Abstract
Patients with carotid stenosis can receive indication for either carotid endarterectomy (CEA) or carotid artery stenting (CAS), with both techniques having an impact on the autonomic function and baroreflex control.
Seventy carotid stenosis patients randomly assigned to CEA or CAS were enrolled. After exclusion of some recordings, 33 CEA (age 67.79 ± 5.32 yrs, 26 males) and 25 CAS (age 70.32 ± 3.63 yrs, 14 males) were admitted to analysis. Autonomic and baroreflex sensitivity markers were derived from the analysis of heart period and systolic arterial pressure spontaneous variability derived in supine position and during active standing (STAND), before (PRE) the intervention and after a 6 and 12-month follow-up (FU6, FU12).
CEA had a preserved response of autonomic and baroreflex control to STAND in PRE and FU6, suggesting an early improvement. CAS had a similar response at PRE but a blunted one at the follow-ups. When directly compared, the two groups had a similar autonomic function, with CAS having a reduced baroreflex control in PRE and lower autonomic function at FU6. All the differences disappeared at the long-term follow-up, showing a similar long term effect of the surgical procedures, suggesting that CEA and CAS induced a similar long-term impairment of autonomic and baroreflex controls.
Keywords: Carotid artery stenting, Carotid artery endarterectomy, Autonomic nervous system, Baroreflex, Cardiovascular risk, Heart rate variability
Subject terms: Biomedical engineering, Carotid artery disease
Introduction
Patients affected by severe stenosis of internal carotid artery (ICA) can take advantage from surgical removal of the atherosclerotic plaque with the benefit of reducing the risk of developing stroke1.
The gold standard treatment for carotid stenosis is carotid endarterectomy (CEA), while carotid artery stenting (CAS) is nowadays considered a valid surgical alternative, also due to its lower invasiveness2,3. Besides a similar risk to develop adverse cerebrovascular events, it is still unclear if there are differences in the autonomic control after the two procedures that could be indicative of reduced cardiovascular risk.
It is known that a lowered autonomic modulation and impaired cardiac baroreflex function are independent risk factors for the development of the most prevalent complications occurring after carotid surgery3,4, namely myocardial infarction5 and stroke6. Furthermore, the modification of the carotid wall related to surgery could modify cardiac baroreflex function, since the adventitia of the ICA wall is the site where carotid sinus baroreceptors are located7. Plaque removal following CEA could damage sensing areas of the carotid artery, leading to a worsening of the autonomic and baroreflex response7,8. Although autonomic function is expected to be less affected by CAS technique9, previous works lead to different conclusions: indeed, Yakhou et al.10 identified a lower autonomic and baroreflex response after CAS but not CEA. As a matter of fact, both techniques can impact on the autonomic control. Considering that the baroreflex is mainly a vagal reflex10, modification of the autonomic control could also affect baroreflex function in CEA and CAS patients. Baroreflex control can also be influenced directly due to manipulation of the carotid arteries.
Autonomic function can be noninvasively inferred by the study of heart period (HP) and systolic arterial pressure (SAP) variability, as derived from electrocardiogram (ECG) and arterial pressure (AP) signals11,12. Baroreflex control is a mechanism fundamental for the maintenance of the homeostasis of the body and can be evaluated from the relation of HP changes to variations of SAP13,14. Several methods have been exploited to assess baroreflex, with the frequency domain ones being among the most powerful15–21.
Different studies so far have investigated HP and SAP variability in patients undergoing CEA or CAS, leading to different results. A previous work22 evaluated patients undergoing CEA showing that autonomic function and baroreflex sensitivity (BRS) were compromised during a postural stimulus evoking sympathetic increase and vagal withdrawal, namely the head-up tilt. The same work22 found that after a 4-months follow-up, autonomic and baroreflex response to the orthostatic stimulus recovered. Eversion-CEA, the procedure considered an alternative to traditional CEA, requiring the oblique circumferential transection of the ICA at the carotid bulb often accompanied by the transection of longitudinal nerve fibers, was also shown to be related to a decreased BRS after surgery8, while other works did not observe any change23.
When CAS and CEA were directly compared, controversial results were found as well, showing that patients undergoing CAS exhibited an increase of vagal modulation as measured via heart rate variability markers, while those undergoing CEA featured the opposite behavior7. Other researchers9 reported that CAS activity was not modified, while a diminished vagal modulation was observed in CEA after up to three months following surgery.
It appears clear that different experimental protocols and follow-up durations led researchers to different conclusions about the autonomic control in patients undergoing carotid revascularization. In this sense, a comparison of the two techniques, with a long-term follow-up (i.e. up to one year) including the evaluation of both autonomic function and baroreflex control as derived from the acquisition of both HP and SAP variability and a maneuver challenging the autonomic nervous system as an orthostatic stressor24, has not been presented so far.
Thus, this work aims to investigate the autonomic control and baroreflex regulation in patients affected by carotid stenosis undergoing the different kind of ICA interventions (e.g., CEA or CAS) to understand whether the two techniques affect equivalently cardiovascular control with special attention to post-surgery recovery. To do so, cardiovascular control was evaluated in patients undergoing CEA or CAS during a postural challenge before surgery and after a 6- and 12-month follow-ups. The main expected outcome was related to see changes in baroreflex and autonomic function at the different time points and compare them between CEA and CAS patients.
Methods of analysis
Experimental protocol
Seventy patients scheduled for CEA or CAS have been enrolled at the Department of Vascular Surgery of IRCCS Policlinico San Donato, San Donato Milanese, Milan Italy. The study adhered to Helsinki principles for studies involving human subjects and was approved by San Raffaele Hospital ethical committee (approval no. 62int/2017, ClinicalTrials.gov registration no. NCT03493971, first trial registration date 11/04/2018). Patients signed an informed consent before participating.
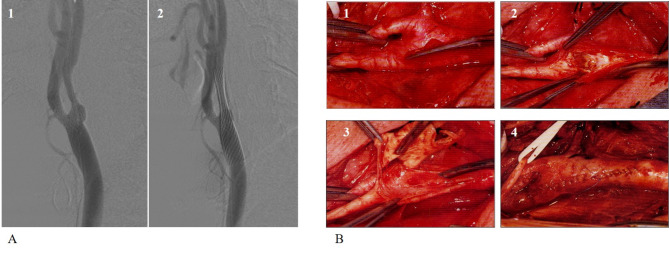
Inclusion criteria were symptomatic ICA stenosis ≥ 70% or asymptomatic ICA stenosis ≥ 80%. Exclusion criteria were age higher than 75 years, previous disabling stroke, non-sinus rhythm, contralateral carotid occlusion or stenosis higher than 70%. Figure 1 illustrates the CEA and CAS procedures.
Fig. 1.
The procedure of CAS (A) and CEA (B) are illustrated. Briefly, the carotid angiography (A1) showing the stenosis at the origin of the internal carotid artery, then in (A2) the placement of the stent with resolution of the stenosis. In (B1), the carotid bifurcation is exposed after a laterocervical longitudinal incision, then after clamping the bifurcation is opened (B2), the plaque is removed (B3) and then the flow is restored after surgical closure of the arteriotomy (B4).
Patients were enrolled in the study if they did not present any contraindication neither to CAS nor to CEA, so that they could potentially undergo both procedures. Then, patients were randomized to perform either CAS or CEA, using a predefined randomization list, with allocation ratio 1:1. The randomization list was computer generated and the randomization was simple. The former responsible of the study (M.M.T.) generated the allocation sequence, enrolled patients and assigned participants to interventions. The enrollment started in March 2018 and the primary completion date, with the completion of the last follow-up was April 2022.
According to Yakhou et al.10, the assumption of a BRS of 5.6 ms/mmHg before the procedure, 8.6 ms/mmHg after the procedure and a standard deviation of 3.1 ms/mmHg, a power of 90% and a level of significance of the first type of 0.05 divided by the number of comparisons, led to a sample size of 30 patients per groups. The supposition of a rate of drop-out of 15% for different reasons, for example bad signal recording or impossibility to perform the follow-up, led to a total sample of 70 patients.
Electrocardiogram (ECG) lead II (BioAmp FE132, ADInstruments, Australia), noninvasive photoplethysmographic AP (CNAP Monitor 500, CNSystems, Austria) and respiration via a piezoelectric thoracic belt (ADInstruments, Australia) were analog-to-digital converted by a polygraph (PowerLab, ADInstruments, Australia) at a sampling frequency of 400 Hz. Patients were monitored at rest in supine position (REST) for 10 min and for other 10 min during active standing (STAND). Acquisitions took place about one week before surgery (PRE) and after a 6- (FU6) and 12-month follow-up (FU12).
Series extraction and data analysis
HP was extracted from the ECG and approximated as the temporal distance between two R-wave peaks, located with minimum jitters via parabolic interpolation. The nth SAP was taken as the maximum of arterial pressure within the nth HP.
Synchronous HP and SAP sequences with a length of 250 beats were randomly selected during the whole recording at REST and during STAND at both PRE, FU6 and FU12. Series were manually checked and corrected for ectopic beats and misdetections via linear interpolation, correcting at maximum the 5% of the entire length of the series.
Time domain indexes were first computed in terms of mean and variance of HP and SAP expressed respectively in ms, ms2, mmHg and mmHg2 and labeled respectively as µHP, σ2HP, µSAP, σ2SAP.
Power spectral density of the series was estimated exploiting an autoregressive approach25,26. After linear detrending, time series were modeled via an autoregressive model whose number of coefficients were optimized in the range between 8 and 14. Power spectral density was then decomposed into bell-shaped components, each characterized by a central frequency, whose sum corresponds perfectly to the overall power spectrum. The power of each component was computed via the residue theorem25,27 and the central frequency of the components corresponded to the phase of the real pole or couple of conjugated poles of the transfer function of the autoregressive model. A spectral component was then labeled as in low frequency (LF) whether its central frequency was comprised in the range between 0.04 and 0.15 Hz and as high frequency (HF) whether its central frequency was in the range 0.15–0.4 Hz11. The total power in LF and HF bands was obtained as the sum of all the components in the same band. The power of SAP in LF band (SAPLF) expressed in absolute units (i.e., mmHg2) was then taken as an index of sympathetic modulation directed to the vasculature12, while the power of HP in HF band (HFHP) in absolute units (i.e., ms2) was taken as an index of parasympathetic modulation directed to the sinus node28.
BRS was first estimated via a spectral approach29, calculating BRS as the square root of the ratio between LF (or HF) power of HP divided by the LF (or HF) power of SAP. In this case the index was labeled as αLF or αHF and expressed in ms/mmHg.
A bivariate cross-spectral approach was also used to characterize baroreflex function15,21. BRS was computed as the transfer function gain of the HP-SAP relationship estimated as the ratio of the modulus of the HP-SAP cross-spectral density function to the modulus of the power spectrum of SAP. Squared coherence (K2) was computed as the ratio of the squared modulus of the HP-SAP cross-spectral density function to the product of the HP and SAP power spectral densities. The phase (Ph) between HP and SAP was also computed with the convention that negative values indicated that HP changes lagged SAP variations. According to a previous work21, the bivariate approach was grounded on a parametric bivariate autoregressive model whose order was fixed to 10. BRS, K2 and Ph were then sampled in correspondence of the maximum of the K2 function14 in, respectively, LF and HF bands and labeled as BRSLF, K2 LF, PhLF, BRSHF, K2 HF, PhHF. BRS was expressed in ms/mmHg regardless the technique used, and Ph and K2 were expressed in radians (rad) and dimensionless units.
Statistical analysis
After testing data for normality of the distribution via Shapiro-Wilk test, a two-way repeated measures analysis of variance (one-factor repetition, Holm-Sidak test for multiple comparisons) was used in either CAS or CEA group to check the differences between REST and STAND within the same time point (i.e., PRE, FU6 or FU12) and across time points within the same experimental condition (i.e., REST or STAND). Similarly, a two-way repeated measures analysis of variance (one-factor repetition, Holm-Sidak test for multiple comparisons) was used either at REST or during STAND to assess differences between CEA and CAS groups within the same time point and across time points within the same group (i.e., CEA or CAS). A commercial statistical software (Sigmaplot 14.5, Systat, Inc., Chicago, US) was used for analysis. A p < 0.05 with the appropriate corrections was always deemed as significant.
Results
After the exclusions of 12 patients for different reasons (e.g. bad signal recordings, arrhythmias, missing data) data coming from 58 patients were analyzed. Thirty-three patients were assigned to CEA (age 67.79 ± 5.32 yrs, 26 males) and 25 to CAS (age 70.32 ± 3.63 yrs, 14 males). As described in Table 1, which resumes demographic and clinical parameters of the population, the two groups were not different for sex composition, while the CAS group was older than the CEA one. Remarkably, all the other parameters were not different between groups.
Table 1.
Clinical and demographic data of CEA and CAS patients.
| Parameter | CEA (n = 33) | CAS (n = 25) |
|---|---|---|
| Age [yrs] | 67.79 ± 5.32 | 70.32 ± 3.63* |
| Gender [male] | 26 (79) | 14 (56) |
| BMI [kg·m− 2] | 27.17 ± 3.62 | 28.85 ± 6.59 |
| Hypertension | 29 (88) | 23 (92) |
| Beta-blockers | 20 (61) | 17 (68) |
| Diabetes under therapy | 7 (21) | 7 (28) |
| Smoke | 4 (12) | 4 (16) |
| Smoke abstinence 1–10 yrs | 7 (21) | 1 (4) |
| Hyperlipidemia under therapy | 22 (67) | 16 (64) |
| Serum creatinine [mg·dl− 1] | 0.94 ± 0.22 | 0.93 ± 0.31 |
| GFR [%] | 76.12 ± 14.01 | 73.33 ± 21.31 |
| Cronic kidney disease | 2 (6) | 0 (0) |
| Ascendent aorta aneurism | 1 (3) | 0 (0) |
| % of stenosis | 75.91 ± 6.31 | 75.60 ± 5.46 |
| % of controlateral stenosis | 43.03 ± 16.77 | 42.29 ± 19.60 |
BMI = body mass index; GFR = glomerular filtration ratio. Continuous data are presented as mean ± standard deviation and categorical data as number (percentage). The symbol § indicates p < 0.05 versus CEA. The symbol * means p < 0.05 versus CEA.
Due to the inability of some patients to participate to the follow-up for different reasons, we acquired 29 CEA and 26 CAS patients in FU6 and 31 CEA and 25 CAS in FU12.
Table 2 shows results of variability markers extracted in CEA patients during PRE, FU6 and FU12 at both REST and STAND. µHP was reduced during STAND at either PRE, FU6 and FU12. Furthermore, during STAND µHP was higher in FU6 with respect to PRE.
Table 2.
Cardiovascular control markers evaluated in CEA patients in PRE, FU6 and FU12 at REST and during STAND.
| CEA | REST | STAND | ||||
|---|---|---|---|---|---|---|
| PRE | FU6 | FU12 | PRE | FU6 | FU12 | |
| µHP [ms] | 932.13 ± 103.76 | 987.74 ± 124.78 | 930.68 ± 138.68 | 819.38 ± 100.85* | 899.09 ± 126.87*,§ | 845.95 ± 147.47* |
| σ2HP [ms2] | 701.13 ± 599 | 938.32 ± 759.35 | 668.56 ± 580.04 | 525.71 ± 500.51 | 773.58 ± 759.02 | 576.76 ± 624.09 |
| HFHP [ms2] | 147.77 ± 192.81 | 246.57 ± 278.18 | 171.18 ± 349.61 | 105.46 ± 252.86* | 145.53 ± 273.7* | 133.84 ± 319.01 |
| LFHP/HFHP | 1.98 ± 2.4 | 1.72 ± 1.99 | 1.57 ± 2.67 | 4.14 ± 7.02 | 2.33 ± 3.64 | 11.89 ± 55.83 |
| µSAP [mmHg] | 152.78 ± 28.61 | 143.78 ± 18.78 | 136.87 ± 18.18§ | 141.69 ± 26.04 | 148.14 ± 23.78 | 142.04 ± 29.72 |
| σ2SAP [mmHg2] | 27.53 ± 28.76 | 25.94 ± 24.79 | 17.78 ± 10.45 | 33.56 ± 30.74 | 80.08 ± 234.09 | 33.21 ± 23.64 |
| LFSAP [mmHg2] | 3.57 ± 8.45 | 5.37 ± 11.76 | 2.83 ± 3.74 | 5.34 ± 5.94 | 6.79 ± 7.51 | 4.83 ± 5.14 |
| αLF [ms/mmHg] | 9.43 ± 8.09 | 12.07 ± 16.25 | 7.89 ± 7.34 | 9.69 ± 19.36 | 6.11 ± 7.16 | 7.13 ± 7.97 |
| αHF [ms/mmHg] | 6.32 ± 5.56 | 7.96 ± 5.03 | 6.59 ± 3.8 | 3.97 ± 1.84* | 4.48 ± 2.58* | 4.64 ± 4.38* |
| K2LF | 0.39 ± 0.21 | 0.43 ± 0.20 | 0.37 ± 0.22 | 0.45 ± 0.23 | 0.37 ± 0.19 | 0.34 ± 0.19 |
| PhLF [rad] | -1.15 ± 1.18 | -0.92 ± 1.64 | -1.26 ± 1.39 | -1.48 ± 0.84 | -1.22 ± 1.58 | -1.01 ± 1.67 |
| BRSLF [ms/mmHg] | 5.14 ± 4.13 | 8.46 ± 12.04 | 6.42 ± 10.28 | 3.96 ± 4.54 | 3.37 ± 2.31* | 2.99 ± 2.36 |
| K2HF | 0.65 ± 0.23 | 0.68 ± 0.21 | 0.64 ± 0.24 | 0.65 ± 0.23 | 0.58 ± 0.23 | 0.62 ± 0.23 |
| PhHF [rad] | -0.18 ± 0.67 | 0.33 ± 0.92 | -0.04 ± 0.90 | -0.05 ± 1.21 | 0.27 ± 0.96 | 0.17 ± 1.24 |
| BRSHF [ms/mmHg] | 5.94 ± 5.38 | 9.93 ± 18.08 | 6.74 ± 8.49 | 5.51 ± 10.45 | 4.38 ± 3.59* | 4.96 ± 5.6 |
HP = heart period; µHP = HP mean; σ2HP = HP variance; SAP = systolic arterial pressure; µSAP = SAP mean; σ2SAP = SAP variance; LF = low frequency; HF = high frequency; HFHP = power of the HP series in the HF band; LFSAP = power of the SAP series in the LF band; BRS = baroreflex sensitivity; α = BRS via the spectral method; αLF = α in the LF band; αHF = BRS in the HF band; K2 = squared coherence; Ph = phase relationship; BRSLF = BRS via the cross-spectral method in the LF band; K2LF = K2 in the LF band; PhLF = Ph in the LF band; BRSHF = BRS via the cross-spectral method in the HF band; K2HF = K2 in the HF band; PhHF = Ph in the HF band. Data are presented as mean ± standard deviation. The symbol * means p < 0.05 versus REST within the same time point, while the symbol § indicates p < 0.05 versus PRE within the same experimental condition.
HFHP was significantly reduced during STAND with respect to REST at PRE and FU6, while it was unchanged at FU12. µSAP at REST was lower in FU12 with respect to PRE.
BRS evaluated as αHF was decreased during STAND at either PRE, FU6 and FU12, while it declined during STAND only at FU6 when evaluated as BRSLF or BRSHF. All the other indexes: σ2vHP, LFHP /HFHP, σ2 SAP, LFSAP and αLF as well as those related to K2 and Ph were unchanged between time points of analysis and experimental conditions.
Table 3 shows results of variability markers extracted in CAS patients in PRE, FU6 and FU12 at REST and during STAND. µHP was reduced during STAND at either PRE, FU6 and FU12 and, during FU6 and FU12 it was higher than PRE both at REST and during STAND. In PRE and during STAND σ2 HP was higher than in PRE during REST and lower than in FU12 during STAND. Similarly, HFHP was reduced during STAND in PRE and increased during STAND in FU6 with respect to PRE. αLF was reduced during STAND with respect to REST only in FU6, while αHF during STAND in FU6 was larger than in PRE during STAND. All the other indexes were unchanged between phases and experimental conditions.
Table 3.
Cardiovascular control markers evaluated in CAS patients in PRE, FU6 and FU12 at REST and during STAND.
| CAS | REST | STAND | ||||
|---|---|---|---|---|---|---|
| PRE | FU6 | FU12 | PRE | FU6 | FU12 | |
| µHP [ms] | 851.20 ± 149.97 | 886.93 ± 111.87§ | 917.64 ± 130.77§ | 768.11 ± 132.42* | 821.81 ± 122.32*,§ | 859.64 ± 108.29*,§ |
| σ2HP [ms2] | 595.97 ± 822.06 | 521.72 ± 610.72 | 559.74 ± 694.53 | 257.66 ± 240.27* | 364.91 ± 414.46 | 549.17 ± 498.72§ |
| HFHP [ms2] | 143.13 ± 267.38 | 125.74 ± 159.58 | 119.27 ± 140.05 | 43.85 ± 42.64* | 97.74 ± 156.67§ | 123.79 ± 172.56 |
| LFHP/HFHP | 1.34 ± 1.94 | 1.11 ± 1.93 | 0.82 ± 0.91 | 1.34 ± 1.64 | 1.47 ± 3.64 | 0.80 ± 1.06 |
| µSAP [mmHg] | 148.97 ± 27.43 | 147.47 ± 21.36 | 147.48 ± 24.41 | 143.24 ± 24.79 | 143.45 ± 30.9 | 146.85 ± 24.94 |
| σ2SAP [mmHg2] | 24.61 ± 18.41 | 30.53 ± 48.49 | 21.82 ± 12.9 | 43.76 ± 29.06 | 30.23 ± 26.55 | 36.09 ± 32.72 |
| LFSAP [mmHg2] | 3.92 ± 4.24 | 2.39 ± 3.40 | 1.89 ± 1.52 | 6.27 ± 7.19 | 4.52 ± 6.76 | 2.92 ± 2.43 |
| αLF [ms/mmHg] | 8.27 ± 20.01 | 7.04 ± 7.64 | 6.47 ± 6.7 | 2.56 ± 1.91 | 3.64 ± 2.7* | 5.06 ± 4.25 |
| αHF [ms/mmHg] | 5.64 ± 5.80 | 6.35 ± 6.06 | 5.9 ± 5.22 | 2.71 ± 1.71 | 6.19 ± 6.87§ | 4.32 ± 2.88 |
| K2LF | 0.31 ± 0.20 | 0.37 ± 0.20 | 0.34 ± 0.2 | 0.31 ± 0.18 | 0.43 ± 0.23 | 0.28 ± 0.18# |
| PhLF [rad] | -0.62 ± 1.66 | -0.97 ± 1.83 | -0.60 ± 1.74 | -0.83 ± 1.70 | -0.98 ± 1.46 | -1.35 ± 1.41 |
| BRSLF [ms/mmHg] | 3.50 ± 3.74 | 4.80 ± 5.03 | 4.77 ± 6.38 | 1.64 ± 1.28 | 2.96 ± 2.61 | 3.00 ± 3.37 |
| K2HF | 0.65 ± 0.25 | 0.69 ± 0.24 | 0.67 ± 0.24 | 0.63 ± 0.23 | 0.66 ± 0.23 | 0.57 ± 0.26 |
| PhHF [rad] | -0.13 ± 0.84 | -0.27 ± 1.14 | 0.13 ± 1.22 | -0.11 ± 1.40 | 0.06 ± 1.23 | 0.35 ± 1.3 |
| BRSHF [ms/mmHg] | 5.36 ± 5.64 | 9.15 ± 15.05 | 6.15 ± 7.50 | 2.42 ± 1.96 | 5.56 ± 5.00 | 5.40 ± 5.15 |
HP = heart period; µHP = HP mean; σ2HP = HP variance; SAP = systolic arterial pressure; µSAP = SAP mean; σ2SAP = SAP variance; LF = low frequency; HF = high frequency; HFHP = power of the HP series in the HF band; LFSAP = power of the SAP series in the LF band; BRS = baroreflex sensitivity; α = BRS via the spectral method; αLF = α in the LF band; αHF = BRS in the HF band; K2 = squared coherence; Ph = phase relationship; BRSLF = BRS via the cross-spectral method in the LF band; K2LF = K2 in the LF band; PhLF = Ph in the LF band; BRSHF = BRS via the cross-spectral method in the HF band; K2HF = K2 in the HF band; PhHF = Ph in the HF band. Data are presented as mean ± standard deviation. The symbol * means p < 0.05 versus REST within the same time point, while the symbols § and # indicate respectively p < 0.05 versus PRE and versus FU6 within the same experimental condition.
Table 4 compares CEA and CAS populations at REST in PRE, FU6 and FU12. µHP was shorter in CAS with respect to CEA solely in PRE and FU6. In FU6 σ2 HP was lower in CAS with respect to CEA. µSAP in CEA patients was lower in FU12 with respect to PRE. In FU6 PhHF was lower and negative in CAS with respect to CEA where it was positive. Remarkably, none of the other indexes was different among time points of analysis and populations.
Table 4.
Cardiovascular control markers in CEA and CAS patients evaluated in PRE, FU6 and FU12 during REST.
| REST | CEA | CAS | ||||
|---|---|---|---|---|---|---|
| PRE | FU6 | FU12 | PRE | FU6 | FU12 | |
| µHP [ms] | 932.13 ± 103.76 | 987.74 ± 124.78 | 930.68 ± 138.68 | 851.20 ± 149.97* | 886.93 ± 111.87* | 917.64 ± 130.77 |
| σ2HP [ms2] | 701.13 ± 599 | 938.32 ± 759.35 | 668.56 ± 580.04 | 595.97 ± 822.06 | 521.72 ± 610.72* | 559.74 ± 694.53 |
| HFHP [ms2] | 147.77 ± 192.81 | 246.57 ± 278.18 | 171.18 ± 349.61 | 143.13 ± 267.38 | 125.74 ± 159.58 | 119.27 ± 140.05 |
| LFHP/HFHP | 1.98 ± 2.4 | 1.72 ± 1.99 | 1.57 ± 2.67 | 1.34 ± 1.94 | 1.11 ± 1.93 | 0.82 ± 0.91 |
| µSAP [mmHg] | 152.78 ± 28.61 | 143.78 ± 18.78 | 136.87 ± 18.18§ | 148.97 ± 27.43 | 147.47 ± 21.36 | 147.48 ± 24.41 |
| σ2SAP [mmHg2] | 27.53 ± 28.76 | 25.94 ± 24.79 | 17.78 ± 10.45 | 24.61 ± 18.41 | 30.53 ± 48.49 | 21.82 ± 12.9 |
| LFSAP [mmHg2] | 3.57 ± 8.45 | 5.37 ± 11.76 | 2.83 ± 3.74 | 3.92 ± 4.24 | 2.39 ± 3.4 | 1.89 ± 1.52 |
| αLF [ms/mmHg] | 9.43 ± 8.09 | 12.07 ± 16.25 | 7.89 ± 7.34 | 8.27 ± 20.01 | 7.04 ± 7.64 | 6.47 ± 6.7 |
| αHF [ms/mmHg] | 6.32 ± 5.56 | 7.96 ± 5.03 | 6.59 ± 3.8 | 5.64 ± 5.8 | 6.35 ± 6.06 | 5.9 ± 5.22 |
| K2LF | 0.39 ± 0.21 | 0.43 ± 0.20 | 0.37 ± 0.22 | 0.31 ± 0.2 | 0.37 ± 0.20 | 0.34 ± 0.2 |
| PhLF [rad] | -1.15 ± 1.18 | -0.92 ± 1.64 | -1.26 ± 1.39 | -0.62 ± 1.66 | -0.97 ± 1.83 | -0.6 ± 1.74 |
| BRSLF [ms/mmHg] | 5.14 ± 4.13 | 8.46 ± 12.04 | 6.42 ± 10.28 | 3.5 ± 3.74 | 4.8 ± 5.03 | 4.77 ± 6.38 |
| K2HF | 0.65 ± 0.23 | 0.68 ± 0.21 | 0.64 ± 0.24 | 0.65 ± 0.25 | 0.69 ± 0.24 | 0.67 ± 0.24 |
| PhHF [rad] | -0.18 ± 0.67 | 0.33 ± 0.92 | -0.04 ± 0.90 | -0.13 ± 0.84 | -0.27 ± 1.14* | 0.13 ± 1.22 |
| BRSHF [ms/mmHg] | 5.94 ± 5.38 | 9.93 ± 18.08 | 6.74 ± 8.49 | 5.36 ± 5.64 | 9.15 ± 15.05 | 6.15 ± 7.5 |
HP = heart period; µHP = HP mean; σ2HP = HP variance; SAP = systolic arterial pressure; µSAP = SAP mean; σ2SAP = SAP variance; LF = low frequency; HF = high frequency; HFHP = power of the HP series in the HF band; LFSAP = power of the SAP series in the LF band; BRS = baroreflex sensitivity; α = BRS via the spectral method; αLF = α in the LF band; αHF = BRS in the HF band; K2 = squared coherence; Ph = phase relationship; BRSLF = BRS via the cross-spectral method in the LF band; K2LF = K2 in the LF band; PhLF = Ph in the LF band; BRSHF = BRS via the cross-spectral method in the HF band; K2HF = K2 in the HF band; PhHF = Ph in the HF band. Data are presented as mean ± standard deviation. The symbol * means p < 0.05 versus CEA within the same time point, while the symbol § indicates p < 0.05 versus PRE within the same group.
Table 5 compares CEA and CAS populations during STAND in PRE, FU6 and FU12. In CEA patients µHP was higher in FU6 with respect to PRE and greater than in CAS patients. In CAS patients it was also higher in FU12 than PRE. αHF in CAS patients was higher in FU6 than in PRE; K2 LF in PRE was lower in CAS than CEA and within CAS it was lower in FU12 with respect to FU6. BRSLF in PRE was lower in CAS with respect to CEA. The other markers did not change between time points of analysis and conditions.
Table 5.
Cardiovascular control markers in CEA and CAS patients evaluated in PRE, FU6 and FU12 during STAND.
| STAND | CEA | CAS | ||||
|---|---|---|---|---|---|---|
| PRE | FU6 | FU12 | PRE | FU6 | FU12 | |
| µHP [ms] | 819.38 ± 100.85 | 899.09 ± 126.87§ | 845.95 ± 147.47 | 768.11 ± 132.42 | 821.81 ± 122.32* | 859.64 ± 108.29§ |
| σ2HP [ms2] | 525.71 ± 500.51 | 773.58 ± 759.02 | 576.76 ± 624.09 | 257.66 ± 240.27 | 364.91 ± 414.46 | 549.17 ± 498.72 |
| HFHP [ms2] | 105.46 ± 252.86 | 145.53 ± 273.7 | 133.84 ± 319.01 | 43.85 ± 42.64 | 97.74 ± 156.67 | 123.79 ± 172.56 |
| LFHP/HFHP | 4.14 ± 7.02 | 2.33 ± 3.64 | 11.89 ± 55.83 | 1.34 ± 1.64 | 1.47 ± 3.64 | 0.80 ± 1.06 |
| µSAP [mmHg] | 141.69 ± 26.04 | 148.14 ± 23.78 | 142.04 ± 29.72 | 143.24 ± 24.79 | 143.45 ± 30.9 | 146.85 ± 24.94 |
| σ2SAP [mmHg2] | 33.56 ± 30.74 | 80.08 ± 234.09 | 33.21 ± 23.64 | 43.76 ± 29.06 | 30.23 ± 26.55 | 36.09 ± 32.72 |
| LFSAP [mmHg2] | 5.34 ± 5.94 | 6.79 ± 7.51 | 4.83 ± 5.14 | 6.27 ± 7.19 | 4.52 ± 6.76 | 2.92 ± 2.43 |
| αLF [ms/mmHg] | 9.69 ± 19.36 | 6.11 ± 7.16 | 7.13 ± 7.97 | 2.56 ± 1.91 | 3.64 ± 2.7 | 5.06 ± 4.25 |
| αHF [ms/mmHg] | 3.97 ± 1.84 | 4.48 ± 2.58 | 4.64 ± 4.38 | 2.71 ± 1.71 | 6.19 ± 6.87§ | 4.32 ± 2.88 |
| K2LF | 0.45 ± 0.23 | 0.37 ± 0.19 | 0.34 ± 0.19 | 0.31 ± 0.18* | 0.43 ± 0.23 | 0.28 ± 0.18# |
| PhLF [rad] | -1.48 ± 0.84 | -1.22 ± 1.58 | -1.01 ± 1.67 | -0.83 ± 1.70 | -0.98 ± 1.46 | -1.35 ± 1.41 |
| BRSLF [ms/mmHg] | 3.96 ± 4.54 | 3.37 ± 2.31 | 2.99 ± 2.36 | 1.64 ± 1.28* | 2.96 ± 2.61 | 3.00 ± 3.37 |
| K2HF | 0.65 ± 0.23 | 0.58 ± 0.23 | 0.62 ± 0.23 | 0.63 ± 0.23 | 0.66 ± 0.23 | 0.57 ± 0.26 |
| PhHF [rad] | -0.05 ± 1.21 | 0.27 ± 0.96 | 0.17 ± 1.24 | -0.11 ± 1.40 | 0.06 ± 1.23 | 0.35 ± 1.3 |
| BRSHF [ms/mmHg] | 5.51 ± 10.45 | 4.38 ± 3.59 | 4.96 ± 5.6 | 2.42 ± 1.96 | 5.56 ± 5.00 | 5.40 ± 5.15 |
HP = heart period; µHP = HP mean; σ2 HP = HP variance; SAP = systolic arterial pressure; µSAP = SAP mean; σ2SAP = SAP variance; LF = low frequency; HF = high frequency; HFHP = power of the HP series in the HF band; LFSAP = power of the SAP series in the LF band; BRS = baroreflex sensitivity; α = BRS via the spectral method; αLF = α in the LF band; αHF = BRS in the HF band; K2 = squared coherence; Ph = phase relationship; BRSLF = BRS via the cross-spectral method in the LF band; K2LF = K2 in the LF band; PhLF = Ph in the LF band; BRSHF = BRS via the cross-spectral method in the HF band; K2HF = K2 in the HF band; PhHF = Ph in the HF band. Data are presented as mean ± standard deviation. The symbol * means p < 0.05 versus CEA within the same time point, while the symbols § and # indicate p < 0.05 versus PRE and FU6 respectively within the same group.
Discussion
The main findings of this work can be resumed as: (i) CEA patients showed the expected autonomic response to the orthostatic stressor before the intervention and at FU6, while their baroreflex control response was elicited especially at FU6; (ii) CAS patients showed a similar response to the postural challenge in PRE but a weaker response at both follow-ups; (iii) one year after the procedure both groups showed a weaker response to the orthostatic challenge; (iv) when directly compared, the two groups of patients showed some differences at PRE that disappeared at the follow-up, especially when extended to one year.
CAS and CEA patients showed a preserved response to STAND before the intervention and a blunted one during a long-term follow-up
Active standing is an orthostatic stimulus evoking a sympathetic enhancement and vagal withdrawal16,24,30. Patients undergoing CEA showed the expected response but with some differences among the three experimental phases (i.e. PRE, FU6 and FU12). In particular, the expected tachycardic response was observed at all the time points, while the reduction of the parasympathetic modulation, representing the vagal withdrawal evoked by the orthostatic maneuver, was significant before the intervention and after six months but not 12 months later. The sympathetic modulation, on the contrary, was not modified by the postural challenge as it should be expected, while the magnitude of the baroreflex response was shown to be reduced during the orthostatic maneuver before the intervention and especially after six months. Remarkably, after 12 months, none of the markers related to the baroreflex resulted significantly modified by STAND and this result was at odds with FU6. We hypothesized that the autonomic response, as shown by the behavior of HFHP, and baroreflex are impaired one year after the procedure as a likely consequence of the long-term effect of the surgical intervention. The reason why the surgical deafferentation of the nervous fibers occurring during the manipulation of the carotid bifurcation in CEA might have impaired exclusively the long-term functioning of the baroreflex arch, while it is much better preserved in FU6, requires further clarification in relation to the strategy followed during manipulation.
Patients undergoing CAS showed the expected increase of heart rate during STAND in all time conditions (i.e. PRE, FU6, FU12), while the vagal withdrawal in response to the postural challenge was present only before the intervention. This finding could indicate a post-procedure impairment of the autonomic control in CAS patients, leading to a lower response of the variability markers to STAND. The post-procedure baroreflex response to STAND was generally weak as well: indeed, spectral technique detected a reduction of the BRS during STAND in FU6 but not in FU12 and cross-spectral method did not find any BRS modification induced by STAND in both FU6 and FU12. It could be hypothesized that, in CAS patients, the autonomic control was preserved before the procedure, as indicated by the vagal withdrawal induced by STAND, but depressed at six-month and one-year follow-ups, while the baroreflex control was depressed regardless of the time points of the analysis. The worsening of the autonomic control and the inability to recover baroreflex function after CAS could be due to the positioning of the stent, which could impair the carotid baroreceptors by intruding a radial force acting on the carotid bifurcation. Even in CAS population, further studies should be needed to confirm these findings. Remarkably, the position of the stenosis with respect to carotid bifurcation is not known in these patients, thus being impossible to hypothesize to what extent the carotid baroreceptors are mechanically affected by the two interventions as well as short-term and long-term consequences on baroreflex function.
It is worth mentioning that this study, by presenting a complete analysis of the response to an orthostatic stimulus of both CEA and CAS, extends previous findings suggesting that after the intervention the autonomic response of CEA patients is enhanced22, while that of CAS ones would be more blunted, especially as related to baroreflex control. An interesting finding is related to the lack of response of both groups to STAND at the longer follow-up, which might be related to different mechanisms in CEA and CAS, being more related to the manipulation strategy in CEA and to the presence of stent in CAS. This study suggests that, regardless of the type of intervention, the final, long-term, impact on autonomic function and baroreflex control is similar. This conclusion could suggest the need of monitoring the patients for a longer period to check whether the increased cardiovascular risk associated to an impaired autonomic function and baroreflex control5,8 plays any role on the long-term prognosis.
CEA and CAS lead to similar long-term impairment of cardiovascular control
After the evaluation of the response of CAS and CEA to STAND separately, we performed a direct comparison of the two populations at REST and during STAND. The two groups did not differ for the most part of the cardiovascular extracted markers at REST and during STAND before the intervention. The few exceptions were related to the µHP, resulting lower in CAS with respect to CEA at REST in PRE and FU6 and to the σ2 HP resulting lower in CAS in FU6 at REST. During STAND no significant differences were observed but, before the intervention, markers of the baroreflex control in the LF band were more depressed during STAND in CAS with respect to CEA patients. Such slight differences in PRE could be at least partially related to the different age of the two groups, since they reflect the tendency observed in previous studies that vagal control was reduced with age31–34. In fact, although the patients were randomly allocated to the procedures, the exclusions of some patients in the analysis led to some difference in age between the two groups.
Anyway, at the 6-months follow-up the lower values of µHP and σ2 HP in CAS than CEA suggest that the stenting procedure would disturb more importantly autonomic control. This difference could not be totally explained by the different age of the two group because their mean ages differ for only a few years. This observation was corroborated by the fact that all the differences disappeared at the 12-months follow-up, showing a reduced response to the postural challenge in both groups and suggesting that both populations featured a less reactive autonomic function in the long-term period compared to PRE. This lower reactivity could expose the two groups to a higher cardiovascular risk suggesting that the severity of the exposition deserves to be more deeply evaluated4,5,30. Interestingly, the most part of the observed differences disappeared at a long follow-up, corroborating the impression that both the interventions, although leading to a slightly different responses to the STAND at short follow-ups, feature similar long-term derangements of the autonomic function and baroreflex control comparable with the one observed in PRE.
Limitations of the study and future developments
The main limitation of the study is related to the difference in age between the two populations that could have masked, or favored, some differences in the autonomic control markers. Although initially balanced at the enrollment, the presence of some dropouts led to this age mismatch. Anyway, since the difference between mean age of the two groups is a few years, the impact on variability markers is expected to be limited, thus allowing us to conclude that the observed behavior of the cardiovascular control markers would be more due to the population than to age.
A second limitation is due to the low statistical power of the analysis associated with the limited sample size. The fact that the study was carried out mainly during the first and second waves of COVID-19 pandemic in Italy accounts for the difficulties we faced in enrolling patients and in recalling patients for the follow-up sessions.
Furthermore, the lack of a gold-standard orthostatic stimulus such as the head-up tilt test could have reduced the possibility of evoking stronger autonomic response in our cohorts that can have limited the statistical power of our study. Anyway, we should remark that active standing is also considered a validated maneuver to evoke a reflex sympathetic response, and it can be more easily carried out in experimental settings compared to head-up tilt24.
We also stress that the LFSAP power is taken in this study as an index of sympathetic modulation. However, since this marker is also driven by the activation of a baroreflex response, speculations about the pure sympathetic origin of this index should be taken with caution, although previous studies found the LFSAP power correlated with a direct measure of the sympathetic modulation such as the LF power derived from the variability of muscle sympathetic nerve activity35.
Finally, the position of the stenosis with respect to the carotid bifurcation is unknown. Thus, it is not possible to directly assess the impact of the procedure and strategy of manipulation on the carotid arteries. Future studies should consider the position of the stenosis and correlate the impact it has on autonomic function and baroreflex.
We remark that our methodologies allow the characterization of the cardiac baroreflex as a global response of the heart rate-arterial pressure relation and do not allow to evaluate the baroreflex function at the carotid site. Future studies are advocated to enroll a higher number of subjects avoiding biases in the populations and to perform a longer follow-up to assess if findings at 12 months will be confirmed and to evaluate the long-term risk associated to CAS or CEA.
Conclusions
This work compared the autonomic and baroreflex controls in patients undergoing CEA and CAS evaluated during an orthostatic challenge under a long-term follow-up. Autonomic control of CEA population responded better than CAS group six months after the intervention, but this improvement was not confirmed with a long-term follow-up, thus leading to similar long-term cardiovascular responses in the two populations.
Only small differences were observed between the two groups and these differences were evident at a 6-month follow-up. On the contrary, the similarly weak autonomic and baroreflex responses to the orthostatic challenge in CEA and CAS groups at the 12-months follow-up suggest that CEA and CAS patients should be monitored during a longer follow-up with respect to previous studies (more than 6 months) and the need to better characterize the impact of a worsened cardiovascular regulation on the risk to develop adverse events in both groups. Future studies should be designed to stratify the risk of these patients and better predict their long-term outcome.
Acknowledgements
Authors acknowledge Dr. Massimiliano Marrocco-Trischitta (M.M.T.), who was the former responsible of the study, participated in funding acquisition, study design, and enrollment/surgery of patients but passed away before the manuscript was completed and the final approval was given.
Author contributions
V.B., M.M.T. and D.M. conceived and designed research; V.B., I.B., G.D.A., B.C., F.G. performed experiments; V.B., B.C., F.G. analyzed data; V.B. and A.P. interpreted results of experiments; V.B., D.M. and A.P. drafted manuscript; V.B., G.N., I.B., G.D.A., B.C., F.G., V.C., M.C., F.S., A.P. and D.M. edited and revised the manuscript; V.B., G.N., I.B., G.D.A., B.C., F.G., V.C., M.C., F.S., A.P. and D.M. approved final version of manuscript.
Data availability
Data are available upon reasonable request to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paciaroni, M. et al. Long-term clinical and angiographic outcomes in symptomatic patients with 70–99% carotid artery stenosis. Stroke31, 2037–2042 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Cao, Q., Zhang, J. & Xu, G. Hemodynamic changes and baroreflex sensitivity associated with carotid endarterectomy and carotid artery stenting. Interv Neurol.3, 13–21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brott, T. G. et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl. J. Med.363, 11–23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranucci, M., Porta, A., Bari, V., Pistuddi, V. & La Rovere, M. T. Baroreflex sensitivity and outcomes following coronary surgery. PLoS One. 12, e0175008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Rovere, M. T., Bigger, J. T. J., Marcus, F. I., Mortara, A. & Schwartz, P. J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (autonomic tone and reflexes after myocardial infarction) investigators. Lancet351, 478–484 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Robinson, T. G., Dawson, S. L., Eames, P. J., Panerai, R. B. & Potter, J. F. Cardiac baroreceptor sensitivity predicts long-term outcome after acute ischemic stroke. Stroke34, 705–712 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Demirci, M. et al. Carotid artery stenting and endarterectomy have different effects on heart rate variability. J. Neurol. Sci.241, 45–51 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Demirel, S. et al. Changes in baroreceptor sensitivity after eversion carotid endarterectomy. J. Vasc Surg.55, 1322–1328 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Amino, M. et al. Prolonged autonomic fluctuation derived from Parasympathetic Hypertonia after Carotid Endarterectomy but not stenting. J. Stroke Cerebrovasc. Dis.28, 10–20 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Yakhou, L. et al. Noninvasive investigation of autonomic activity after carotid stenting or carotid endarterectomy. J. Vasc Surg.44, 472–479 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur. Heart J.17, 354–381 (1996). [PubMed] [Google Scholar]
- 12.Pagani, M. et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res.59, 178–193 (1986). [DOI] [PubMed] [Google Scholar]
- 13.Laude, D. et al. Comparison of various techniques used to estimate spontaneous baroreflex sensitivity (the EuroBaVar study). Am. J. Physiol. Regul. Integr. Comp. Physiol.286, 226 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Cohen, M. A. & Taylor, J. A. Short-term cardiovascular oscillations in man: measuring and modelling the physiologies. J. Physiol. (Lond). 542, 669–683 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bari, V. et al. Comparison of causal and non-causal strategies for the Assessment of Baroreflex Sensitivity in Predicting Acute kidney dysfunction after coronary artery bypass grafting. Front. Physiol.10, 1319 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bari, V. et al. Characterization of cardiovascular and cerebrovascular controls via spectral causality analysis in patients undergoing surgical aortic valve replacement during a three-month follow-up. Physiol. Meas.4410.1088/1361-6579/acf992 (2023). [DOI] [PubMed]
- 17.Pinna, G. D. et al. Different estimation methods of spontaneous baroreflex sensitivity have different predictive value in heart failure patients. J. Hypertens.35, 1666–1675 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Porta, A. et al. Model-based causal closed-loop approach to the estimate of baroreflex sensitivity during propofol anesthesia in patients undergoing coronary artery bypass graft. J. Appl. Physiol.115, 1032–1042 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Faes, L. et al. Causal transfer function analysis to describe closed loop interactions between cardiovascular and cardiorespiratory variability signals. Biol. Cybern. 90, 390–399 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Saul, J. P., Berger, R. D., Chen, M. H. & Cohen, R. J. Transfer function analysis of autonomic regulation. II. Respiratory sinus arrhythmia. Am. J. Physiol.256, 153 (1989). [DOI] [PubMed] [Google Scholar]
- 21.Porta, A., Baselli, G., Rimoldi, O., Malliani, A. & Pagani, M. Assessing baroreflex gain from spontaneous variability in conscious dogs: role of causality and respiration. Am. J. Physiol. Heart Circ. Physiol.279, 2558 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Dalla Vecchia, L. et al. Favorable effects of carotid endarterectomy on baroreflex sensitivity and cardiovascular neural modulation: a 4-month follow-up. Am. J. Physiol. Regul. Integr. Comp. Physiol.304, 1114 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Marrocco-Trischitta, M. et al. Peripheral baroreflex and chemoreflex function after eversion carotid endarterectomy. J. Vasc Surg.58, (2013). 136 – 44.e1. [DOI] [PubMed]
- 24.Matsushima, R., Tanaka, H. & Tamai, H. Comparison of the active standing test and head-up tilt test for diagnosis of syncope in childhood and adolescence. Clin. Auton. Res.14, 376–384 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Baselli, G. et al. Spectral decomposition in multichannel recordings based on multi-variate parametric identification. IEEE Trans. Biomed. Eng.44, 1092–1101 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Kay, S. M. In Spectrum Analysis: A Modern Perspective (Englewood Cliffs, NJ, 1988).
- 27.Zetterberg, L. H. Estimation of parameters for a linear difference equation with application to EEG analysis. Math. Biosci.5, 227–275 (1969). [Google Scholar]
- 28.Akselrod, S. et al. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science213, 220–222 (1981). [DOI] [PubMed] [Google Scholar]
- 29.Pagani, M. et al. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension12, 600–610 (1988). [DOI] [PubMed] [Google Scholar]
- 30.Porta, A. et al. Evaluation of the impact of surgical aortic valve replacement on short-term cardiovascular and cerebrovascular controls through spontaneous variability analysis. PLoS ONE 15 (2020). [DOI] [PMC free article] [PubMed]
- 31.Milan-Mattos, J. C. et al. Influence of age and gender on the phase and strength of the relation between heart period and systolic blood pressure spontaneous fluctuations. J. Appl. Physiol. (1985). 124, 791–804 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Barantke, M. et al. Effects of gender and aging on differential autonomic responses to orthostatic maneuvers. J. Cardiovasc. Electrophysiol.19, 1296–1303 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Beckers, F., Verheyden, B. & Aubert, A. E. Aging and nonlinear heart rate control in a healthy population. Am. J. Physiol. Heart Circ. Physiol.290, 2560 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Laitinen, T., Niskanen, L., Geelen, G., Länsimies, E. & Hartikainen, J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J. Appl. Physiol. (1985). 96, 2333–2340 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Marchi, A. et al. Calibrated variability of muscle sympathetic nerve activity during graded head-up tilt in humans and its link with noradrenaline data and cardiovascular rhythms. Am. J. Physiol. Reg. Integr. Comp. Physiol.310, 1134–1143 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request to the corresponding author.