partial purification and characterization. J Biol Chem. 1978 Oct 10;253(19):6744–6750. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Feigelson P., Schutz G. Analysis of the complexity and diversity of mRNA from chicken liver and oviduct. Cell. 1976 Feb;7(2):247–254. doi: 10.1016/0092-8674(76)90024-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Garoff H., Simons K., Dobberstein B. Assembly of the Semliki Forest virus membrane glycoproteins in the membrane of the endoplasmic reticulum in vitro. J Mol Biol. 1978 Oct 5;124(4):587–600. doi: 10.1016/0022-2836(78)90173-0. [DOI] [PubMed] [Google Scholar]
- Harris S. E., Rosen J. M., Means A. R., O'Malley B. W. Use of a specific probe for ovalbumin messenger RNA to quantitate estrogen-induced gene transcripts. Biochemistry. 1975 May 20;14(10):2072–2081. doi: 10.1021/bi00681a006. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M. Effects of testosterone on messenger ribonucleic acid and protein synthesis in rat seminal vesicle. Biochem J. 1978 Aug 15;174(2):543–551. doi: 10.1042/bj1740543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Testosterone control of nucleic acid content and proliferation of epithelium and stroma in rat seminal vesicles. Biochem J. 1976 Oct 15;160(1):43–48. doi: 10.1042/bj1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Parker M. G., Herries D. G. Effects of testosterone on sequence complexity of polyadenylated RNA from rat seminal vesicle. Eur J Biochem. 1978 Nov 15;91(2):327–334. doi: 10.1111/j.1432-1033.1978.tb12683.x. [DOI] [PubMed] [Google Scholar]
- Jones R., Brown C. R., Von Glós K. I., Parker M. G. Hormonal regulation of protein synthesis in the rat epididymis. Characterization of androgen-dependent and testicular fluid-dependent proteins. Biochem J. 1980 Jun 15;188(3):667–676. doi: 10.1042/bj1880667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- McKnight G. S. The induction of ovalbumin and conalbumin mRNA by estrogen and progesterone in chick oviduct explant cultures. Cell. 1978 Jun;14(2):403–413. doi: 10.1016/0092-8674(78)90125-3. [DOI] [PubMed] [Google Scholar]
- Monahan J. J., Harris S. E., O'Malley B. W. Effect of estrogen on gene expression in the chick oviduct. Effect of estrogen on the sequence and population complexity of chick oviduct poly(A)-containing RNA. J Biol Chem. 1976 Jun 25;251(12):3738–3748. [PubMed] [Google Scholar]
- Orgebin-Crist M. C. Studies on the function of the epididymis. Biol Reprod. 1969 Jun;1(Suppl):155–175. doi: 10.1095/biolreprod1.supplement_1.155. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Moore P. B., Mulvihill E. R. A significant lag in the induction of ovalbumin messenger RNA by steroid hormones: a receptor translocation hypothesis. Cell. 1976 Aug;8(4):557–572. doi: 10.1016/0092-8674(76)90224-5. [DOI] [PubMed] [Google Scholar]
- Parker M. G. Hormonal control of mRNA synthesis studied by nucleic acid hybridization. Mol Cell Endocrinol. 1978 Apr;10(2):119–133. doi: 10.1016/0303-7207(78)90119-3. [DOI] [PubMed] [Google Scholar]
- Parker M. G., Mainwaring W. I. Effects of androgens on the complexity of poly(A) RNA from rat prostate. Cell. 1977 Oct;12(2):401–407. doi: 10.1016/0092-8674(77)90116-7. [DOI] [PubMed] [Google Scholar]
- Parker M. G., Scrace G. T., Mainwaring W. I. Testosterone regulates the synthesis of major proteins in rat ventral prostate. Biochem J. 1978 Jan 15;170(1):115–121. doi: 10.1042/bj1700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. G., Scrace G. T. Regulation of protein synthesis in rat ventral prostate: cell-free translation of mRNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1580–1584. doi: 10.1073/pnas.76.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. G., Scrace G. T. The androgenic regulation of abundant mRNA in rat ventral prostate. Eur J Biochem. 1978 Apr 17;85(2):399–406. doi: 10.1111/j.1432-1033.1978.tb12252.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]