Abstract
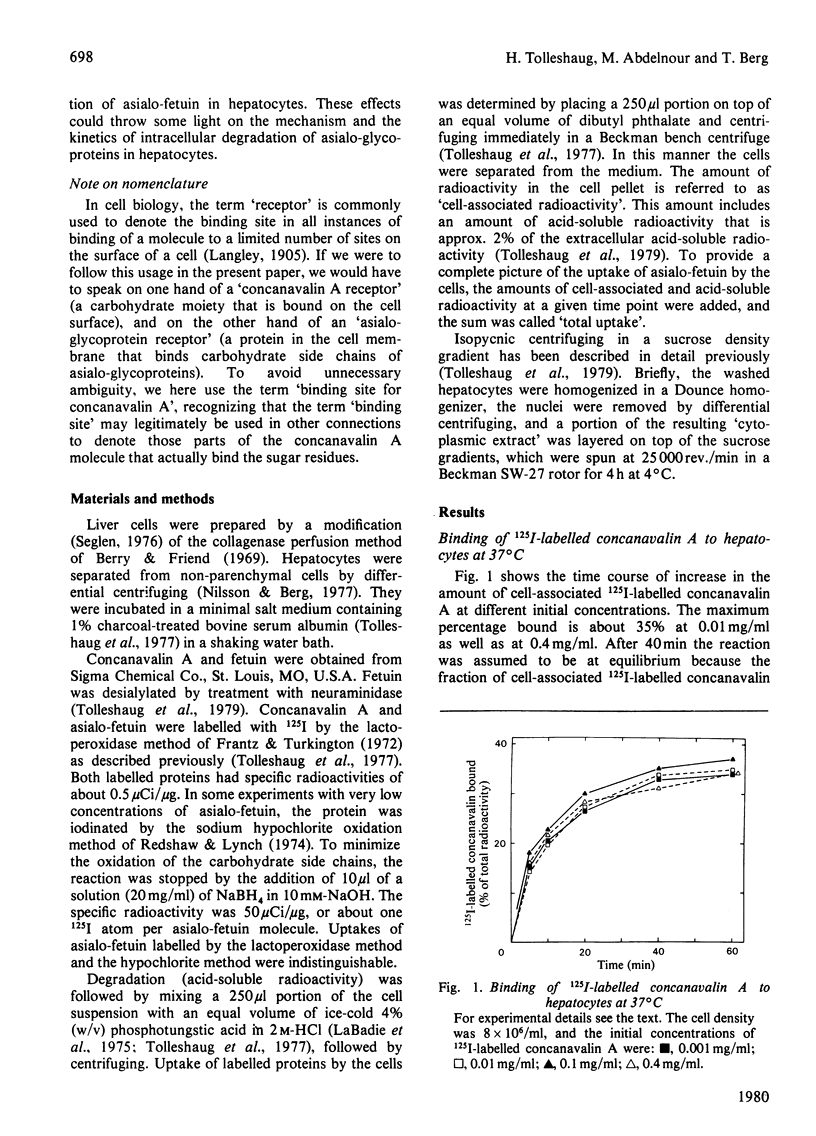
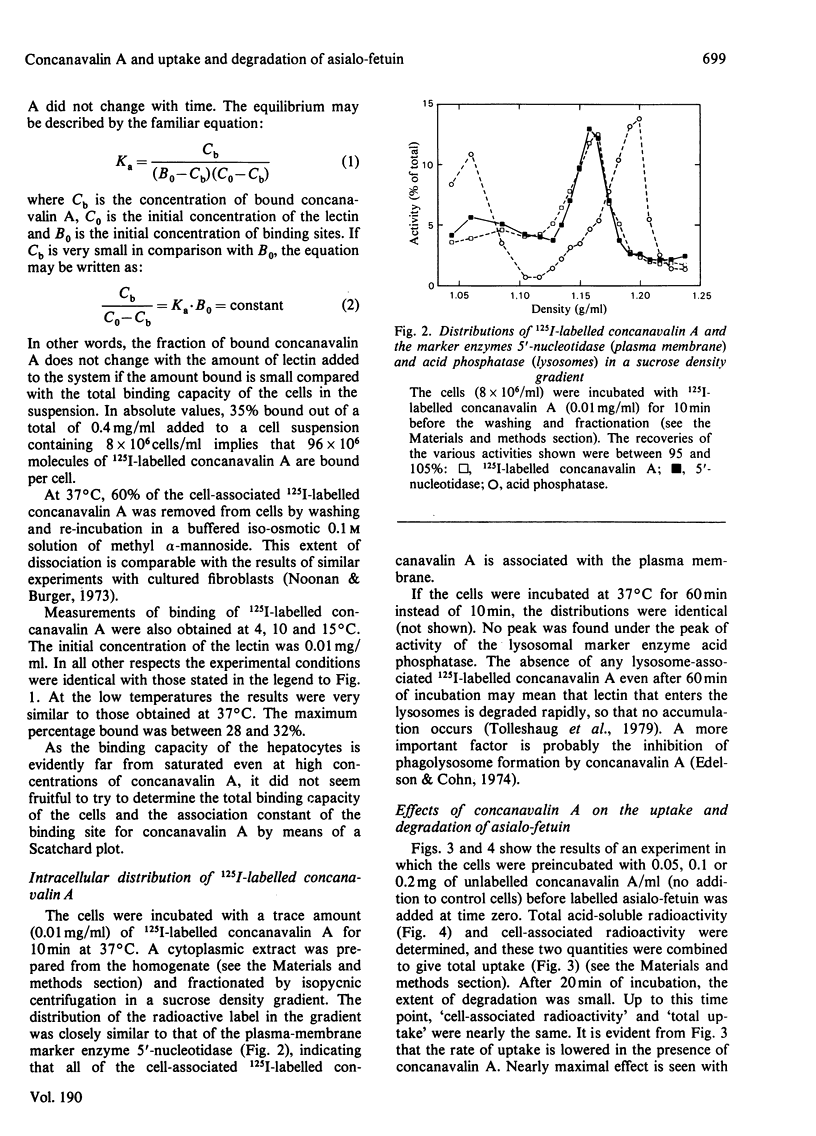
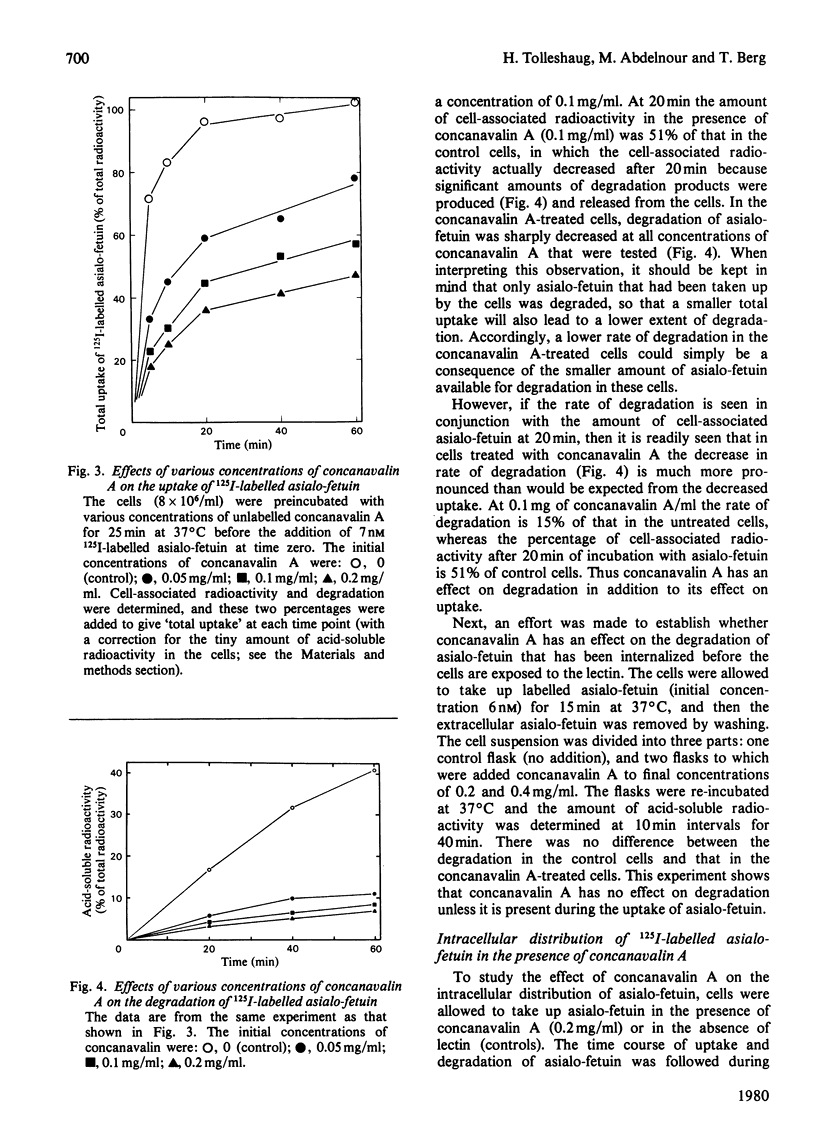
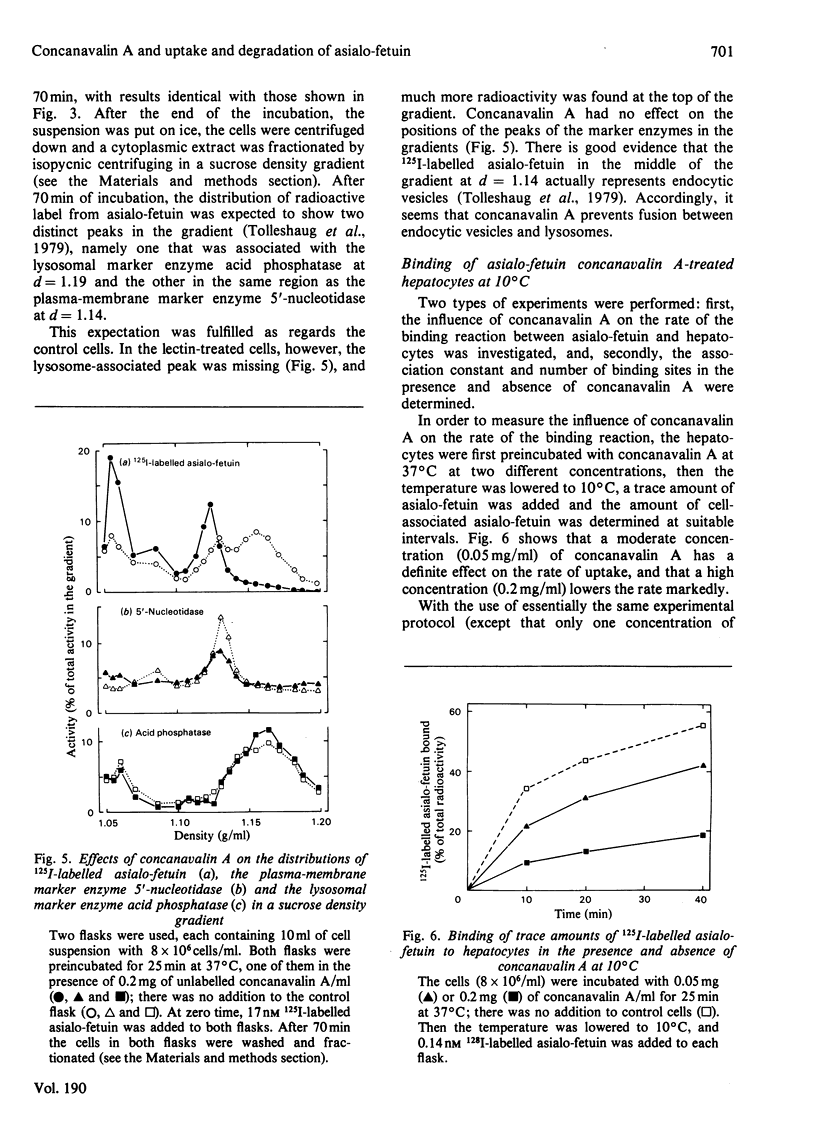
1. The binding of 125I-labelled concanavalin A to isolated rat hepatocytes was studied at temperatures between 4 degrees C and 37 degrees C. At the latter temperature, concentrations of concanavalin A from 0.01 to 0.4 mg/ml were used. In all of these experiments, binding reached a plateau after 40--60 min, when 28--35% of the concanavalin A added was bound to the cells (cell density 8 x 10(6) cells/ml). 2. The rate of uptake of 125I-labelled asialo-fetuin by the hepatocytes was lowered to 30% of control values when the cells were preincubated with 0.1 mg of concanavalin A/ml. This decrease could be accounted for by a decrease in the rate of binding of asialo-fetuin to the beta-galactoside receptor of the cells. The binding capacity of the cells was not influenced by preincubation with concanavalin A. 3. Degradation of asialo-fetuin was decreased only if concanavalin A was present during the uptake of asialo-fetuin by the cells. Subcellular fractionation revealed that concanavalin A lowered the rate of entry of endocytosed asialo-fetuin into the lysosomes. The effect of concanavalin A on degradation is distinct from its effect on the rate of uptake of asialo-fetuin by hepatocytes.
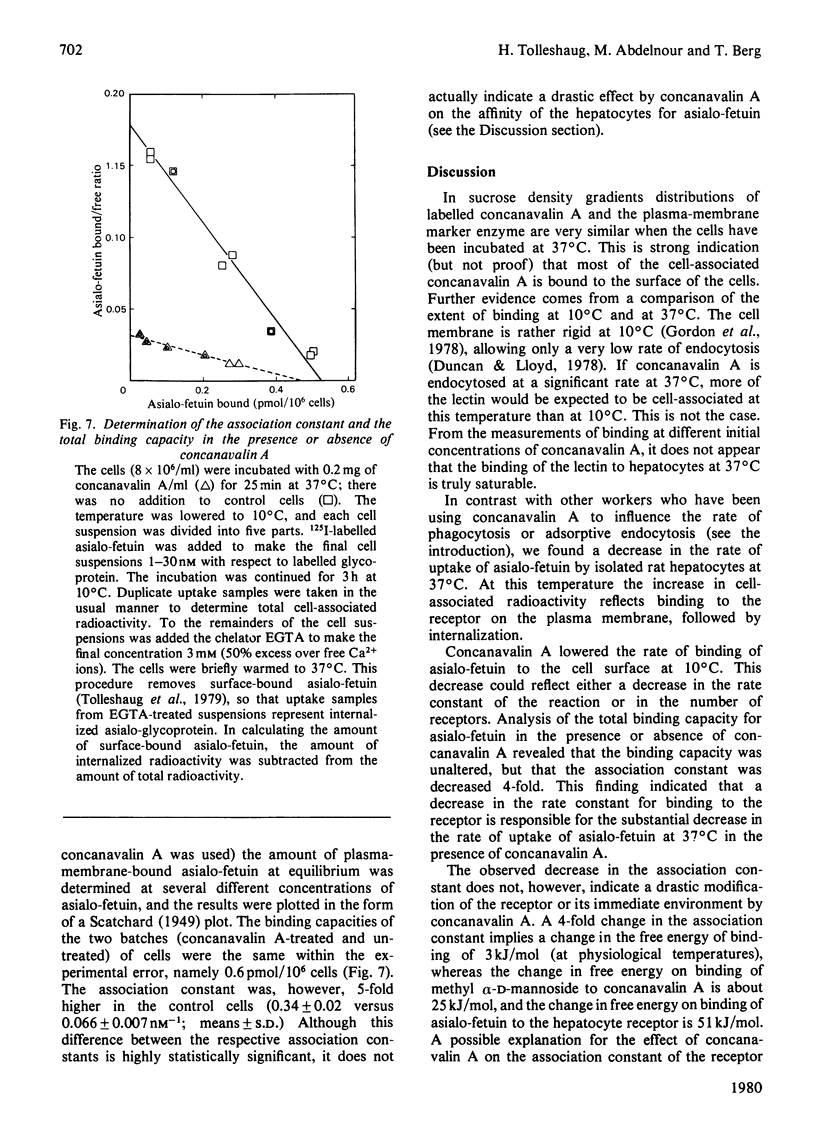
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Beeck H., Ullrich K., von Figura K. Effect of lectins on endocytosis and secretion of lysosomal enzymes by cultured fibroblasts. Biochim Biophys Acta. 1979 Mar 7;583(2):179–188. doi: 10.1016/0304-4165(79)90425-2. [DOI] [PubMed] [Google Scholar]
- Berg T., Tolleshaug H. The effects of ammonium ions and chloroquine on uptake and degradation of 125I-labeled asialo-fetuin in isolated rat hepatocytes. Biochem Pharmacol. 1980 Mar 15;29(6):917–925. doi: 10.1016/0006-2952(80)90222-1. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Freychet P., Le Cam A., Orci L. Lysosomal association of internalized 125I-insulin in isolated rat hepatocytes. Direct demonstration by quantitative electron microscopic autoradiography. J Clin Invest. 1979 Jun;63(6):1249–1261. doi: 10.1172/JCI109420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato S. D., Wiesmann U. N., Herschkowitz N. Membrane adsorption and internalization of (14C)chloroquine by cultured human fibroblasts. Biochem Pharmacol. 1977 Jan 1;26(1):7–10. doi: 10.1016/0006-2952(77)90122-8. [DOI] [PubMed] [Google Scholar]
- Duncan R., Lloyd J. B. Pinocytosis in the rat visceral yolk sac. Effects of temperature, metabolic inhibitors and some other modifiers. Biochim Biophys Acta. 1978 Dec 18;544(3):647–655. doi: 10.1016/0304-4165(78)90339-2. [DOI] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz W. L., Turkington R. W. Formation of biologically active 125 I-prolactin by enzymatic radioiodination. Endocrinology. 1972 Dec;91(6):1545–1548. doi: 10.1210/endo-91-6-1545. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Sly W. S. Concanavalin A mediated uptake of enzymes by fibroblasts. Biochem Biophys Res Commun. 1978 Nov 14;85(1):174–182. doi: 10.1016/s0006-291x(78)80026-6. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D., Esgate J. A. Spin label studies on rat liver and heart plasma membranes: effects of temperature, calcium, and lanthanum on membrane fluidity. J Supramol Struct. 1978;9(3):299–326. doi: 10.1002/jss.400090303. [DOI] [PubMed] [Google Scholar]
- Guillouzo A., Feldmann G. Surface and intracellular localization of concanavalin A binding sites in rat liver cells. J Histochem Cytochem. 1977 Dec;25(12):1303–1310. doi: 10.1177/25.12.336784. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Moore M. R., Callahan J. W., Lowden J. A. Concanavalin A promotes the uptake of lysosomal hydrolases by human fibroblasts. Biochim Biophys Acta. 1978 Nov 2;513(2):285–291. doi: 10.1016/0005-2736(78)90180-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Carbohydrate structure of glycopeptides isolated from an hepatic membrane-binding protein specific for asialoglycoproteins. J Biol Chem. 1976 Sep 10;251(17):5292–5299. [PubMed] [Google Scholar]
- Kolset S. O., Tolleshaug H., Berg T. The effects of colchicine and cytochalasin B on uptake and degradation of asialo-glycoproteins in isolated rat hepatocytes. Exp Cell Res. 1979 Aug;122(1):159–167. doi: 10.1016/0014-4827(79)90570-6. [DOI] [PubMed] [Google Scholar]
- LaBadie J. H., Chapman K. P., Aronson N. N., Jr Glycoprotein catabolism in rat liver: Lysosomal digestion of iodinated asialo-fetuin. Biochem J. 1975 Nov;152(2):271–279. doi: 10.1042/bj1520271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J Physiol. 1905 Dec 30;33(4-5):374–413. doi: 10.1113/jphysiol.1905.sp001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M., Berg T. Uptake and degradation of formaldehyde-treated 125I-labelled human serum albumin in rat liver cells in vivo and in vitro. Biochim Biophys Acta. 1977 Mar 29;497(1):171–182. doi: 10.1016/0304-4165(77)90150-7. [DOI] [PubMed] [Google Scholar]
- Noonan K. D., Burger M. M. Binding of ( 3 H)concanavalin A to normal and transformed cells. J Biol Chem. 1973 Jun 25;248(12):4286–4292. [PubMed] [Google Scholar]
- Redshaw M. R., Lynch S. S. An improved method for the preparation of iodinated antigens for radioimmunoassay. J Endocrinol. 1974 Mar;60(3):527–528. doi: 10.1677/joe.0.0600527. [DOI] [PubMed] [Google Scholar]
- Roth J. Distribution of concanavalin a receptors on normal rat liver cells and zajdela ascites hepatoma cells. Int J Cancer. 1974 Dec 15;14(6):762–770. doi: 10.1002/ijc.2910140610. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So L. L., Goldstein I. J. Protein-carbohydrate interaction. XX. On the number of combining sites on concanavalin A, the phytohemagglutinin of the jack bean. Biochim Biophys Acta. 1968 Oct 15;165(3):398–404. doi: 10.1016/0304-4165(68)90218-3. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Frölich W., Norum K. R. Intracellular localization and degradation of asialofetuin in isolated rat hepatocytes. Biochim Biophys Acta. 1979 Jun 1;585(1):71–84. doi: 10.1016/0304-4165(79)90326-x. [DOI] [PubMed] [Google Scholar]
- Tolleshaug H., Berg T., Nilsson M., Norum K. R. Uptake and degradation of 125I-labelled asialo-fetuin by isolated rat hepatocytes. Biochim Biophys Acta. 1977 Aug 25;499(1):73–84. doi: 10.1016/0304-4165(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Ullrich K., Basner R., Gieselmann V., Von Figura K. Recognition of human urine alpha-N-acetylglucosaminidase by rat hepatocytes. Involvement of receptors specific for galactose, mannose 6-phosphate and mannose. Biochem J. 1979 May 15;180(2):413–419. doi: 10.1042/bj1800413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweig S., Singer S. J. Concanavalin A-induced endocytosis in rabbit reticulocytes, and its decrease with reticulocyte maturation. J Cell Biol. 1979 Feb;80(2):487–491. doi: 10.1083/jcb.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]


