Abstract
Benign prostatic hyperplasia (BPH) is an age-related disease that affects millions of aging males globally. While the pathogenesis of BPH remains incompletely understood, emerging evidence suggests a pivotal role for the androgen receptor (AR) in mediating prostate growth and function. Understanding age-related AR signaling alteration may inform novel BPH treatments. Here, we analyzed the prostatic protein expressions of AR, NKX3.1, and Ki-67 in young (2 months) and aged (24 months) mice. We also examined the potential mechanism of AR protein expression. Compared to young mice, decreased AR and NKX3.1 protein expression was observed in the anterior prostate (AP) and ventral prostate (VP) of aged mice, indicating reduced AR signaling in these prostate lobes. Additionally, we observed decreased protein expression of proliferation maker Ki-67 in aged AP, VP, and dorsal-lateral prostate (DLP), with no difference in apoptosis as compared to young counterparts. We conclude that prostatic androgen receptor signaling shows an age-related and lobe-specific alteration in mice.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79879-x.
Keywords: Benign prostatic hyperplasia, Androgen receptor, Lower urinary tract symptoms, Prostatic proliferation, Aging, Prostate, Urology, Hormone
Subject terms: Urology, Prostate
Introduction
Benign prostatic hyperplasia (BPH) is one of the most prevalent and clinically important urological conditions affecting aging men worldwide1. In 2019, about 94 million cases of BPH were reported globally, rising from about 51.1 million cases in 20002. Published data indicated that the annual cost of BPH-related management exceeded 1.5 billion dollars in the United States3. Symptomatic BPH can manifest lower urinary tract symptoms (LUTS) such as urinary hesitancy, increased voiding frequency and urgency, and nocturia, which significantly impacts patients’ quality of life4,5. In addition, despite being a non-malignant condition, the progressive nature of BPH often leads to complications such as bladder stones, kidney failure, and urinary tract infections6.
It has been shown that the occurrence of BPH is closely related to age, and aging has been identified as a risk factor for BPH/LUTS7. Approximately 50% of men over the age of 50 and around 80% of men over the age of 70 are under the influence of BPH/LUTS8,9. During aging, the prostate gland undergoes physiological changes, including glandular enlargement and increased smooth muscle tone, which can result in LUTS10–12. Age-related alterations in prostatic structure and function are accompanied by steroid hormonal changes, particularly involving androgens. As men age, the level of serum androgens, such as testosterone (T) and dihydrotestosterone (DHT), decreases13,14. It has been believed that the androgen receptor (AR) signaling axis plays a pivotal role in the pathology of BPH since T and DHT are ligands of AR15,16. However, the exact mechanisms by which aging influences androgen metabolism and action in the prostate remain unclear.
The prostate gland is an androgen-dependent organ, and AR activation is essential for normal prostatic development and function17. Dysregulation of AR signaling contributes to the aberrant proliferation and survival of prostatic cells observed in BPH18. Clinically, the use of 5-alpha reductase inhibitors (5ARIs), which inhibit the process of T-to-DHT conversion, has shown success in BPH/LUTS management19. In some patients, 5ARI reduces the clinical risk of disease progression and improves symptom scores, compared with placebo19,20. However, as 5ARI abates AR signaling, the effectiveness of 5ARI as a BPH treatment seems to contradict androgen reduction during aging. Thus, having a better understanding of AR signaling characteristics during aging is of great importance.
For decades, mice have been used as a model for human diseases. However, histologic BPH in men is commonly described as nodular growth, due to the types of glandular and stromal prostatic nodules observed. These nodules are obvious in humans but not apparent in rodents. Nonetheless, prostate growth, hyperplasia, and hypertrophy occur in prostate enlargement found in aged men and mice21. Additionally, urinary symptoms (e.g. increased urinary frequency) found in men with BPH also occur in aged mice21. Although the mouse prostate is anatomically distinct from the human prostate, the true nature of the zones and lobes of these species remains enigmatic. Our previous work demonstrated that mice could represent the cellular and functional aspects of BPH21,22. In addition, a decrease in serum T level with age has been observed in mice, suggesting that mice may experience similar hormone changes to those seen in humans during the aging process21,23.
Our earlier work has characterized AR protein expression in BPH and normal prostate22. Still, due to a limited source of young human samples, the impact of aging on prostatic AR signaling remains unexplored. In this study, we evaluated and quantified the localization and expression of prostatic AR and AR downstream target NKX3.1 in mice at 2 months (young) and 24 months (aged) of age. We also determined the effect of aging on prostatic hyperplasia by using the proliferation marker Ki-67 and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). Our work provides a better understanding of the role of androgen signaling in the aging prostate.
Materials and methods
Animals
All animal experiments were conducted under the protocols approved by the University of Wisconsin Animal Care and Use Committee. All authors comply with the ARRIVE guidelines, and all methods were performed in accordance with the relevant guidelines and regulations. Male C57Bl6/J mice (cat# 000664) were obtained from the Jackson Laboratory (Bar Harbor, ME) or directly from the National Institute of Aging (Wilmington, MA). Animals were housed under standard laboratory conditions with a 12:12 light/dark cycle and provided with food and water ad libitum. Young mice were euthanized at 2 months of age and aged mice were euthanized at 24 months of age. Mice were euthanized with carbon dioxide followed by cardiac puncture. The anterior prostate (AP, young n = 9, aged n = 9), ventral prostate (VP, young n = 9, aged n = 9), and dorsal-lateral prostate (DLP, young n = 9, aged n = 9) were carefully dissected, fixed in 10% normal buffered formalin, and embedded in paraffin.
Immunohistochemistry
All procedures were performed as previously described24. Briefly, 5µm sections were cleared and rehydrated. Antigen retrieval was performed using a Decloaking ChamberTM (Biocare Medical, Pacheco, CA) in 10 mM citrate buffer for 15 minutes at 110°C. Sections were incubated with anti-androgen receptor (Abcam, cat#: ab227678, 1:1000), anti-NKX3.1 (Proteintech, cat#: 13069-1-AP, 1:1000), anti-DDX3 (Bethyl, cat#: a300-474a, 1:250), and anti-Ki-67 (Cell Signaling Technology, cat# 12202, 1:1000) at 4°C overnight. Horseradish peroxidase (HRP)-conjugated horse anti-rabbit IgG polymer reagent (Vector Laboratories, cat# MP-7401) was used with 3,3’-Diaminobenzidine (DAB) chromogen (Cell Signaling Technology, cat# 8059) and hematoxylin counterstain.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
All procedures followed the Abcam TUNEL Assay Kit-HRP-DAB protocol (Abcam, cat#: ab206386). Briefly, 5 μm sections were cleared and rehydrated. Permeabilization was performed using proteinase K solution, followed by 5 min of quenching using 3% H2O2. Sections were incubated with TdT Labeling Reaction Mix for 1.5 h at room temperature. The apoptotic cells were visualized by DAB with hematoxylin counterstain.
Image analysis
Multispectral images were captured using Mantra (Perkin-Elmer, Waltham, MA) and quantified using inForm software (Perkin-Elmer). Images were taken with 20x magnification. Tissue segmentation (epithelium vs. stroma), cell segmentation, and determination of cell number were performed with inForm software. 18% of the total images were trained in the algorithm for the segmentation. Cells at the edges of images were excluded from segmentation. The 4-bin scoring on the percentages of positive cells over the total number of cells was used based on the negative (0), mild (1), moderate (2), and strong (3) staining of AR, NKX3.1, and DDX3. The expression of Ki-67 and the number of TUNEL-positive cells were measured by positivity using inForm software. The optical density (OD, intensity/pixel) of AR, NKX3.1, DDX3, and Ki-67 was automatedly determined by inForm software utilizing the equation: optical density= -log10 (pixel value / white reference pixel value). 6 pixels from the cell nuclei were defined as the thickness of the cytoplasm for the quantification of cytoplasmic DDX3 expression. Mean nuclear size was calculated by dividing the total area of the nuclear tissue compartment measured in pixels (epithelium and stroma) by the number of cells in the respective tissue compartment. The area was then converted from pixels into µm2. The following images were excluded from analysis: less than 5% epithelial component, significant tissue loss or folding, and images with more than 5% poorly segmented nuclei.
Statistics
Grubbs’ test was performed for outlier detection using GraphPad outlier calculator (GraphPad Software, Boston, MA). Outliers having values of p < 0.05 in Grubbs’ test and greater than two standard deviations of the mean were removed from the analysis. Statistical significance for the expressions of AR, NKX3.1, DDX3, and Ki-67 and the numbers of TUNEL-positive cells was calculated using F-test and two-tailed Student’s t-test (equal variances)/Welch’s t-test (unequal variances). A value of p < 0.05 was considered statistically significant.
Results
Young and aged mice have different prostate tissue compartmentation, cell numbers, and nuclear sizes in a lobe-specific manner
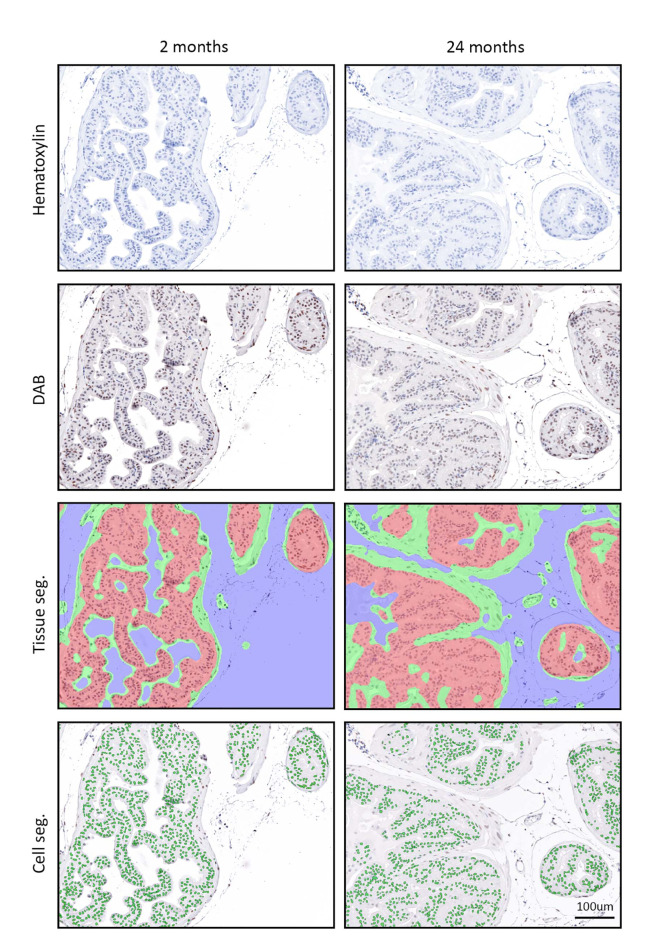
To better understand the influence of aging on the histological features of mouse prostate, we characterized prostate tissue area and cell numbers in mice at 2 months (young) and 24 months (aged) of age. Tissue and cell segmentation was performed using inForm software (Fig. 1). The areas of epithelium and stroma were quantified in the units of millimeters and percentages, which represent the absolute area and the relative area of compartments to the overall tissue area respectively (Table 1). In the anterior prostate (AP), epithelium was nearly twice as large as stroma, accounting for around 60% of the entire AP area. Compared with young mice, aged mice had a larger tissue area in the field of view of the ventral prostate (VP, p = 0.0112). Tissue segmentation indicated that aged mice had larger areas of epithelium and stroma in the field of view of VP (p < 0.05). However, aging did not alter the compositional ratio of VP, as epithelial and stromal area percentages did not change with age. Even though the overall areas in the field of view of the dorsal-lateral prostate (DLP) were similar in young and aged mice, aged mice had a larger area and a higher area percentage of stroma than young mice in DLP (p < 0.05). In terms of cell numbers, aged mice had more cells in the field of view of VP (p = 0.027). Tissue segmentation showed more stromal cells in the field of view of VP in aged mice (p = 0.013). In contrast, aged mice had fewer epithelial cells in the field of view of DLP, leading to an overall lower number of cells in DLP (p < 0.05). There was no difference in either the areas or cell numbers in the field of view when young AP was compared to the aged counterpart. Moreover, we normalized the cell numbers to areas and calculated cell densities. No significant difference was observed between young and aged prostate in any prostate lobe in terms of cell density. Additionally, there was no difference in cell nuclear size between young and aged AP and DLP. However, the nuclear size of stromal cells in VP was significantly larger in aged mice than in young mice (p = 0.0011).
Fig. 1.
Unmixed signals of chromogen and segmentation images from immunohistochemistry of young (2 months) and aged (24 months) anterior prostate. Hematoxylin (blue), 3, 3’-diaminobenzidine (DAB, brown), tissue segmentation (epithelium: red; stroma: green; non-tissue area: blue), cell segmentation (cell nuclei: green). Segmentations were processed using inForm software.
Table 1.
Tissue compartment, areas, cell counts, cell density, and nuclear size in young (2 months, n = 8) and aged (24 months, n = 9) prostates. Values are mean (SD). Bold values have statistical significance in young vs. aged by Student’s t-test (equal variances)/Welch’s t-test (unequal variances). *p < 0.05, **p < 0.01, ****p < 0.0001.
| Prostate lobe | Anterior prostate | Ventral prostate | Dorsal-lateral prostate | |||
|---|---|---|---|---|---|---|
| Age | 2 months | 24 months | 2 months | 24 months | 2 months | 24 months |
| A. Area (mm2) | ||||||
| Total | 218.47 (45.24) | 283.06 (81.45) | 61.24(22.55)* | 106.41 (41.57)* | 166.40 (33.22) | 167.46 (33.97) |
| Epithelium | 147.75 (32.07) | 199.65 (86.92) | 38.76 (13.32)* | 58.67 (23.52)* | 109.17 (22.27) | 90.39 (20.68) |
| Stroma | 70.72 (19.26) | 83.41 (22.03) | 22.48 (11.92)* | 47.74 (31.19)* | 57.22 (12.63)* | 77.07 (16.22)* |
| B. Area (%) | ||||||
| Total | 1 | 1 | 1 | 1 | 1 | 1 |
| Epithelium | 67.69 (4.74) | 69.09 (10.14) | 64.26 (9.48) | 57.13 (14.07) | 65.61 (2.88)**** | 46.34 (5.64)**** |
| Stroma | 32.31 (4.74) | 30.91 (10.14) | 35.74 (9.48) | 42.87 (14.07) | 34.39 (2.88)**** | 53.66 (5.64)**** |
| C. Cell number | ||||||
| Total | 1,411 (268) | 1,643 (204) | 560 (178)* | 783 (208)* | 1,024 (245)* | 747 (183)* |
| Epithelium | 1,232 (223) | 1,442 (200) | 432 (126) | 568 (177) | 867 (134)** | 635 (126)** |
| Stroma | 179 (85) | 201 (67) | 128 (71)* | 215 (60)* | 157 (128) | 113 (77) |
| D. Cell density (per mm2) | ||||||
| Total | 7,294 (535) | 6,995 (1243) | 6,687 (447) | 6,008 (1256) | 7,817 (952) | 7,891 (1875) |
| Epithelium | 8,435 (624) | 8,551 (1605) | 11,058 (1357) | 9,936 (2171) | 8,231 (1033) | 7,448 (2155) |
| Stroma | 2,720 (1183) | 2,698 (810) | 8,246 (2749) | 6,222 (2185) | 3,329 (2094) | 2,276 (1177) |
| E. Nuclear size (µm2) | ||||||
| Epithelium | 24.65 (2.14) | 25.55 (1.28) | 26.55 (1.92) | 25.21 (2.80) | 25.55 (1.41) | 24.78 (0.90) |
| Stroma | 22.18 (4.11) | 18.49 (3.18) | 12.08 (1.51)** | 16.09 (2.64)** | 21.03 (3.24) | 21.52 (1.65) |
The expression of androgen receptors with age shows lobe-specific changes in mouse prostate
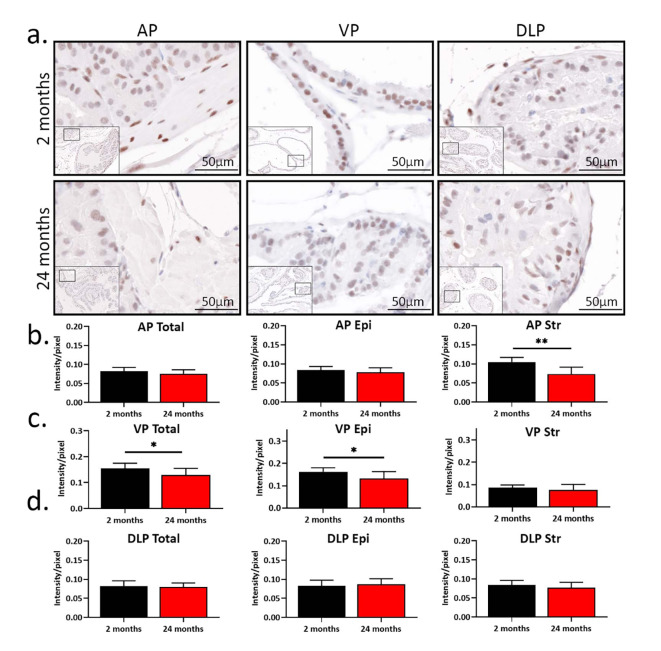
Previous work has shown that testosterone (T) levels decrease with age in humans and mice12,13,21,23. Thus, we hypothesized that prostatic AR protein expression would decrease in aged mice. To test our hypothesis, we determined the expression of prostatic AR in mice using immunohistochemistry (IHC). Consistent with previous studies, AR was localized to nuclei within the epithelium and stroma (Fig. 2a)22,25. The total prostatic AR expression in AP was higher in young mice than in aged mice, with more cells having strong AR positivity observed in young AP (p = 0.025, Table 2). In VP, aged mice had more cells with mild AR positivity, while young mice had more cells with moderate and strong positivity (p < 0.05). Thus, the overall AR expression was higher in young VP. There was no difference in overall AR expression in DLP when young mice were compared to aged mice. Tissue segmentation analysis showed a decrease in the percentage of AR-positive cells in the stroma of AP with age (p = 0.002), while no difference in the percentage of AR-positive cells was observed in AP epithelium. Aged AP also had fewer cells with moderate and strong staining in the stroma (p < 0.01). Compared to young VP, aged VP had fewer AR-positive epithelial cells (p < 0.05). There was no difference in the percentage of AR-positive cells in the epithelium or stroma of DLP between young and aged mice.
Fig. 2.
The age-related expression of androgen receptor (AR) shows lobe-specific changes in the mouse prostate. (a) AR immuno-staining in young (2 months, n = 8) and aged (24 months, n = 9) prostates. AP, anterior prostate; VP, ventral prostate; DLP, dorsal-lateral prostate. (b) The intensity of AR expression in total, epithelial, and stromal AP. (c) The intensity of AR expression in total, epithelial, and stromal VP. (d) The intensity of AR expression in total, epithelial, and stromal DLP. *p < 0.05, **p < 0.01.
Table 2.
4-bin scoring of androgen receptor (AR) positivity in young (2 months, n = 8) and aged (24 months, n = 9) prostates. Values are percentages (%) of AR-negative (0) and mild- (1), moderate- (2), and strong- (3) AR-positive cells. Values are presented as mean (SD). Bold values have statistical significance in young vs. aged by Student’s t-test (equal variances)/Welch’s t-test (unequal variances). *p < 0.05, **p < 0.01, ***p < 0.001.
| Prostate lobe | Anterior prostate | Ventral prostate | Dorsal-lateral prostate | |||
|---|---|---|---|---|---|---|
| Age | 2 months | 24 months | 2 months | 24 months | 2 months | 24 months |
| A. Total (%) | ||||||
| 0 | 16.12 (7.19) | 21.02 (10.39) | 9.35 (5.02) | 15.15 (10.27) | 19.17 (9.59) | 23.74 (5.95) |
| 1 | 38.36 (10.91) | 43.16 (9.98) | 21.42 (8.53)** | 39.55 (13.09)** | 36.04 (10.54) | 36.72 (6.07) |
| 2 | 41.77 (14.98) | 34.39 (16.90) | 41.55 (7.75)* | 32.57 (9.51)* | 35.24 (10.72) | 31.37 (8.53) |
| 3 | 3.75 (2.27)* | 1.43 (1.56)* | 27.69 (14.21)* | 12.73 (12.73)* | 9.55 (9.10) | 8.17 (8.26) |
| B. Epithelium (%) | ||||||
| 0 | 11.41 (5.43) | 17.42 (13.64) | 8.19 (2.76)* | 16.79 (8.62)* | 15.25 (7.50) | 16.78 (6.92) |
| 1 | 41.20 (12.38) | 43.45 (11.55) | 18.07 (8.92)*** | 40.66 (14.47)*** | 35.84 (11.32) | 31.99 (8.95) |
| 2 | 44.51 (15.06) | 37.78 (19.87) | 44.07 (8.20)* | 32.63 (11.95)* | 39.35 (8.69) | 39.74 (8.01) |
| 3 | 2.87 (2.10) | 1.34 (1.65) | 29.67 (15.52)** | 9.92 (11.48)** | 9.56 (9.84) | 11.50 (10.48) |
| C. Stroma (%) | ||||||
| 0 | 13.53 (5.09)** | 33.15 (14.06)** | 39.03 (11.88) | 57.82 (28.17) | 23.61 (5.88) | 29.09 (13.62) |
| 1 | 27.06 (10.73)* | 38.15 (5.04)* | 49.68 (12.60) | 32.71 (19.29) | 27.83 (6.36) | 29.67 (9.31) |
| 2 | 41.65 (8.92)** | 25.07 (13.51)** | 12.46 (6.13) | 8.20 (9.93) | 36.26 (5.23) | 33.49 (16.46) |
| 3 | 17.76 (9.12)*** | 3.64 (3.17)*** | 0.84 (1.15) | 1.27 (2.27) | 12.30 (9.39) | 8.75 (6.16) |
In addition to AR percentage positivity, we also quantified AR expression in terms of intensity. Interestingly, the overall AR intensity did not change with age in AP (Fig. 2b), even though AR was more intense in the stromal cells (p = 0.0013). This is likely due to epithelial cells outnumbering stromal cells. In VP, aged mice had lower AR intensity in the epithelium, leading to a decrease in overall AR intensity with age (Fig. 2c). No difference in stromal AR intensity was observed in VP between young and aged mice. Moreover, DLP did not show differences in AR intensity in any compartment (Fig. 2d).
Our previous study in double-negative prostate cancer indicated that DEAD (Asp-Glu-Ala-Asp)-box helicase 3 (DDX3) represses AR translation at stress granules under stressful conditions26. Since oxidative stress accumulation correlates with aging, we next examined whether the expression of DDX3 increases with age, which may lead to a decrease in prostatic AR expression in aged mice27. Cytoplasmic DDX3 was quantified using inForm software (Supplementary Figure S1). There was no meaningful difference in DDX3 expression between young and aged prostate (Supplementary Table S1), suggesting that DDX3 may not participate in AR downregulation in non-malignant mouse prostate during aging. Only a higher percentage of cells with moderate expression of DDX3 was observed in aged DLP (p = 0.028).
Taken together, these results demonstrate that AR protein expression is reduced in aged mouse AP and VP, but not DLP. DDX3-mediated translational inhibition of AR mRNA may not be involved in AR reduction in aged prostate.
AR signaling decreases with age in mouse AP and VP
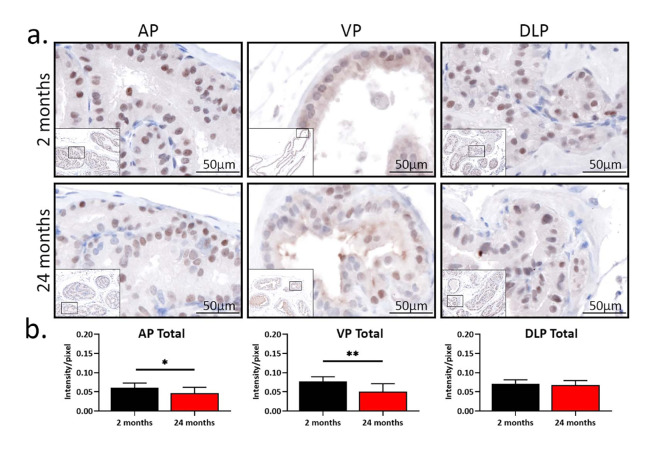
We next examined the expression of NKX3.1, a downstream target of AR, to assess AR signaling in young and aged mice28. As shown in Fig. 3a, NKX3.1 was only expressed in the nuclei of epithelial cells, consistent with previously reported localization of NKX3.129. In addition, consistent with a decrease in AR, the percentage of NKX3.1-negative cells was higher in AP and VP from aged mice than in young counterparts (p < 0.05, Table 3). More epithelial cells with moderate expression of NKX3.1 were observed in young VP. There was no difference in the percentages of NKX3.1-negative and -positive cells when young DLP was compared to the aged counterpart. In terms of NKX3.1 intensity, young AP and VP had more intense NKX3.1 expression (p < 0.05, Fig. 3b). No difference in the intensity of NKX3.1 between young and aged mice was observed in DLP. These results suggest AR signaling decreases with age in the mouse prostate in a lobe-specific manner.
Fig. 3.
The expression of NKX3.1 decreases with age in mouse anterior prostate and ventral prostate. (a) Pictures of NKX3.1 staining from immunohistochemistry in young (2 months, n = 9) and aged (24 months, n = 9) mice. AP, anterior prostate; VP, ventral prostate; DLP, dorsal-lateral prostate. (b) The intensity of NKX3.1 expression in total, epithelial, and stromal AP, VP, and DLP. *p < 0.05, **p < 0.01.
Table 3.
4-bin scoring of NKX3.1 positivity in young (2 months, n = 9) and aged (24 months, n = 9) prostates. Values are percentages (%) of NKX3.1-negative (0) and mild- (1), moderate- (2), and strong- (3) NKX3.1-positive cells. Values are presented as mean (SD). Bold values have statistical significance in young vs. aged by Student’s t-test (equal variances)/Welch’s t-test (unequal variances). *p < 0.05, ***p < 0.001.
| Prostate lobe | Anterior prostate | Ventral prostate | Dorsal-lateral prostate | |||
|---|---|---|---|---|---|---|
| Age | 2 months | 24 months | 2 months | 24 months | 2 months | 24 months |
| Epithelial (%) | ||||||
| 0 | 19.12 (6.72)* | 27.19 (5.60)* | 30.10 (12.18)* | 54.37 (26.43)* | 27.91 (6.86) | 30.72 (10.41) |
| 1 | 38.05 (12.09) | 41.73 (13.09) | 29.25 (11.12) | 30.85 (17.86) | 42.33 (10.38) | 42.99 (8.46) |
| 2 | 29.30 (10.35) | 24.73 (8.88) | 33.06 (13.51)*** | 10.31 (7.03)*** | 23.78 (7.37) | 21.28 (6.98) |
| 3 | 13.53 (9.62) | 6.35 (5.60) | 7.58 (7.22) | 4.47 (5.08) | 5.97 (6.96) | 5.01 (6.22) |
Prostatic proliferation decreases with age with no change in apoptosis in mice
AR signaling plays a pivotal role in cell proliferation30. The alterations of expression of AR and NKX3.1 prompted the question of whether total proliferation is affected by altered AR signaling during aging. To test this, we examined the expression of the proliferation marker Ki-67 and determined apoptosis using terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)31.
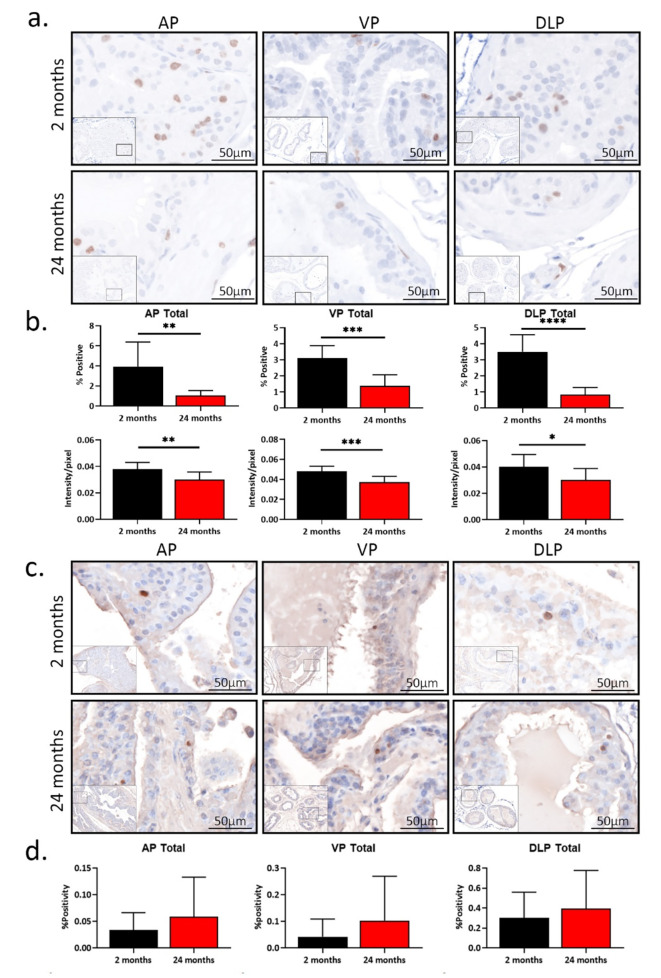
As shown in Fig. 4a, Ki-67 was localized to epithelial and stromal nuclei, consistent with its previously reported localization32. Overall, compared to young mice, aged mice had lower percentages of Ki-67-positive cells in the AP, VP, and DLP (p < 0.05). The difference in Ki-67 expression between young and aged mice was only from epithelium, not stroma. In addition, the young prostate had a higher intensity of Ki-67 expression in all three lobes than the aged prostate (p < 0.05, Fig. 4b). TUNEL assay showed little to no apoptotic cells in the AP, VP, and DLP (Fig. 4c). No statistical difference was found between young and aged prostates (Fig. 4d), consistent with our previous report21. These results suggest young prostate lobes are more proliferative, compared to aged prostate lobes.
Fig. 4.
Total proliferation decreases with age with no change in apoptosis in mouse prostate (a) Pictures of prostatic Ki-67 staining from immunohistochemistry in young (2 months, n = 9) and aged (24 months, n = 9) mice. (b) The percentages of Ki-67-positive cells and the intensity of Ki-67 protein expression in young and aged anterior prostate (AP), ventral prostate (VP), and dorsal-lateral prostate (DLP). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (c) Pictures of TUNEL assay in young (2 months, n = 9) and aged (24 months, n = 8) prostates. (d) The percentages of apoptotic cells in young and aged AP, VP, and DLP.
Discussion
Aging has been shown to correlate with the incidence of multiple prostate diseases in humans, including benign prostatic hyperplasia (BPH)1,33,34. Thus, understanding the cellular and molecular effects of aging on the prostate is of importance to the development of novel therapeutics and better treatment strategies. However, due to the low incidence of prostate diseases in young men, the ability to obtain prostate specimens from young men is limited. Therefore, the identification of relevant aging models for studying BPH is critical. Serum androgens are reported to reduce with age in both humans and mice14,21,23. In addition, our previous study indicated that in aged male mice, the prostates were enlarged, and the mice had altered urinary functions, which modeled key aspects of lower urinary tract symptoms in human21. These findings suggest mice mimic humans in the hormonal regulation of prostate growth. Further insights into steroid hormone receptor expression in mice could be helpful to urologic research in humans. Hence, we characterized age-related androgen signaling changes in young (2 months) and aged (24 months) mice.
Previously, manual segmenting of mouse prostatic tissue proved to be challenging as mice show less stroma as compared to human prostate. With the help of cutting-edge software, we were able to segment tissues and cells in an automated manner. Here, we determined epithelial and stromal areas in mouse prostate, as well as the cell counts in each compartment. About 60% of the tissue was epithelium in all three lobes in young and aged mice. An increased percentage of stromal area with age was observed in the dorsal-lateral prostate (DLP). No significant difference was found in cell density in the anterior prostate (AP), ventral prostate (VP), or DLP. These characteristics provide insight into morphometric changes that occur with age in the mouse prostate.
Androgen receptor (AR) has been widely acknowledged to play an important role in prostate development and maintenance, sexual function, and prostatic diseases22,34,35. Age-related alterations in AR expression have been observed in multiple organs and tissues of mice, including the brain, bone, and skeletal muscle36–38. However, it is unclear whether AR expression is altered in mouse prostate and whether it is associated with prostatic hyperplasia. We demonstrate here that in the aged prostate, a lobe-specific alteration of AR expression exists. Traditionally, researchers have used optical density (OD) as a measurement of the intensity of protein expression39. We found that the overall OD of AR in AP and DLP did not change with age, while aged VP had less intense AR expression than young VP. Because OD averages the intensity by pixels, it does not necessarily stratify the cell groups that have different intensities of AR39. To better understand the pattern of AR expression, we used 4-bin scoring based on OD thresholds. More cells with strong AR expression were observed in AP and VP, but not in DLP in young mice. Tissue segmentation revealed that the reduction of AR primarily existed in the stroma of AP and the epithelium of VP. To further assess the effects of aging on AR signaling, we next examined the expression of NKX3.1, a transcription factor whose expression is under the regulation of AR40. NKX3.1 declined with age in AP and VP, while no significant difference was observed in DLP. Our findings suggest that prostatic AR signaling decreases along with serum testosterone (T) reduction and age in a lobe-specific manner in the mouse. The prostate weights were not taken into consideration while analyzing the data, since the age-related prostate enlargement has been published previously21. In addition, our analysis raises the question of how the expression of AR is regulated during aging. Earlier work in double-negative prostate cancer demonstrated that the RNA helicase, DEAD (Asp-Glu-Ala-Asp)-box helicase 3 (DDX3), inhibited AR mRNA translation under stress conditions26,41. As oxidative stress accumulates with age, DDX3 may translationally regulate AR expression in the prostate. However, we found the expression of DDX3 protein was not different between young and aged prostates, suggesting DDX3 might not be involved in AR protein reduction in aged non-malignant tissues. Studies have shown other mechanisms that may regulate AR expression, including promoter methylation and microRNA-mediated regulation42,43. The roles of these mechanisms in prostatic AR expression during aging are yet to be demonstrated.
The clinical use of 5-alpha reductase inhibitors as a BPH treatment suggests that AR signaling could be involved in prostatic hyperplasia19,20. Thus, we determined the expression of proliferation maker Ki-67 in young and aged prostates. We found that compared to young mice, aged mice had less expression of Ki-67 protein in AP, VP, and DLP. It should be noted that although AR did not change with age in DLP, Ki-67 level was higher in young mice than in aged mice. These results are consistent with our published results using bromodeoxyuridine (BrdU) staining to determine proliferation in the mouse21. Additionally, we observed nuclear enlargement in the stroma of aged VP. Studies have shown that a decreased Ki-67 expression and enlarged nuclei could be signs of cellular senescence, which in recent years has been considered to accelerate the progression of prostatic diseases25,44. We emphasize that the enlargement of mouse prostate lobes may be a result of accumulated proliferation over the years as the proliferation rate decreases with age. Meanwhile, we do not mean to rule out the possibility that other factors, such as inflammation and fibrosis, are playing roles in prostate enlargement. In fact, BPH has been considered as a stromal disease, and stroma is the primary site where fibrosis occurs45. We also noticed an increased stromal area in VP and DLP in this study, supporting that claim.
A limitation of our study is that we do not determine the specific cell types that show different AR expressions with age. A normal mouse prostate is composed of multiple cell types, including luminal cells, basal cells, smooth muscle cells, endothelial cells, fibroblasts, immune cells, and others46,47. Co-staining the target proteins with cell markers would further clarify the effects of aging on different cells. In addition to AR, researchers argue that the estrogen receptor (ER) is involved in the pathology of prostatic diseases, and it is believed that an imbalanced AR/ER signaling contributes to the transition of cells from normal state to disease state48. As mentioned earlier, endogenous T production in males declines with age in humans and mice, while the level of estrogen remains stable14,21,23,49. This change can lead to a hormonal imbalance that favors estrogenic signaling to become the dominant hormone signaling in the prostate. Our previous study indicated that ERα could be pathogenic, as knockdown of ERα ameliorated hormone treatment-induced urinary dysfunctions in male mice48. Additionally, a study implicating estrogen and G protein-coupled estrogen receptors induced fibrosis and promoted BPH50. Future studies combining estrogenic signaling with androgenic signaling would provide a more complete understanding of how aging is associated with prostatic diseases. Moreover, the site of the tissue section may affect the results, which has been a concern of histological studies for years. In this regard, we ensured that the prostate lobes were embedded in the same direction and used sections in similar positions to reduce possible bias.
Taken together, our analysis shows that aging alters prostatic AR signaling in a lobe-specific manner in the mouse, associated with reduced proliferation. Our findings provide insight into the expression pattern of AR in benign tissues and may impact the usage of mice as a model of BPH. Further validation is needed to confirm whether humans have a similar change in AR expression during natural aging.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Hannah Miles, Avan Colah, Alexis Adrian, and Ajinkya Limkar for their review of the manuscript.
Author contributions
H.Z., T.T.L., and W.A.R. conceived, designed, and wrote the manuscript. T.T.L., E.A.R., and W.A.R. provided intellectual input and edited the manuscript. E.A.R. made the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Institute of Aging, grant number K01AG059899; the National Institute of Diabetes and Digestive and Kidney Diseases, grant numbers U54DK104310, R01DK127081, and R01DK131175; and the National Cancer Institute, grant numbers P30CA014520 and P50CA269011.
Data availability
The data that support the findings of this study are available within the article.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cannarella, R., Condorelli, R. A., Barbagallo, F., La Vignera, S. & Calogero, A. E. Endocrinology of the aging prostate: current concepts. Front. Endocrinol. (Lausanne). 12, 554078. 10.3389/fendo.2021.554078 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborators, G. B. D. B. P. H. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Healthy Longev.3, e754–e776. 10.1016/S2666-7568(22)00213-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chughtai, B. et al. Cost-effectiveness and Budget Impact of emerging minimally invasive Surgical treatments for Benign Prostatic Hyperplasia. J. Health Econ. Outcomes Res.8, 42–50. 10.36469/jheor.2021.22256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langan, R. C. Benign Prostatic Hyperplasia. Prim. Care. 46, 223–232. 10.1016/j.pop.2019.02.003 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Girman, C. J. et al. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology. 51, 428–436. 10.1016/s0090-4295(97)00717-6 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Speakman, M. J. & Cheng, X. Management of the complications of BPH/BOO. Indian J. Urol.30, 208–213. 10.4103/0970-1591.127856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platz, E. A. et al. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J. Urol.188, 496–501. 10.1016/j.juro.2012.03.125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litman, H. J. & McKinlay, J. B. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int.100, 820–825. 10.1111/j.1464-410X.2007.07018.x (2007). [DOI] [PubMed] [Google Scholar]
- 9.Berry, S. J., Coffey, D. S., Walsh, P. C. & Ewing, L. L. The development of human benign prostatic hyperplasia with age. J. Urol.132, 474–479. 10.1016/s0022-5347(17)49698-4 (1984). [DOI] [PubMed] [Google Scholar]
- 10.Allen, K. S., Kressel, H. Y., Arger, P. H. & Pollack, H. M. Age-related changes of the prostate: evaluation by MR imaging. AJR Am. J. Roentgenol.152, 77–81. 10.2214/ajr.152.1.77 (1989). [DOI] [PubMed] [Google Scholar]
- 11.Lee, S. N. et al. Age related differences in responsiveness to Sildenafil and Tamsulosin are due to myogenic smooth muscle tone in the human prostate. Sci. Rep.7, 10150. 10.1038/s41598-017-07861-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida, M. et al. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp. Gerontol.36, 99–109. 10.1016/s0531-5565(00)00175-3 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Grandys, M. et al. Age-related decrease in serum dihydrotestosterone concentration is accompanied by impaired vascular status. Exp. Gerontol.173, 112104. 10.1016/j.exger.2023.112104 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Harman, S. M. et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J. Clin. Endocrinol. Metab.86, 724–731. 10.1210/jcem.86.2.7219 (2001). [DOI] [PubMed] [Google Scholar]
- 15.White, J. W. I. The results of double castration in hypertrophy of the prostate. Ann. Surg.22, 1–80. 10.1097/00000658-189507000-00001 (1895). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park, W. Y. et al. Ellagic acid improves benign prostate hyperplasia by regulating androgen signaling and STAT3. Cell. Death Dis.13, 554. 10.1038/s41419-022-04995-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke, P. S., Young, P. & Cunha, G. R. Androgen receptor expression in developing male reproductive organs. Endocrinology. 128, 2867–2873. 10.1210/endo-128-6-2867 (1991). [DOI] [PubMed] [Google Scholar]
- 18.Wang, X. et al. Increased infiltrated macrophages in benign prostatic hyperplasia (BPH): role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J. Biol. Chem.287, 18376–18385. 10.1074/jbc.M112.355164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gormley, G. J. et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl. J. Med.327, 1185–1191. 10.1056/NEJM199210223271701 (1992). [DOI] [PubMed] [Google Scholar]
- 20.McConnell, J. D. et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl. J. Med.349, 2387–2398. 10.1056/NEJMoa030656 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Liu, T. T. et al. Prostate enlargement and altered urinary function are part of the aging process. Aging (Albany NY). 11, 2653–2669. 10.18632/aging.101938 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholson, T. M., Sehgal, P. D., Drew, S. A., Huang, W. & Ricke, W. A. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation. 85, 140–149. 10.1016/j.diff.2013.02.006 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machida, T., Yonezawa, Y. & Noumura, T. Age-associated changes in plasma testosterone levels in male mice and their relation to social dominance or subordinance. Horm. Behav.15, 238–245. 10.1016/0018-506x(81)90013-1 (1981). [DOI] [PubMed] [Google Scholar]
- 24.Nicholson, T. M. et al. Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice. Endocrinology. 153, 5556–5565. 10.1210/en.2012-1522 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ewald, J. A. et al. Androgen deprivation induces senescence characteristics in prostate cancer cells in vitro and in vivo. Prostate. 73, 337–345. 10.1002/pros.22571 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vellky, J. E., McSweeney, S. T., Ricke, E. A. & Ricke, W. A. RNA-binding protein DDX3 mediates posttranscriptional regulation of androgen receptor: a mechanism of castration resistance. Proc. Natl. Acad. Sci. U S A. 117, 28092–28101. 10.1073/pnas.2008479117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vida, C. et al. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol.12, 423–437. 10.1016/j.redox.2017.03.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan, P. Y. et al. Integration of regulatory networks by NKX3-1 promotes androgen-dependent prostate cancer survival. Mol. Cell. Biol.32, 399–414. 10.1128/MCB.05958-11 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia-Gaur, R. et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev.13, 966–977. 10.1101/gad.13.8.966 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, Q., Fung, K. M., Day, W. V., Kropp, B. P. & Lin, H. K. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell. Int.5, 8. 10.1186/1475-2867-5-8 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerdes, J., Schwab, U., Lemke, H. & Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer. 31, 13–20. 10.1002/ijc.2910310104 (1983). [DOI] [PubMed] [Google Scholar]
- 32.Masoodi, K. Z. et al. 5alpha-reductase inhibition suppresses testosterone-induced initial regrowth of regressed xenograft prostate tumors in animal models. Endocrinology. 154, 2296–2307. 10.1210/en.2012-2077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, C. C. et al. Relationship between serum testosterone and measures of benign prostatic hyperplasia in aging men. Urology. 70, 677–680. 10.1016/j.urology.2007.05.025 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Welen, K. & Damber, J. E. Androgens, aging, and prostate health. Rev. Endocr. Metab. Disord. 23, 1221–1231. 10.1007/s11154-022-09730-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naamneh Elzenaty, R., du Toit, T. & Fluck, C. E. Basics of androgen synthesis and action. Best Pract. Res. Clin. Endocrinol. Metab.36, 101665. 10.1016/j.beem.2022.101665 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Kumar, R. C. & Thakur, M. K. Androgen receptor mRNA is inversely regulated by testosterone and estradiol in adult mouse brain. Neurobiol. Aging. 25, 925–933. 10.1016/j.neurobiolaging.2003.10.011 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Serra, C. et al. Testosterone improves the regeneration of old and young mouse skeletal muscle. J. Gerontol. Biol. Sci. Med. Sci.68, 17–26. 10.1093/gerona/gls083 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu, J. et al. The androgen receptor is required for maintenance of bone mass in adult male mice. Mol. Cell. Endocrinol.479, 159–169. 10.1016/j.mce.2018.10.008 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Fedchenko, N. & Reifenrath, J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue - a review. Diagn. Pathol.9, 221. 10.1186/s13000-014-0221-9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie, Q. & Wang, Z. A. Transcriptional regulation of the Nkx3.1 gene in prostate luminal stem cell specification and cancer initiation via its 3’ genomic region. J. Biol. Chem.292, 13521–13530. 10.1074/jbc.M117.788315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, H. et al. DDX3X and stress granules: emerging players in Cancer and Drug Resistance. Cancers (Basel). 16. 10.3390/cancers16061131 (2024). [DOI] [PMC free article] [PubMed]
- 42.Jarrard, D. F. et al. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res.58, 5310–5314 (1998). [PubMed] [Google Scholar]
- 43.Nadiminty, N. et al. MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of myc expression in prostate cancer cells. J. Biol. Chem.287, 1527–1537. 10.1074/jbc.M111.278705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pernicova, Z. et al. Androgen depletion induces senescence in prostate cancer cells through down-regulation of Skp2. Neoplasia. 13, 526–536. 10.1593/neo.11182 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohr, H. P. & Bartsch, G. Human benign prostatic hyperplasia: a stromal disease? New perspectives by quantitative morphology. Urology. 16, 625–633. 10.1016/0090-4295(80)90577-4 (1980). [DOI] [PubMed] [Google Scholar]
- 46.Joseph, D. B. et al. Single-cell analysis of mouse and human prostate reveals novel fibroblasts with specialized distribution and microenvironment interactions. J. Pathol.255, 141–154. 10.1002/path.5751 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowley, L. et al. A single-cell atlas of the mouse and human prostate reveals heterogeneity and conservation of epithelial progenitors. Elife. 910.7554/eLife.59465 (2020). [DOI] [PMC free article] [PubMed]
- 48.Nicholson, T. M. et al. Estrogen receptor-alpha is a key mediator and therapeutic target for bladder complications of benign prostatic hyperplasia. J. Urol.193, 722–729. 10.1016/j.juro.2014.08.093 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenblatt, R. B., Oettinger, M. & Bohler, C. S. Estrogen-androgen levels in aging men and women: therapeutic considerations. J. Am. Geriatr. Soc.24, 173–178. 10.1111/j.1532-5415.1976.tb04294.x (1976). [DOI] [PubMed] [Google Scholar]
- 50.Yang, Y. et al. Estrogen and G protein-coupled estrogen receptor accelerate the progression of benign prostatic hyperplasia by inducing prostatic fibrosis. Cell. Death Dis.13, 533. 10.1038/s41419-022-04979-3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the article.