Abstract
The circadian clock and its output pathways play a pivotal role in optimizing daily processes. To obtain insights into how diverse rhythmic physiology and behaviors are orchestrated, we have generated a comprehensive connectivity map of an animal circadian clock using the Drosophila FlyWire brain connectome. Intriguingly, we identified additional dorsal clock neurons, thus showing that the Drosophila circadian network contains ~240 instead of 150 neurons. We revealed extensive contralateral synaptic connectivity within the network and discovered novel indirect light input pathways to the clock neurons. We also elucidated pathways via which the clock modulates descending neurons that are known to regulate feeding and reproductive behaviors. Interestingly, we observed sparse monosynaptic connectivity between clock neurons and downstream higher-order brain centers and neurosecretory cells known to regulate behavior and physiology. Therefore, we integrated single-cell transcriptomics and receptor mapping to decipher putative paracrine peptidergic signaling by clock neurons. Our analyses identified additional novel neuropeptides expressed in clock neurons and suggest that peptidergic signaling significantly enriches interconnectivity within the clock network.
Subject terms: Circadian rhythms and sleep, Neural circuits, Computational neuroscience
Circadian clocks are a fundamental biological system which orchestrate various physiological and behavioral processes in response to daily environmental changes. Here, the authors utilize a multipronged approach to generate a comprehensive neural connectome of an animal circadian clock.
Introduction
Almost all living organisms from humans to bacteria possess a circadian clock1. This internal timekeeping system enables organisms to anticipate and adapt to the rhythmic environmental changes that occur over a 24-hour cycle. At their molecular core, these clocks are comprised of cell-autonomous transcription-translation negative feedback loops2. In most animals, the master circadian clock in the brain receives light cues via the eyes which enable synchronization (or entrainment) with the external 24-hour light-dark cycles. The master clock sits at the top of the hierarchy and in turn modulates the activity of downstream neurons, as well as the peripheral clocks located in tissues throughout the body via endocrine and systemic signaling. In vertebrates, the master clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus and is comprised of approximately 20,000 neurons3. Extensive intercellular coupling between these neurons likely via neurotransmitters, neuropeptides, and gap junctions forms a neuronal network that is resilient to internal and environmental perturbations4,5. Systematic characterization of these diverse coupling mechanisms between clock neurons is thus crucial to understanding circadian clock entrainment and mutual coupling of the clock neurons. In addition, unraveling the clock output pathways which generate rhythmic behaviors and hormonal signaling can provide mechanistic insights into circadian regulation of organismal physiology and homeostasis.
These aims are particularly challenging to accomplish in vertebrates due to the large neuronal network size and resultant increase in complexity. However, the molecular clock architecture as well as the neuronal network motifs are highly conserved from humans to insects6,7. Hence, Drosophila melanogaster with its powerful genetic toolkit and a complete brain connectome represents an ideal system for deciphering the clock network and its input and output pathways8–10. Not surprisingly, the fly circadian clock, generally regarded to be comprised of approximately 150 neurons, is extremely well characterized11. These neurons have historically been classified into different groups of Lateral and Dorsal Neurons (LN and DN, respectively) based on their size, anatomy, location in the brain, and differences in gene expression12. Further, these neuronal subgroups are active at different times during the day and are consequently distinct functionally13. Single-cell transcriptome sequencing analyses of clock neurons recently revealed additional heterogeneity within some of these clusters, which can largely be explained by neuronal signaling molecules that they express14,15. Comparing the synaptic connectivity of different clock neurons can determine if the molecular heterogeneity based on gene expression also translates into heterogenous synaptic connectivity. Nonetheless, given the rich array of neuropeptides expressed in clock neurons, both synaptic and paracrine signaling appear crucial in mediating the connectivity between clock neurons as well as their output pathways. While recent work has begun to uncover some of this connectivity16–21, global analyses encompassing entire neuronal networks across both brain hemispheres are lacking.
Here, we harnessed the power of connectomics to generate the first comprehensive connectivity map of the circadian clock. Intriguingly, we identified additional DN in the network, thus showing that the Drosophila circadian clock is in fact comprised of at least 240 neurons instead of 150 neurons. In addition, our analyses revealed that light input from extrinsic photoreceptors to the clock neurons is largely indirect. Furthermore, we discovered extensive ipsilateral synaptic connectivity between the clock neurons and identified a subset of DN as an important hub that link the clock network across the two brain hemispheres via contralateral projections. We also elucidated the output pathways from the clock network that could affect general behavioral activity levels and organismal physiology. In particular, we elucidated clock output pathways to descending neurons that are known to regulate feeding and reproductive behaviors. Additionally, we observed sparse monosynaptic connectivity between clock neurons and higher-order brain centers, suggesting that multi-synaptic connections and peptidergic signaling account for most of the connectivity, as is characteristic of the vertebrate clock output pathways22. Hence, as a complementary approach, we also deciphered putative paracrine signaling pathways within the clock network by extensively mapping the expression of clock neuropeptides and their receptors, and filtering them based on the topographical constraints determined by the connectome. This peptidergic signaling greatly enriches the connectivity within the clock network and appears to be the major output pathway to the neuroendocrine center23,24 which regulates systemic physiology.
Results
Identification of the circadian neuronal network in the FlyWire and hemibrain connectomes
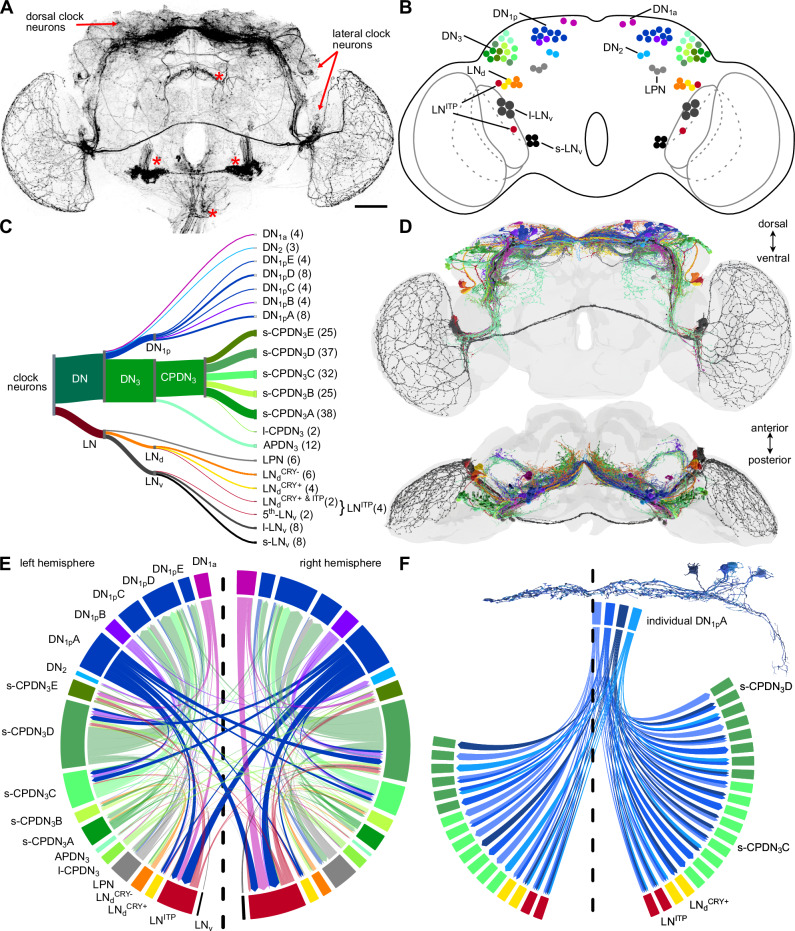
The master clock in the Drosophila brain is widely regarded to be comprised of approximately 150 neurons. This number is derived from neuronal expression of different clock genes14. Clk856-Gal4, based on the Clk promoter, faithfully recapitulates expression in most of these clock neurons (Fig. 1A). These neurons can be broadly classified into four classes each of LN and DN (Fig. 1B and Table 1). The LN comprise Lateral Posterior Neurons (LPN), dorsoLateral Neurons (LNd), Ion Transport Peptide (ITP)-expressing LN (LNITP), and Pigment-Dispersing Factor (PDF)-expressing ventroLateral Neurons (LNvPDF). Conversely, the DN include anterior Dorsal Neurons 1 (DN1a), posterior DN1 (DN1p), DN2 and DN3. These clock neuron classes can be further subdivided into different cell types (Fig. 1C and Table 1). Morphological characterization of some DN1p and DN3 subtypes is currently lacking18. Moreover, the estimated number of clock neurons in Drosophila is likely an underestimation as the precise number of DN3 has not been determined thus far25. This is partly because drivers like Clk856-Gal4 only include a small proportion of DN314. In addition, most DN3 have small somata that are densely packed together, making it difficult to count. The majority of these small DN3 project to the central brain, and hence they are aptly called small Central Projecting DN3 (s-CPDN3) (Table 1)26. Similarly, a pair of DN3 with large somata project to the central brain (large Central Projecting DN3; l-CPDN3), whereas about 6 neurons per brain hemisphere project to the anterior brain (Anterior Projecting DN3; APDN3)26. These latter cells also have larger somata than the s-CPDN318. Despite the large number of DN3, previous studies suggest that they are less important for behavioral rhythmicity compared to some of the LN, which are regarded as the master pacemaker neurons27–29.
Fig. 1. Drosophila melanogaster circadian clock network.
A Clk856-Gal4 drives GFP expression in most of the circadian clock neurons and some non-clock neurons (asterisk). Only a few DN3 are included in this Gal4-line. Scale bar = 50μm. B Drosophila clock neurons can be divided into four classes each of Dorsal neurons (DN) and Lateral neurons (LN) based on their cell body location. These can be further subdivided into different cell types based on their morphology and gene expression. Subtypes of clock neurons are color-coded. This color code is used throughout the manuscript. C Classification and numbers of all clock neurons identified in the FlyWire connectome. Refer to Table 1 for details. D The morphology of identified clock neurons in the connectome largely resembles the morphology of clock neurons marked by Clk856-Gal4. E Broad synaptic interconnectivity between different cell types within the clock network. The direction of the arrow indicates the flow of information. Strong connectivity is observed from DN1a to LNITP, from s-CPDN3C and s-CPDN3D to DN1pC-E, as well as from DN1pA to LNITP, LNdCRY+, s-CPDN3C and s-CPDN3D. Note extensive contralateral connectivity between different cell types, most of which can be accounted for by the DN1pA. The dashed line indicates the brain midline. F Individual DN1pA in the right hemisphere forms both ipsilateral and contralateral connections with s-CPDN3C, s-CPDN3D, LNdCRY+, and LNITP. Source data for panels E and F are provided in the Source Data file. Brain mesh is from Dorkenwald et al., 2024.
Table 1.
Identification and classification of Drosophila clock neurons in the FlyWire brain connectome
| Clock Super Class | Clock Class | Clock Cell Type | Expected | Observed |
|---|---|---|---|---|
| Dorsal neurons (DN) | anterior DN1 (DN1a) | DN1a | 4 | 4 |
| posterior DN1 (DN1p) | DN1pA | ~8-10 | 8 | |
| DN1pB | ~4 | 4 | ||
| DN1pC | ~12-14 | 4 | ||
| DN1pD | 8 | |||
| DN1pE | 4 | |||
| DN2 | DN2 | 4 | 3 | |
| DN3 | small Central Projecting DN3 (s-CPDN3)A | ~80 | 38 | |
| s-CPDN3B | 25 | |||
| s-CPDN3C | 32 | |||
| s-CPDN3D | 37 | |||
| s-CPDN3E | 25 | |||
| large Central Projecting DN3 (l-CPDN3) | 2 | |||
| Anterior Projecting DN3 (APDN3) | 12 | |||
| Lateral neurons (LN) | Lateral Posterior Neurons (LPN) | LPN | 6 | 6 |
| dorsoLateral Neurons (LNd) | Cryptochrome-negative LNd (LNdCRY-) | 6 | 6 | |
| Cryptochrome-positive LNd (LNdCRY+) | 4 | 4 | ||
| ITP-positive Lateral Neurons (LNITP) | Cryptochrome- and ITP-positive LNd (LNdCRY+ & ITP) | 2 | 2 | |
| 5th ventroLateral Neuron (5th-LNv) | 2 | 2 | ||
| PDF-positive ventroLateral Neurons (LNvPDF) | large ventroLateral Neuron (l-LNv) | 8 | 8 | |
| small ventroLateral Neuron (s-LNv) | 8 | 8 | ||
| Total | ~152 | 242 | ||
As a first step in determining the synaptic connectivity within the clock network, we used a systematic approach (Supplementary Fig.1) to identify most, if not all, of the clock neurons in the FlyWire connectome generated in our companion papers9,10. In total, we successfully identified 242 clock neurons based on a combination of morphology, previously determined connectivity, and location of their cell soma (Fig. 1C, D, Supplementary Movies 1, 2, Supplementary Data 1)17–19,26,30. The number of clock neurons that we identified in the connectome was considerably higher than the expected number of clock neurons (~152) in the adult brain (Table 1). This was mainly due to the presence of more s-CPDN3 in the connectome than estimated, which prompted us to accurately quantify the total number of DN3 in adult brains. To comprehensively quantify DN3, we used antibodies against clock proteins Period (PER) and Vrille (VRI) in tim-Gal4 > GFP expressing flies. Since PER and VRI exhibit peak expression at different Zeitgeber times (Fig. 2A), the inclusion of three clock markers (PER, VRI, and timeless (tim)) can provide a reliable estimate of the total number of DN3 (Fig. 2B, C, Supplementary Movie 3). Indeed, our analyses revealed that there are more than 70 DN3 per hemisphere in both males and females (Fig. 2D). Thus, the number of DN3 identified in the connectome (171 neurons) is in line with our DN3 estimate (~166 neurons) based on anatomical analysis. The majority of these are s-CPDN3 (157 neurons) which we now classified into five subtypes (s-CPDN3A-E) based on morphological similarity (Fig. 2E–I). These five DN3 subtypes are comprised of 26 distinct cell types9,10 and could thus be subdivided further in the future. During the revision of this manuscript, another study reported the existence of 12 DN3 clusters/subtypes based on single-cell RNA sequencing31, further supporting our analyses.
Fig. 2. Morphology of newly identified s-CPDN3 and DN1p subtypes.
A Schematic cycling of Period (PER) and Vrille (VRI) protein abundance in clock neurons101. B tim-(UAS)-Gal4 drives GFP expression in all DN3, many of which coexpress PER and about 12-19 coexpress VRI. When VRI staining is strong, PER staining is weak or not detectable. Image based on a female brain fixed at ZT1. Representative images are based on expression analyzed in 5 male and 5 female brains. Scale bar = 10 μm (C) DN3 are closely associated with the lateral horn. D The number of DN3 per hemisphere accounts for s-CPDN3, l-CPDN3, and APDN3 identified in the connectome. On average, females have slightly more DN3 (83 ± 5 standard deviation) compared to males (76 ± 6 standard deviation), which could be attributed to DN3 being more densely packed (and thus difficult to quantify) in males. n = 10 hemispheres from 5 brains. Error bars depict standard deviation. E–I s-CPDN3 cell types identified in the FlyWire dataset. Numbers in brackets represent the number of neurons in the total brain. s-CPDN3 could be subdivided into five subtypes. Each subtype comprises two to eight different cell types that have unique morphological characteristics. J Clk4.1M-Gal4 reliably drives GFP expression in 14-15 DN1p per hemisphere. K Multi-color flip-out (MCFO) analysis reveals previously characterized DN1pA (4-5 neurons/hemisphere) and DN1pB (2 neurons) subtypes. MCFO analysis additionally reveals the morphology of uncharacterized DN1p subtypes. DN1pC and DN1pE comprise two cells each per hemisphere L, N. DN1pD comprises four cells per hemisphere two of which project over the midline while the other two do not, suggesting that the DN1pD is comprised of two subtypes M. Scale bar = 50μm. Source data for panel D are provided in the Source Data file. Brain mesh is from Dorkenwald et al., 2024.
Additionally, we also identified several candidate DN1p which could not be classified into previously characterized DN1p cell types (Table 1). To verify if these neurons identified in the connectome are indeed DN1p, we characterized them anatomically. Specifically, we performed multi-color flip-out (MCFO) analysis of DN1p using Clk4.1M-Gal4 to elucidate the morphology of this densely packed neuronal cluster. In total, about 14 DN1p per hemisphere are labeled with the Clk4.1M-Gal4 (Fig. 2J). MCFO analysis revealed DN1pA (4-5 neurons) and DN1pB (2 neurons) subtypes characterized previously (Table 1 and Fig. 2K). Our analysis additionally revealed the morphology of previously uncharacterized DN1p subtypes (Fig. 2L–N). DN1pC and DN1pE are each comprised of two neurons per hemisphere (Fig. 2L, N). DN1pD is comprised of four neurons per hemisphere (Fig. 2M). Two of these project over the midline while the other two remain ipsilateral, suggesting that the DN1pD is comprised of two morphologically distinct subtypes. Notably, our analysis confirmed that the candidate DN1p identified from the connectome are in fact morphologically similar to DN1pC-E subtypes labeled by Clk4.1M-Gal4. Taken together, our identification and morphological characterization of novel DN3 and DN1p subtypes provide a solid framework to comprehensively examine the connectivity of clock neurons.
The FlyWire connectome combines automatically detected chemical synapses with proofread neurons. These synapses represent an additional anatomical feature that could potentially distinguish neuronal groups. Consequently, we asked whether the classification of clock neurons based on differences in their synaptic connectivity aligns with the traditional anatomical and recent gene expression-based classification. To address this, we clustered clock neurons based on cosine similarity between their total synaptic inputs and outputs. Our clustering analysis shows that neurons of a given clock cell type (Table 1) usually cluster together, suggesting that neurons from the same group are more similar (in terms of synaptic connectivity) to each other than to other clock neurons (Supplementary Fig. 2A, B). For example, all three LNdCRY- from one hemisphere are part of the same clade. Similarly, the two DN2 are part of a clade. Exceptions to this are the clades containing DN1p and DN3. For DN1p, this can be explained by our findings which show that this group comprises five morphologically distinct subtypes. In the case of DN3, while some of them form their own cluster, other DN3 cluster together with different clock neuron subtypes. On one hand, this is not unexpected since it is unlikely for such a large group of neurons to have similar connectivity patterns. On the other hand, this is quite interesting since it provides insights into their possible function. For instance, heterogenous clusters comprising clock neurons of different classes have similar synaptic inputs and outputs, and may thus play similar roles in the clock network and beyond. Moreover, the connectivity-based clustering does not resolve the five subtypes of the s-CPDN3. This suggests that these five s-CPDN3 subtypes comprise synaptically heterogeneous cell populations. Nonetheless, synaptic connectivity-based classification of clock neurons largely aligns with the ones determined based on anatomical and gene expression differences.
Having identified all the clock neurons, we next sought to determine their synaptic interconnectivity which could facilitate intercellular coupling within the network. Generally, we regarded >4 common synapses per neuron as significant connections and >9 synapses as strong connections. We first wanted to validate our analysis by comparing it with previously reported connections. In agreement with previous reports18,19, we observed strong synaptic connectivity from DN1a to LNITP, from DN1pA to LNITP and LNd, and from DN1pB to DN2 clusters (Fig. 1E and Supplementary Fig.3), highlighting the robustness of our approach. Importantly, our analysis also uncovered novel connections between the different subgroups. Specifically, we observed strong contralateral and ipsilateral connectivity from DN1pA to LNITP, as well as additional significant connections with s-CPDN3C, s-CPDN3D, and LNdCRY+ across both hemispheres. Similarly, LNITP also provide synaptic inputs to s-CPDN3 clusters in both hemispheres (Fig. 1E and Supplementary Fig.3). This raised the question of whether DN1pA represents a heterogeneous population where one subgroup forms ipsilateral connections and the other contralateral. To address this, we examined their connectivity at cellular resolution (Supplementary Fig. 4,5) which revealed that individual DN1pA indeed form both ipsilateral and contralateral connections (Fig. 1F). Our analysis thus identified DN1pA as an important center which links the clock network across the two brain hemispheres. These results are in line with previous reports of contralateral projections of DN1p32. In contrast, there are virtually no synaptic connections between the s-LNv and the LNd neurons (Fig. 1E and Supplementary Fig.3-5), which control morning and evening activities, respectively. This is consistent with previous analyses using the hemibrain connectome19.
To assess the extent of inter-individual differences in the numbers, neuronal projections, and synaptic connectivity of clock neurons, we next performed comparisons with the partial hemibrain connectome33. Several groups of clock neurons were previously identified in the hemibrain connectome including all s-LNv, l-LNv, LNd, LNITP, LPN, DN1a, and some DN2, DN1p, and DN317–19. Here, we identified additional DN1p and DN3 (Supplementary Fig.6A–D). In total, 64 clock neurons can be identified in the hemibrain connectome, with the majority of missing neurons belonging to the DN3 subgroups (Supplementary Fig.6A–D, Supplementary Data 2). Comparison of different subgroups revealed stable neuronal numbers across the two connectomes (Table 1 and Supplementary Fig.6D). Similarly, there is a high degree of stereotypy in the connectivity between the clock clusters (Supplementary Fig.6E, F). For instance, l-LNv and DN2 form the least synaptic contacts with other clock clusters. At the opposite end of the spectrum, s-CPDN3A are connected to all the clock clusters except for s-LNv, l-LNv, DN1a, and DN1pE. Given its partial nature, the hemibrain connectome lacks information about all contralateral connections, reiterating the significance of characterizing information flow across entire networks. Taken together, our analyses revealed hitherto unknown connectivity between the clock neurons which could contribute to the robustness of the master clock. Moreover, the identification of the complete circadian neuronal network in the FlyWire connectome underscores the power of the fruit fly in pushing forward the frontier of our understanding of chronobiology.
Validating clock neuron connectivity using trans-synaptic tracing
While the FlyWire and hemibrain connectomes exhibit a high degree of stereotypy, we further used an independent approach to validate our connectivity analyses. We performed light microscopy-based trans-synaptic circuit tracing by expressing trans-Tango34 using specific driver lines for different populations of clock neurons (Supplementary Figs. 7,8). Upon driving trans-Tango with Clk4.1M-Gal4 which labels most DN1p (Supplementary Fig. 7B), we observed post-synaptic signals in DN1p, DN2, DN3, LPN, LNd, 5th-LNv, and s-LNv (Supplementary Fig. 9A). However, l-LNv and DN1a were not post-synaptic to DN1p. Thus, our trans-Tango analysis of DN1p agrees with the connectivity of DN1p based on the connectomes (Supplementary Fig. 3 and Supplementary Fig. 6F). Similarly, a split-Gal4 line targeting DN3 drives post-synaptic signals in DN1a, DN1p, DN2, DN3, LPN, LNd, and l-LNv (Supplementary Fig. 9B), which mirrors the connectivity seen in the connectomes. While post-synaptic signal was not detected in most clock neurons of control flies, occasionally, a false post-synaptic signal was detected in two clock neurons (LNd) from the entire network (Supplementary Fig. 10A). Hence, any potential synaptic output to LNd should be treated with caution. Overall, we observed similar congruency between the two approaches with other Gal4-lines including those targeting DN2 (Supplementary Fig.10B), LPN (Supplementary Fig. 10C), and LNITP (Supplementary Fig.10D).
Differences compared to the connectomes were observed when driving trans-Tango with Pdf-Gal4 (for s-LNv and l-LNv) (Supplementary Fig. 10E) and with the DN1a-specific split-Gal4 line (Supplementary Fig.10F). In both cases, trans-Tango generated post-synaptic signals in more clock neurons than anticipated based on the connectomes. This discrepancy could be explained by: (1) the presence of additional neurons in the Gal4-line (e.g. PDF tritocerebrum neurons35), (2) daily remodeling of neural circuits, as shown previously for s-LNv and DN1a36,37 and/or (3) connections persisting through development. In summary, our trans-Tango analysis is largely in agreement with the clock network generated using the connectomes.
Deciphering light input pathways to clock neurons
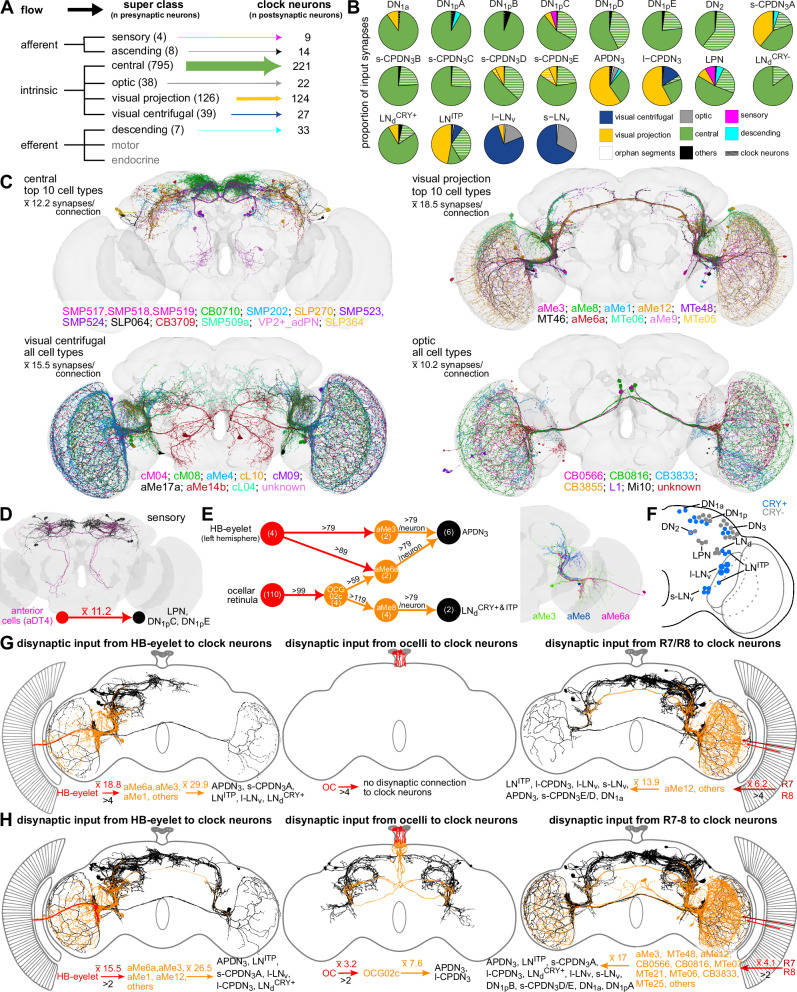
Following the successful validation of our connectivity data, we next identified all the major classes of neurons providing inputs to the clock network. To this end, we utilized the annotation scheme of our companion paper9, which provides a hierarchical classification of all neurons in the connectome (Fig. 3A). We found that neurons intrinsic to the brain provide the majority of the inputs to the clock network (Fig. 3A–C). This includes visual centrifugal neurons projecting from the central brain to the optic lobes, visual projection neurons projecting from the optic lobes to the central brain, as well neurons intrinsic to the optic lobes and central brain (Fig. 3C). Examining inputs to specific clock clusters, we observed differential inputs across all the subgroups (Fig. 3B). As expected, s-LNv and l-LNv receive most of their input from optic lobe and visual centrifugal neurons as they have a large number of input synapses in the optic lobes and the accessory medulla (AME) (Supplementary Fig. 11). In contrast, APDN3, l-CPDN3, and LNITP populations receive major inputs from visual projection neurons. The remaining clock clusters receive most of their inputs from central brain neurons (Fig. 3B, Supplementary Figs.11, and 12). In some cases, such as DN1pC-E, DN2, and s-CPDN3A-E, a significant portion of these central neurons are clock neurons themselves (Supplementary Fig.13), confirming prominent intercellular synaptic connectivity between some clock clusters. Interestingly, only 4 sensory neurons provide direct inputs to the clock network. These are anterior cells (aDT4) (Dr. Gregory Jefferis, personal communication) (Fig. 3A, D) which provide temperature inputs to LPN, DN1pC, and DN1pE38,39. Therefore, most of the strong thermosensory input to clock neurons is indirect via thermosensory projection neurons40.
Fig. 3. Light and other inputs to the clock network.
A Input to the clock neurons grouped by the nine neuronal super classes annotated in the FlyWire connectome. Intrinsic neurons, specifically the central and visual projection neurons, are the largest groups providing inputs to the clock. B Breakdown of inputs to different types of clock neurons. C Neurons from the four different super classes (central, visual projection, visual centrifugal, and optic) which provide major inputs (based on the number of neurons) to the clock. D Anterior cells (AC, magenta) providing temperature inputs to LPN, DN1pC, and DN1pE. (black). E aMe neurons provide the strongest inputs to clock neurons. Upstream partners of aMe neurons with strong connectivity include HB-eyelet and OCG02c. Numbers above the arrows indicate the number of synapses and numbers in circles indicate the number of neurons. Note that the connection from the OC to OCG02c is below the threshold of 5 synapses/connection. Neuronal reconstructions of aMe3, aMe6a, and aMe8 are shown in one hemisphere. F Cryptochrome (CRY)-positive clock neurons are shown in blue. G Disynaptic inputs to the clock from the three types of extrinsic photoreceptors (HB-eyelet, ocelli, photoreceptor cells of the compound eye) using a threshold of >4 synapses. H Disynaptic inputs to the clock from the three types of extrinsic photoreceptors using a threshold of >2 synapses. For C, D, G, and H, numbers represent the average number of synapses. All cell types are listed according to their input strength (from high to low) to clock neurons. Note: HB eyelet was only identified in the left hemisphere. HB Hofbauer-Buchner, OC ocellar retinula cells, OCG ocellar ganglion cell. Source data for panels B–D, G, and H are provided in the Source Data file. Brain mesh is from Dorkenwald et al., 2024.
Having broadly classified the inputs from different neuronal super classes to the clock network, we probed further and identified individual cells providing the strongest synaptic inputs to clock neurons. For this purpose, we used a stringent threshold of 80 synapses to obtain a narrow list of candidate inputs. Our analysis discovered 13 neurons, including 7 aMe neurons (a subset of visual projection and visual centrifugal neurons) that are strongly connected to specific downstream clock neurons (Fig. 3E). For example, individual aMe3 and aMe6a neurons can form more than 79 synapses per neuron with APDN3, while aMe8 are similarly connected to LNdCRY+ & ITP clock neurons (Fig. 3E). The unifying feature of these aMe neurons is their dense arborization in the AME and posterior lateral protocerebrum (Fig. 3E), where they anatomically interact with clock neuron dendrites (Supplementary Figs. 11 and 12)18,41. Interestingly, the aMe neurons themselves receive strong inputs from the extraretinal photoreceptors. Specifically, aMe3 and aMe6a neurons receive strong inputs directly from the Hofbauer-Buchner (HB) eyelets (Fig. 3E). Conversely, aMe8 receive indirect inputs from ocellar retinula cells via the ocellar ganglion neurons (OCG) type 2c (OCG02c) (Fig. 3E). Therefore, the clock receives strong light inputs from extrinsic photoreceptor cells, albeit indirectly. This is not surprising since light is the most important Zeitgeber for circadian clocks42. Flies synchronize their circadian clocks with the light-dark cycles using these extrinsic photoreceptor cells as well as via the blue-light photoreceptor Cryptochrome (CRY), which is expressed in about half of the clock neurons (Fig. 3F)42. While CRY interacts with the core clock protein Timeless and can quickly reset the clock, the different photoreceptor cells are important for sensing dawn, dusk, high light intensities, and day length, and for adapting morning and evening activities to the appropriate time of day. Regardless, we found little direct inputs from the photoreceptor cells and other sensory cells to the clock neurons (Fig. 3A). This is consistent with previous findings which revealed that most of the light input to the clock appears to be indirect43. In light of this and our in-silico circuit tracing analysis described above, we comprehensively characterized indirect connectivity between photoreceptor cells and clock neurons. Therefore, we traced all the disynaptic connections between them. Using the normal threshold of >4 synapses, we again recovered the strong connections from the H-B eyelets via the aMe3/aMe6a to the APDN3, and additional weaker connections to the s-CPDN3A, LNITP, l-LNv, and LNdCRY+ (Fig. 3G). Furthermore, we revealed connections from R7/R8 compound eye photoreceptors to several clock neurons via aMe1244 and other interneurons. While we did not observe any disynaptic connections from the ocellar retinula cells to clock neurons using the normal threshold (Fig. 3G), reducing the threshold to >2 synapses revealed connections from the ocelli to APDN3 and l-CPDN3 via OCG02c (Fig. 3H). The synaptic connections from the ocelli to OCG and beyond are extensively characterized in our companion paper10 and demonstrate interesting details that may also be valid for the other photoreceptor inputs to clock neurons. The majority of ocellar photoreceptors are synaptically connected to ocellar ganglion neurons with thick axons (OCG01a-f) or directly to descending neurons (DNp28). These connections likely enable fast behavioral responses. In contrast, axons of OCG02c that connect to the clock neurons are rather thin and not suited for fast neurotransmission. Instead, these neurons appear suited for collecting light information over time – a property needed for entraining the circadian clock. Further, collecting light information over larger time intervals may not require a high synapse density. Thus, 3 to 4 synapses between retinula cells and the relevant downstream OCG observed here could be sufficient for this purpose (Fig. 3H). The same is also true for the photoreceptor cells of the compound eyes. Reducing the threshold of significant connections from 5 to 3 synapses revealed indirect clock input from additional photoreceptor cells, including those that project from the dorsal rim area of the eye (Fig. 3H). These photoreceptor cells are involved in polarized vision and might contribute to time-compensated sun compass orientation45. Whether the connectivity observed with a lower threshold of >2 synapses is functional in vivo remains to be seen; however, this is very likely since there are usually many photoreceptor cells that synapse onto only a few aMe neurons. For example, theoretically, the ~300 pale R8 cells project to only 3–4 aMe12 neurons44, resulting in ~100 connections on average per aMe neuron. Even if each of these connections were mediated via only 3 synapses, each aMe neuron could potentially receive inputs from R8 cells via 300 synapses, which is quite substantial (Fig. 3H).
Identification of clock network output pathways
Delineating the output pathways that translate daily 24-hour oscillations of the molecular clock into physiological and behavioral rhythms remains a major focus in chronobiology. Using the same strategy as above to identify the inputs, we systematically classified all the neurons downstream of the clock network. Most synaptic output from the clock network is directed to intrinsic brain neurons, and in particular, the central brain neurons (Fig. 4A–C). Except for l-LNv, all clock clusters have a majority of their output onto central brain neurons. l-LNv mostly provide inputs to Medullary intrinsic neurons in the optic lobe (Mi 1, Fig. 4B, C), consistent with their role in adapting the sensitivity of the visual system to the time of day46,47. Further, examination of individual postsynaptic partners of clock neurons (Supplementary Fig. 14) reveals that the majority of the output from DN1pA is onto other clock neurons, namely LNITP, LNdCRY+, and s-CPDN3C-D (Fig. 4B and D). s-CPDN3C-D, in turn, provide strong output to DN1pC-E (Fig. 4D, E), thus linking CRY-negative DN1p clusters to CRY-positive DN1p clusters. After broadly classifying clock outputs, we next focused on specific cell types which receive the strongest synaptic inputs (>49 synapses) from clock neurons using an approach similar to the one used earlier for clock inputs. Our analysis identified the enigmatic Clamp neurons (Fig. 4F), which receive strong synaptic inputs from APDN3. While the functions of most of these clamp neurons are still unknown, some of them output onto descending neurons, while others promote sleep26. Moreover, DN1pB provide strong inputs to Tubercle-innervating neurons (Fig. 4G), which are part of the anterior visual pathway48. Lastly, several clock neurons are strongly connected to diverse neurons from different neuropil regions (Fig. 4H).
Fig. 4. Direct and indirect clock output pathways.
A Clock output grouped by the nine neuronal super classes annotated in the FlyWire connectome. Intrinsic neurons, specifically the central neurons, are the largest group of neurons downstream of clock neurons. B Breakdown of outputs from different types of clock neurons. All clock neurons, except for l-LNv, provide a majority of their output to central neurons. l-LNv mostly provides inputs to optic neurons. C Neurons from the four different super classes (central, optic, visual projection, and visual centrifugal) which receive inputs from the clock. D Individual postsynaptic partners of DN1pA and s-CPDN3C-D are sorted based on the number of synapses. Postsynaptic clock neurons are colored based on their identity. E CRY-positive DN1pA provide major inputs to CRY-negative DN1pC-E via s-CPDN3C-D. Contralateral connections from DN1pA to s-CPDN3 are stronger than ipsilateral connections. F APDN3 forms the most output synapses in the clock network and is highly connected to several types of Clamp neurons (CL). G DN1pB-E provides strong outputs to Tubercle-innervating neurons. H Several clock neurons provide strong inputs to neurons from diverse neuropils. Mono- and di-synaptic clock inputs to neurons associated with the I central complex, J mushroom bodies, and K neurosecretory cells. Note that there are only a few direct connections from the clock to downstream higher brain centers. Numbers above the arrows indicate the number of synapses and numbers in circles indicate the number of neurons. Note: all numbers refer to neurons across both hemispheres. For C–I, cell types are listed according to the strength of the clock input (from high to low). EB ellipsoid body, FB fan-shaped body, NO noduli, EPG ellipsoid body-protocerebral bridge-gall neuron, PFGs protocerebral bridge glomerulus-fan-shaped body-ventral gall surround, KC Kenyon cell, DAN dopaminergic neuron, MBON mushroom body output neuron, SEZ-NSC suboesophageal neurosecretory cells, l-NSC lateral neurosecretory cells, m-NSC medial neurosecretory cells. Source data for panels B–K are provided in the Source Data file. Brain mesh is from Dorkenwald et al., 2024.
The circadian clock is also known to modulate behaviors such as activity/sleep, spatial orientation, and learning and memory49,50. These behaviors are regulated by higher brain centers such as the central complex and mushroom bodies. The clock also influences systemic physiology via modulation of endocrine/neurosecretory cells (NSC)16,21. Consistent with previous results17,18, we found few direct connections from the clock to the central complex (Fig. 4I), mushroom bodies (Fig. 4J), and NSC (Fig. 4K). Consequently, we predicted that the clock output to these brain regions is either indirect or paracrine via neuropeptides. In line with this prediction, we found prominent disynaptic connectivity from clock neurons to central complex neurons (mainly fan-shaped body (FB) and ellipsoid body neurons (EB)), Kenyon cells (KC), dopaminergic neurons (DANs), mushroom body output neurons (MBONs) and NSC (Fig. 4I–K). Taken together, circadian modulation of neurons regulating diverse behaviors and physiology is largely indirect.
Circadian modulation of behaviors via descending neurons
Next, we focused on clock output pathways to descending neurons which could influence diverse behaviors such as locomotion, feeding, and egg-laying. Interestingly, clock neurons provide direct inputs to 18 descending neurons (Fig. 5A). These descending neurons include those that have not yet been classified (Fig. 5B), as well as Allatostatin-C (AstC) and SIFamine (SIFa) peptidergic neurons (Fig. 5C), the latter of which modulate feeding, mating, and sleep51. When considering disynaptic connections, the connectivity between the clock network and descending neurons increased drastically, with approximately 24% of all descending neurons receiving indirect inputs from most of the clock neurons (Fig. 5A).
Fig. 5. Clock output to descending neurons.
A Mono- and di-synaptic clock output to descending neurons. B Novel and C peptidergic descending neurons receiving direct clock input. All cell types are listed according to the strength of the clock input (from high to low). D–F Neurotransmitters expressed in clock neurons and their pre- and post-synaptic partners. D Neurotransmitter prediction from Eckstein et al. 202452 based on electron microscopic data for presynaptic neurons of the clock network (left), the clock neurons (middle), and their postsynaptic neurons (right). E Most of the lateral clock neurons are cholinergic and most of the dorsal clock neurons are glutamatergic, while no clock neurons are GABAergic. Electron microscopy-based neurotransmitter predictions (Em) of clock neurons agree with F anatomical (An) expression mapping with T2A-Gal4 lines for neurotransmitter markers. G Weighted direct connections of clock neurons to descending neurons colored according to their neurotransmitter identity. s-CPDN3 and LPN are strongly connected to three types of descending neurons. H Proportion of input synapses of the three descending neuron types that receive the strongest input from clock neurons. Each cell type gets about 25% input from clock neurons. I Schematic showing pathways via which CCAP-positive DNpe048 integrate time cues from the clock network with hunger and thirst signals to regulate feeding behavior. J Reconstructions of neurons that transmit time cues (black) and hunger and thirst-related signals (grey) to DNpe048 (magenta). K Pathways from clock neurons to descending neurons which could modulate the timing of reproductive behaviors. ACh acetylcholine, Glut glutamate, GABA γ-Aminobutyric acid, ChAT choline acetyltransferase, VGlut glutamate vesicle transporter, CCAP crustacean cardioactive peptide; Scale bar represents 5 µm. Source data for panels A, D, E, G, H, and K are provided in the Source Data file. Brain mesh is from Dorkenwald et al., 2024.
In order to obtain a better understanding of how the clock modulates descending neurons, and other downstream neurons in general, we first sought to characterize the neurotransmitters expressed in clock neurons. To address this, we used a combination of neurotransmitter predictions based on electron microscopy52 and anatomical expression mapping of neurotransmitter markers (Fig. 5D–F), which are largely in agreement53,54. For some clock neurons such as DN1pA and LNITP, anatomical mapping (Fig. 5F) allowed us to fill the gap left by uncertain neurotransmitter predictions based on electron microscopy (Fig. 5D). Remarkably, most of the lateral clock neurons express the excitatory neurotransmitter acetylcholine, while most of the dorsal clock neurons express glutamate which can either be excitatory or inhibitory in Drosophila52. The only exception to these are the cholinergic l-CPDN3. None of the clock neurons are predicted to be GABAergic. We also examined the neurotransmitters predicted to be expressed in presynaptic and postsynaptic partners of clock neurons (Fig. 5D). As seen for clock neurons, acetylcholine and glutamate appear to be the major neurotransmitters expressed in their synaptic partners. In addition to neurotransmitters identified here, previous work has revealed that s-LNv and l-LNv utilize the inhibitory transmitter glycine55. It is noteworthy that most of the postsynaptic partners of s-LNv and l-LNv are glutamatergic and cholinergic, respectively. Taken together, this comprehensive classification of neurotransmitters expressed in clock neurons allows valence assignment to the clock output pathways such as those to descending neurons.
We next utilized this information to characterize output pathways from the clock to downstream descending neurons. Direct synaptic output to descending neurons predominantly derives from s-CPDN3A-D (glutamatergic), and LPN (cholinergic) (Fig. 5G). Three descending neuron types, namely DNpe048, DNpe033, and DNpe041, receive the majority of clock inputs. These descending neurons receive a substantial portion (about 25%) of their total input from clock neurons (Fig. 5H). We focussed further on DNpe048 which receives strong cholinergic and glutamatergic inputs from clock neurons. DNpe048 express crustacean cardioactive peptide (CCAP) and have recently been shown to regulate sugar and water ingestion by integrating inputs from other modulatory interneurons56 (Fig. 5I). Allatostatin-A-expressing Janu-AstA neurons which regulate hunger and thirst57 also provide inputs to DNpe048. It is thus tempting to speculate that DNpe048 integrate time and temperature cues via s-CPDN3A-D and LPN along with hunger and thirst signals from diverse modulatory interneurons to regulate daily feeding rhythms (Fig. 5I, J).
Having elucidated major direct clock output to descending neurons, we decided to focus on the 312 descending neurons that receive clock inputs indirectly. These include oviposition descending neurons (oviDNa), vaginal plate opening descending neurons (vpoDN), and DNg30 that have previously been implicated in the regulation of reproductive behaviors. oviDNa regulate egg-laying58, vpoDN control female sexual receptivity during courtship59, and DNg30 express the neuropeptide natalisin which regualtes mating60 (Fig. 5K). A recent study elucidated the pathway from LNdCRY+ to oviDNa which controls rhythmic egg-laying61. Here, we show that glutamatergic DN1a and cholinergic l-CPDN3 provide strong inputs to AVLP594 which in turn provide strong inputs to mating-regulating DNg30. Moreover, several types of clock neurons including s-CPDN3C and APDN3 provide strong inputs to vpoDN via pC1 neurons which integrate inputs regarding the female mating status58,59. Lastly, SIFa neurons, which receive both direct and indirect clock signals, also independently affect sexual receptivity. It remains to be seen if mating and sexual rhythms exist in Drosophila, and the contribution of these pathways towards them. In summary, our connectivity analysis indicates that the clock can have a major influence on diverse behaviors via outputs to descending neurons.
Identifying the molecular basis of paracrine clock output pathways
Given the large repertoire of neuropeptides previously shown to be expressed in clock neurons12,14,15, we predicted that peptidergic paracrine signaling is crucial in mediating intercellular coupling within the clock network as well as output to downstream neurons such as NSC. First, we determined if any additional neuropeptides are expressed in clock neurons. To address this, we used a publicly available single-cell transcriptome dataset of clock neurons14 combined with immunohistochemical localization and T2A-Gal4 lines. Unsupervised clustering of all clock neuron transcriptomes using t-SNE analysis yields 32 independent clusters (data not shown), 16 of which have high expression of clock genes (tim and Clk) and can be reliably identified based on known markers (Fig. 6A, B). Our analysis revealed that most clock clusters express at least one neuropeptide (Fig. 6B). Consistent with previous studies, l-LNv express high levels of Pdf, whereas s-LNv express both Pdf and short neuropeptide F (sNPF) (Fig. 6B). Similar coexpression of neuropeptides is also observed in other clusters including the DN1pCNMa & AstC cluster which coexpresses CNMamide (CNMa), AstC, and Diuretic hormone 31 (Dh31) neuropeptides. In total, at least 12 neuropeptides are highly expressed in the clock network (Fig. 6B, C). Importantly, this includes novel clock-related neuropeptides, namely DH44 and Proctolin (Proc). Dh44 is expressed in several clock clusters including DN1a, DN1pAstA, DN1psNPF, DN3VGlut, LPN and LNdNPF (Fig. 6B, C). We independently confirmed the presence of DH44 peptide in these clusters using a combination of DH44 antibody or DH44-T2A-Gal4 (Fig. 6D, Supplementary Fig. 15, and Supplementary Data 3). Proc, on the other hand, is strongly expressed in DN1pCNMa and weakly in DN2 clusters which was verified by driving GFP using a Proc-T2A-LexA driver (Fig. 6E and Supplementary Fig.15). Proc expression in other clock clusters such as LNdNPF and DN3VGlut, remains to be validated. Lastly, we also detected AstC expression in additional clock neurons. AstC immunoreactivity was previously localized in DN1p, DN3, and LPN62, which is in agreement with AstC transcript expression in DN1pRh7 and DN1pCNMa & AstC clusters (Fig. 6B, C). Here, we show that AstC is additionally expressed in DN2, which were labeled using an antibody against VRI (Fig. 6B, F, Supplementary Fig.15, and Supplementary Data 3). Our expression analyses revealed the comprehensive neuropeptide complement of clock neurons (Fig. 6B–F, Supplementary Fig.15, and Supplementary Data 3) and provides the basis to explore paracrine targets of clock neurons.
Fig. 6. Circadian clock neuropeptidome.
A Single-cell RNA sequencing of clock neurons reveals 16 distinct clock neuron clusters (shown in a t-SNE plot) that can be reliably identified based on known markers. B, C Clock neurons express at least 12 different neuropeptides. Based on average scaled expression (red = high and grey = low). D DH44 is a new clock-related neuropeptide which is expressed in APDN3, DN1a, LNd, and LPN (arrowheads). E Proctolin is a new clock-related neuropeptide which is expressed in one DN1p (open arrowhead) and two DN2 (filled arrowheads). F AstC is expressed in DN2 in addition to other clock neurons. Double arrowheads indicate DN3 labeled by anti-AstC, filled arrowheads indicate DN2 and open arrowheads indicate DN1p. Scale bars = 50 μm for overview and 10 μm for higher magnification images. PER Period, TIM Timeless, VRI Vrille, PDF Pigment dispersing factor, DH44 Diuretic hormone 44, AstC Allatostatin-C. G Single-cell transcriptomes of NSC express receptors for clock-related neuropeptides. Based on average scaled expression (red = high and grey = low). Source data for panels A, C, and G are provided in the Source Data file.
As a first step in this direction and to validate our approach, we focused on select NSC which have been extensively characterized previously23. We predicted that NSC are targeted by clock-related neuropeptides since they receive sparse monosynaptic inputs from clock neurons despite being closely associated with them anatomically. To investigate potential paracrine signaling between clock neurons and NSC, we again turned our attention to single-cell transcriptomics. We identified single-cell RNA transcriptomes of medial NSC expressing DH44 (m-NSCDH44) and insulin-like peptides (m-NSCDILP), as well as lateral NSC expressing corazonin (l-NSCCRZ), DH31 (l-NSCDH31) and ITP (l-NSCITP)63 based on previously identified markers, and quantified the expression of clock peptide receptors (Fig. 6G). Consistent with our prediction, our analysis indicated that multiple receptors for clock peptides are indeed expressed in different populations of NSC. Modulatory inputs to m-NSCDILP have been examined extensively and our analysis is in agreement with previous expression and functional studies16,64–67. Taken together, our analysis uncovers the molecular substrates of paracrine signaling between clock neurons and NSC.
We next explored the magnitude of peptidergic paracrine signaling between clock neurons themselves. To predict putative paracrine connections, we extensively mapped the expression of clock neuropeptide receptors within the clock network. Consistent with promoter and genomic fragment-based Pdfr-Gal4 expression68, Pdf receptor (Pdfr) transcript is highly enriched in most clock clusters (Fig. 7A, B), which we verified independently by expressing GFP using Pdfr[RA]-T2A-Gal4 (Fig. 7C, D). Other receptors are more sparsely expressed within the clock network (Fig. 7A, B). However, most clock clusters express at least two receptors, with DN1psNPF expressing at least 7 receptors. We validated our single-cell transcriptome analysis by mapping the expression of select receptors using T2A-Gal4 knock-in lines. Our anatomical mapping of receptors (Fig. 7D and Supplementary Data 4) is largely in agreement with transcriptome data. In some cases, however, receptor mapping can provide additional insights. For instance, there are four transcript variants (A, B, C, and D) encoding the Drosophila Neuropeptide F receptor (NPFR). Using Gal4 lines specific for NPFR-A/C and NPFR-B/D isoforms, we showed that these isoforms are differentially expressed across the clock clusters, with A/C isoforms expressed more broadly than B/D isoforms (Fig. 7D and Supplementary Data 4).
Fig. 7. Paracrine signaling within the clock network.
A Expression of clock-related neuropeptide receptors in different clock clusters was identified using single-cell transcriptome analysis. B t-SNE plots showing the expression of receptors in different clock clusters. Clustering is based on Fig. 6A. C Pdfr[RA]-T2A-Gal4 drives GFP expression in all clock cell types. Arrowheads indicate different types of clock neurons that express PDFR[RA]. Scale bars = 20 μm. PDF, Pigment dispersing factor, TIM Timeless. D Schematics depicting expression of receptors in different clock neurons. The schematics are based on GFP expression using T2A-Gal4 lines for different receptors (see Supplementary Data 4 for confocal images). The numbers refer to neurons expressing that receptor in one hemisphere. E Chord diagrams showing synaptic (filled boxes) and putative peptidergic paracrine connections (arrows) between clock neurons. This figure is based on synaptic connectivity in Fig. 1E, and peptide and receptor expression (following thresholding) mapping reported in Figs. 6B–F, 7A–D, Supplementary Fig. 15, and Supplementary Data 3, 4. See Supplementary Fig.16 for a detailed explanation of the filtering of putative paracrine connections. Source data for panels A, B, and E are provided in the Source Data file.
Finally, we utilized our expression data of neuropeptides and their cognate receptors in clock neurons to delineate putative paracrine signaling pathways within the network. For this, we utilized an approach (Supplementary Fig. 16) similar to the one used recently to predict the Caenorhabditis elegans neuropeptide connectome69. Briefly, we used expression data based on independent methods to conservatively localize the expression of neuropeptides and their receptors across all the clock clusters. We also utilized the connectome to factor in the distance between neurons of different clusters. This was done to ensure that the cells releasing the peptide and those expressing its receptor are not further apart than a cut-off of 14μm, which was set based on a previous paracrine connectivity study16. Lastly, we only considered strong peptide-receptor interactions by disregarding ligands with EC50 values for receptor activation higher than 500 nM. This stringent in silico approach allowed us to predict paracrine connectivity within the clock network with high confidence. Taking s-LNv as an example, this cluster expresses both PDF and sNPF. Following our expression thresholding, PDFR is expressed in most clock clusters, whereas sNPF receptor is expressed in DN1p, DN3, LPN, and l-LNv (Fig. 7 and Supplementary Fig.16). Thus, in addition to providing synaptic inputs to DN3, s-LNv can potentially provide paracrine inputs to most clock clusters across both hemispheres (Fig. 7E). Connectivity from l-LNv is also enhanced by paracrine signaling, although not to the same extent as it is for s-LNv. Expanding this analysis to other clock clusters allowed us to comprehensively identify putative peptidergic signaling pathways between clock clusters (Fig. 7E). These pathways, however, can only be considered putative for three main reasons: (1) all clock neuropeptides are also expressed in other non-clock neurons70, suggesting likely inputs from neurons extrinsic to the clock network, (2) we don’t account for peptide efficacies and receptor affinities since these values were independently determined in distinct systems thus making comparisons difficult and (3) despite our best efforts to account for it, distance of peptide diffusion may vary. To put our peptide diffusion threshold of 14μm into perspective, we performed an additional stringent analysis where we lowered this threshold to 1μm. In this scenario, long-range peptide diffusion is not necessary and two neurons could communicate via paracrine signaling only if they are touching or almost within touching distance and express the peptide-receptor pair. Even using these highly stringent criteria, we see significant putative paracrine connections (~92% of those seen with the 14μm threshold) between different clock classes (Supplementary Fig. 16C). We speculate that the actual magnitude of paracrine signaling is somewhere between no peptide diffusion and brain-wide peptide diffusion, and our threshold of 14μm appears to be a good starting point to elucidate paracrine signaling pathways. In summary, peptidergic signaling greatly enriches the connectivity between different subsets of clock neurons. Additional investigations are necessary to determine which of these putative connections are functional in vivo.
Discussion
Peptidergic signaling supplements synaptic connectivity within the circadian clock network
Using a multipronged approach centered around the FlyWire connectome9,10, we describe the whole-brain neural connectome of an animal circadian clock. Our analysis using the complete brain connectome eluded identification of one DN2, and a couple of s-CPDN3. Nevertheless, given the fact that we identified almost all of the expected clock neurons as well as several additional DN3 (Table 1), our clock neuron synaptic wiring diagram is complete enough to be regarded as a connectome. Our clock connectome is also a significant upgrade (10-fold larger numerically) compared to the partial connectivity diagram based on the hemibrain connectome reported earlier19. The previous analysis was based on only 24 clock neurons and largely focused on LN clusters, while excluding l-LNv, and several DN1p, DN2, and DN3 clusters due to the incomplete nature of the dataset. However, as evident from our analysis here, the DN in fact represents an important hub in the clock network and displays high synaptic connectivity. In particular, DN1p plays a large role in clock cluster interconnectivity and DN3 appears important for clock output pathways. Our analysis also sheds light on the precise number of DN3 in the clock network. Although approximately 80 DN3 were previously estimated in the entire clock network, there are in total about 170 DN3 based on our connectome and anatomical analyses. We anticipate that resources such as NeuronBridge71, which allow for comparisons between electron and light microscopy datasets, will facilitate the identification of Gal4 drivers that target the novel s-CPDN3 subtypes identified here. In addition, we also characterized the molecular basis for neuropeptide connectivity between the clock neurons, consequently highlighting putative peptidergic pathways within the clock network. Similar to vertebrates22,72, the Drosophila clock network is highly peptidergic, with all clock neuron clusters expressing at least one neuropeptide. Notably, a majority of the clock clusters express two neuropeptides, and several express three, while the LPN expresses four neuropeptides. Similar neuropeptide coexpression is also evident in SCN neurons and thus appears to be a common feature of clock neurons22. Surprisingly, there is little to no overlap in the neuropeptide complement of the Drosophila and vertebrate clock neurons (Fig. 8). Orthologs of vertebrate clock neuropeptides including vasoactive intestinal peptide, arginine vasopressin, neuromedin S, cholecystokinin, gastrin-releasing peptide, and prokineticin 2 are either absent in the Drosophila genome or expressed outside the clock network. Hence, Drosophila and vertebrates have evolved to utilize different signaling molecules while still conserving the diversity of neuropeptide signaling within the clock networks. Remarkably, except for PDF, there appears to be little conservation in neuropeptide identities of clock neurons across different insects49,73. This suggests that it is more important to conserve the mode of communication (paracrine signaling) rather than the messenger (specific neuropeptide).
Fig. 8. Parallels in Drosophila and vertebrate clock input and output pathways.

Direct and indirect light input pathways to the Drosophila clock (comprised of LN and DN) and vertebrate suprachiasmatic nucleus (SCN). Note that the pineal gland receives light input only in non-mammalian vertebrates. Drosophila and vertebrate clocks utilize different neuropeptides (grey box). Output from the clock to downstream targets is either synaptic (direct in red and indirect in orange) or paracrine (grey arrow). CRY Cryptochrome, MPS Melanopsin, RHT Retinohypothalamic tract, ipRGC Intrinsically-photosensitive retinal ganglion cells, aMe Accessory medulla neurons, NSC neurosecretory cell, AVP arginine vasopressin, CCK cholecystokinin, ENK met-enkephalin, GRP gastrin-releasing peptide, NMS neuromedin S, PROK2 prokineticin 2, SST somatostatin, VIP vasoactive intestinal peptide.
Contralateral connectivity within the network prevents decoupling of clock neurons across the hemispheres
Analysis of interconnectivity within the clock network revealed extensive contralateral synaptic connectivity between the clock neurons, which is largely mediated by DN1pA and to a lesser extent by s-CPDN3C, s-CPDN3D, l-CPDN3, and LNITP. Furthermore, paracrine peptidergic signaling amongst clock neurons has the potential to further strengthen this contralateral connectivity. Such a strong bilateral coupling of clock neurons could prevent the internal desynchronization of clock neuron oscillations between the two hemispheres – a phenomenon that can happen in other insects and even in mammals, but so far has not been observed in fruit flies7. In addition, consistent with previous analysis using the hemibrain connectome19, we did not observe any synaptic connectivity between the s-LNv and the LNd neurons, which control morning and evening activities, respectively. In line with this observation, the phase relationship between morning and evening oscillators is plastic, consequently facilitating seasonal adaptations as in mammals28. Our data may also explain how the morning and evening oscillators in flies internally desynchronize under certain conditions74. One such relevant condition is increased PDF signaling during long days21, which was shown to delay the evening oscillators13,75,76 and may lead to internal desynchronization77. Here, we confirm the presence of PDFR in the evening neurons. Thus, enhanced PDF signaling could delay the evening neurons and bring them out of phase with the morning neurons, especially because the two sets of neurons are not connected via synapses. Taken together, the lack of interconnectivity between s-LNv and LNd could potentially be a factor promoting adaptation to different seasons and contexts.
Light and other inputs to Drosophila and vertebrate circadian clocks
Our analyses reveal that extrinsic light input from the photoreceptor cells of the compound eyes, HB eyelets, and ocelli to the clock neurons is largely indirect, with the former two transmitting light inputs via aMe neurons. This situation may appear to be different from mammals where intrinsically photosensitive retinal ganglion cells in the retina project directly through the retinohypothalamic tract onto the SCN neurons (Fig. 8)78. However, even the mammalian clock receives indirect photoreceptor inputs from rods and cones via bipolar cells and retinal ganglion cells. Furthermore, indirect light inputs may reach the SCN also via the intergeniculate leaflets of the thalamus79. Altogether, this suggests that the fly and mammalian systems are not fundamentally different. One apparent difference is the presence of CRY, a cell-autonomous circadian photoreceptor, in subsets of Drosophila clock neurons that is sufficient for light entrainment in eyeless mutants80. Mammals lack light-sensitive CRY. Instead, they possess light-sensitive melanopsin in retinal ganglion cells, and mice lacking rods and cones can still entrain to light/dark cycles due to melanopsin81. Thus, flies and mice possess several redundant and partly parallel light-input pathways to entrain their clocks. The similarity is even higher when comparing flies with vertebrates in general. For instance, fish, birds, and reptiles possess additional photoreceptors in the pineal gland, which is reminiscent of the extraretinal HB eyelets or even the ocelli of flies (Fig. 8). Most importantly, vertebrates and flies use their eyes for both vision and entraining their circadian clocks, tasks that require completely different properties of light inputs. Vision requires image formation and fast neurotransmission, whereas circadian entrainment is dependent on integrating light collection over a longer time that can be at a slower rate. The connectome reveals that the number of synapses as well as the axon thickness of the neurons mediating this connectivity are very different between the two types of photoreception. Hence, they are aptly suited to perform their required functions.
Limitations of our approach
The synaptic and putative paracrine connections reported here can only be considered predictions until they are functionally verified. In the case of paracrine connectivity, functional connectivity experiments (in normal and peptide mutant flies) using electrophysiological methods or genetically encoded secondary messenger sensors (calcium and cAMP) are needed to confidently establish functional paracrine connectivity. Additionally, the synaptic connectivity reported here is likely an underestimation due to several factors: (1) we did not explore connectivity via gap junctions, (2) proofreading of photoreceptor axons was not complete in the version utilized here10, and (3) we generally used a connectivity threshold of >4 synapses. Preliminary expression analysis using the single-cell transcriptomes of clock neurons suggests that gap junction genes are enriched in the clock network (data not shown) and they can influence activity-rest rhythms82. It remains to be seen which clock neurons are additionally coupled via gap junctions and how this electrical connectivity complements synaptic and peptidergic connectivity detailed here. Moreover, as discussed earlier, fewer than 5 synapses could also represent functional connections which were largely disregarded in our analyses. Further, during the revision of this manuscript, additional synapses were predicted in the FlyWire connectome by Princeton University. Some of these additional synapses increase the connections between clock neurons, specifically those mediated by LNITP (not shown). Crucially, these additional synapses and connections do not change the conclusions reported here. While the FlyWire and hemibrain connectomes exhibit a high degree of stereotypy, they are both based on an adult female brain. The lack of a male brain connectome currently prevents any comparisons on sex-specific differences within the circadian network and its output pathways which could influence sexually dimorphic behaviors and physiology. Further, the connectome provides a singular snapshot of connectivity which could change depending on the time of day, the age of the animal as well as its internal state.
Conclusion
In conclusion, our circadian clock connectome, the first of its magnitude, is a significant milestone in chronobiology. Given the high conservation of circadian network motifs between Drosophila and vertebrates, this connectome provides the framework to systematically investigate circadian dysregulation which is linked to various health issues in humans including sleep, metabolic, and mood disorders. Moreover, it will also facilitate the development and experimental validation of novel hypotheses on clock function.
Methods
Fly strains
Drosophila melanogaster strains used in this study are listed in Supplementary Table 1. T2A-Gal4 and T2A-LexA knock-in lines generated previously 83,84 were obtained from the Bloomington Drosophila Stock Center (BDSC) and Dr. Shu Kondo. Flies were maintained under LD12:12. Flies for peptide and receptor mapping were reared at 25 °C on Drosophila medium containing 0.7% agar, 8.0% glucose, 3.3% yeast, 4.0% cornmeal, 2.5% wheat embryo, and 0.25% propionic acid. Flies for trans-Tango and Gal4 verification were raised at 18 °C and 25 °C, respectively, on a standard medium containing 8.0% malt extract, 8.0% corn flour, 2.2% sugar beet molasses, 1.8% yeast, 1.0% soy flour, 0.8% agar and 0.3% hydroxybenzoic acid. 2-week-old flies were used for trans-Tango analysis.
Immunohistochemistry and confocal imaging
Neuropeptide and receptor mapping
Immunostainings were performed as described previously85. Briefly, male flies were entrained in LD12:12 at 25 °C for at least 3 days. Whole flies sampled at Zeitgeber time (ZT) 20 were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) with 0.1% Triton X-100 (PBS-T) for 2.5 h at room temperature (RT). Fixed flies were washed three times with PBS, before dissecting their brains. Samples were washed with PBS-T three times. The samples were blocked in PBS-T containing 5% normal donkey serum for 1 hour at RT and subsequently incubated in primary antibodies at 4 °C for 48 h. Following six washes with PBS-T, the brains were incubated in secondary antibodies at RT for 3 h. Lastly, the samples were washed six times in PBS-T and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). At least five brains for each strain were used for immunostaining to characterize the clock cells stained by anti-GFP antibodies (Gal4 or LexA expression) in the first experiment and the clock cells were briefly characterized with PDP1 and PDF antibodies. In the second experiment, we conducted the same immunostaining with anti-TIM antibodies, but only for positive strains, to confirm the prior results. Images were taken from at least three different brains using laser scanning confocal microscopes (Olympus FV1200 and FV3000, Olympus, Tokyo, Japan), and analyzed using Fiji.
Mapping DH44 and AstC expression in clock neurons, verification of clock neuron Gal4 lines, and trans-Tango analysis
Flies for PER staining were fixed at ZT23 (1 hour before lights-on) since PER levels are maximal at this ZT86. Flies for VRI staining were fixed at ZT 20. Immunohistochemistry was performed as described previously17,18. Samples were scanned with a Leica TCS SP8 confocal microscope equipped with a photon multiplier tube and hybrid detector. A white light laser (Leica Microsystems, Wetzlar, Germany) was used for excitation. We used a 20-fold glycerol immersion objective (0.73 NA, HC PL APO, Leica Microsystems, Wetzlar Germany) for wholemount scans and obtained confocal stacks with 2048 × 1024 pixels with a maximal voxel size of 0.3 × 0.3 × 2 µm and an optical section thickness of 3.12 µm. For noise reduction, we used a frame average of 3. Images were acquired using Leica Application Suite X (v3.5.7.23225) and analyzed using Fiji. All the primary and secondary antibodies are listed in Supplementary Table 2.
Multi-color flip-out
Multi-color flip-out (MCFO) analysis was performed to identify the morphology of the 8 so far uncharacterized DN1p87. Clk4.1M-Gal4 flies were crossed to MCFO7 flies. The F1 generation was kept at 25 °C and 1-7 days old male and female flies were used for staining. Immunohistochemistry was performed as described above. All the primary and secondary antibodies are listed in Supplementary Table 2.
DN3 quantification
To quantify the number of DN3 in the adult brain, tim-(UAS)-Gal4 > JFRC81-GFP male and female flies were entrained at 25 °C for 3 days and then fixed at ZT1. Brains were stained for GFP, PER, and VRI as described above. Images were acquired using a Leica TCS SP8 confocal microscope as described above. A 63-fold glycerol immersion objective (1.3 NA, HC PL APO, Leica Microsystems, Wetzlar Germany) was used to acquire detailed images of the DN3 cluster. Images were recorded at 1024 × 1024 pixels with a maximum voxel size of 0.18 × 0.18 × 0.33 µm and an optical section thickness of 0.99 µm. Cells were counted using the Multi-Point tool in Fiji. 5 brains each for males and females were used for quantification.
Connectome datasets and neuron identification
For all analyses, we used the v783 snapshot of the FlyWire whole-brain connectome and its annotations (annotations last updated: 26.12.2023)9,10. We also used the adult hemibrain connectome (v1.2.1) for comparisons (Scheffer et al. 2020). The pipeline for identifying clock neurons is summarized in Supplementary Fig.1. Briefly, neurons were first identified based on morphological and/or connectivity features described previously17,18,26,30,32, or based on NBLAST similarity to identified neurons in the hemibrain9. Morphology clustering (v630) for 124,988 FlyWire neurons9 was then used to identify neurons that were morphologically similar to the clock neurons identified above. Several clock neuron types (e.g. APDN3, s-LNv, LNd, etc.) formed their own morphology cluster, suggesting that additional neurons similar to them do not exist in the dataset. If a morphology cluster comprised more neurons besides the clock neurons identified earlier, all the additional neurons were considered putative clock neurons (Supplementary Fig.1), which were subsequently filtered based on different features. First, the cell body position was determined by mapping the coordinates of the nuclei88 to the root IDs of the neurons. The average distance between the cell body position of neurons in one clock cluster was determined per hemisphere and candidate neurons laying less than twice the average distance away were retained for manual comparison of morphological features and connectivity described in previous studies. In addition, hemilineage and cell type information was used to determine whether the candidate neurons could be additional clock neurons. If new clock neurons were identified, the whole procedure was repeated. By definition, a cell type is a uniquely identifiable neuron in the dataset9. Based on our analysis, if a certain cell type was part of a clock cluster, all other neurons of that cell type were also part of this clock cluster. Thus, the likelihood of other cells that have a similar morphology to the clock neurons identified here is extremely low. FlyWire and Hemibrain cell IDs of identified clock neurons are provided in Supplementary Data 1 and 2, respectively.
Paracrine connectivity prediction
For paracrine connectivity analysis, peptide and receptor expression was first determined based on single-cell RNA sequencing analyses14. A gene was considered to be expressed in a given cluster if 1) it was expressed in more than 49% of the cells within that cluster and 2) the expression level was above the set threshold. A threshold of 0.208 average scaled expression was used for peptides and 0.067 for receptors to align single-cell RNA sequencing data with previously established expression data. Further, T2A-Gal4 expression patterns for peptides and receptors were analyzed in conjunction with antibody staining. This information, together with published antibody staining and live imaging data for receptors (see Source Data for Supplementary Fig. 16), was used to generate expression matrices based on the different methods (Supplementary Fig. 16A). To reduce the number of false positives, we only considered the expression of peptides when shown by two independent methods. A receptor was deemed present if its expression was demonstrated using two independent methods or based on live imaging data (Supplementary Fig. 16A). Next, we calculated the distance between neurons of different clock clusters based on their skeletons (v783_L2). To calculate a threshold for the distance that a peptide can diffuse, we used the closest distance (14 µm) between the s-LNv and the m-NSCDILP since paracrine PDF signaling between these neurons has been demonstrated previously16. If two clock clusters included neurons that were located closer than this distance, we assumed that peptidergic paracrine signaling was possible between these clusters (Supplementary Fig. 16B). Additionally, some Drosophila receptors are activated by multiple ligands. Hence, based on the previously reported EC50 values for receptor activation (see Source Data for Supplementary Fig. 16), we set a stringent threshold of 500 nM to determine if a peptide can activate a receptor. Lastly, to identify putative paracrine connections, we combined the distance matrix with the peptide-receptor expression matrix (Supplementary Fig. 16C). Additionally, we used a distance threshold of 1 µm for a more stringent analysis.
Neurotransmitter predictions
Neurotransmitter predictions are based on Eckstein et al. (2024)52. As suggested by Eckstein et al. (2024), we only considered neurotransmitters that were predicted with more than 62% certainty. Further, we followed Dale’s principle89 and Lacin’s law90. Hence, we assume that acetylcholine, glutamate, and GABA are not coexpressed, and all neurons belonging to the same hemilineage express the same neurotransmitter. We only considered the fast-acting neurotransmitters (i.e., acetylcholine, glutamate, and GABA) for our analyses as their predictions were the most reliable. If a neurotransmitter was not predicted for a given neuron, we used predictions from neurons of the same cell type and hemilineage for which only one type of neurotransmitter was predicted. If several neurotransmitters were predicted for the same cell type/hemilineage, we did not use this information to classify neurons with uncertain predictions. Lastly, we used T2A-Gal4 lines for ChAT, VGlut, and VGAT to examine the expression of these neurotransmitter markers in clock neurons. We used neurotransmitter identities determined based on these anatomical data in cases where neurotransmitter predictions were not possible based on the electron microscope data.
Data visualization
Data was visualized using ggplot2 (v 3.4.2, Wickham, 2016) and circlize (v 0.4.15) for R (v 4.2.2) in RStudio (2022.12.0)91. Reconstructions were downloaded using the navis library (v 1.0.4, https://github.com/navis-org) and cloud-volume library (v 8.10.0, https://github.com/seung-lab/cloud-volume) for python (v 3.8.5), and visualized using blender (v 3.01, Community, B. O. 2018). For visualizing a large number of neurons, Codex (doi: 10.13140/RG.2.2.35928.67844) and FlyWire neuroglancer were used92.
Connectivity analyses
Connectivity data was analyzed using the natverse libraries (v 0.2.4) for R in RStudio93. Synaptic connectivity similarity for clock neurons was analyzed based on all their input and output synapses. Cosine similarity analyses were conducted with coconat (v 0.1.1.9; https://github.com/natverse/coconat) for R. Filtered synapses were retrieved in Python using the navis and pandas (v 1.1.394,) libraries. Unless stated otherwise, a connection with more than 4 synapses was considered significant.
Single-cell transcriptome analyses
Expression of neuropeptides and their cognate receptors in clock neurons was mined using the single-cell RNA sequencing dataset and analysis pipeline established earlier14. NSC transcriptomes were identified from the brain transcriptomes generated previously63. The parameters used to identify the different cell types were based on previous studies and provided below64,65,70,95–99.
DH31 (6 cells): ITP > 2 & Dh31 > 4 & amon > 0 & Phm > 0.
DH44 (12 cells): Dh44 > 1 & CG13248 > 1 & CG13743 > 0 & Lkr > 0
CRZ (4 cells): Crz > 3 & sNPF > 3 & Dh44 = 0 & ITP = 0
& ChAT = 0 & Gr64a = 0
ITP (7 cells): Tk > 1 & sNPF > 1 & ITP > 1 & ImpL2 > 1 & Crz = 0
IPC (19 cells): Ilp2 > 3 & Ilp3 > 3 & Ilp5 > 3
Expression was scaled based on all genes in the dataset. All analyses were performed in R-Studio (v2022.02.0) using the Seurat package (v4.1.1100).
Statistics and Reproducibility
All micrographs shown in the figures are representative images based on at least five independent samples.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
We would like to thank Selina Hilpert and Barbara Mühlbauer for technical assistance, Maria Steigmeier for preliminary investigations on PDFR expression, and Tatsuya Yokosako for generating the LNITP split-Gal4 line. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We are also grateful to Drs. Fumika Hamada for Clk.9M-Gal4;pdf-Gal80, Shu Kondo for T2A-Gal4 lines, Paul E. Hardin for VRI antibody, Ralf Stanewsky for PER antibody, Jadwiga Giebultowicz for TIM antibody, Justin Blau for PDP1 antibody, Heinrich Dircksen for ITP antibody, Susan Morton for RFP antibody, and Jan Veenstra for AstC and DH44 antibodies. We thank the Princeton FlyWire team and members of the Murthy and Seung labs, as well as members of the Allen Institute for Brain Science, for the development and maintenance of FlyWire (supported by BRAIN Initiative grants MH117815 and NS126935 to Murthy and Seung). We also acknowledge members of the Princeton FlyWire team and the FlyWire consortium, especially Drs. Gregory Jefferis, Sven Dorkenwald, and Philipp Schlegel for troubleshooting and guidance. Special thanks to Austin T Burke and Mareike Selcho from the FlyWire consortium for contributing >10% effort towards editing and proofreading at least 10% of clock neurons. We are also thankful to Drs. Theresa McKim and Dick Nässel for helpful feedback during the preparation of this manuscript, as well as the Division of Instrumental Analysis, Okayama University for the laser scanning confocal microscopes (FV1200 and FV3000). M.Z. was supported by funding from the University of Würzburg, Deutsche Forschungsgemeinschaft (DFG; ZA1296/1-1), and NV INBRE grant from the National Institute of General Medical Sciences (GM103440). D.R. (DFG; RI 2411/1-1) and C.H.F. (DFG; FO 207/16-1) were supported by DFG. T.Y. was supported by JSPS (KAKENHI 19H03265). A.S. and M.S. were supported by the OU fellowship (JST SPRING, Grant Number JPMJSP2126). We also acknowledge funding from the DFG for the Leica TCS SP8 microscope (251610680, INST 93/809-1 FUGG).
Author contributions
N.R., C.H.F., T.Y., and M.Z. conceived the study. N.R., D.R., C.H.F., T.Y., and M.Z. supervised the project. N.R., A.F., G.Manoli, E.D., A.S., G.Möller, M.S., and M.Z. performed the experimental work and analyzed the data. N.R. and M.Z. performed computational analyses. M.Z. wrote the manuscript with input from C.H.F and N.R. All authors read, provided feedback, and approved the final manuscript.
Peer review
Peer review information
Nature Communications thanks Xitong Liang, Amita Sehgal and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Connectivity analyses can be performed using the cell IDs provided at https://codex.flywire.ai/ and with the scripts provided (see below). Source data for plots are provided with this paper. Source data are provided with this paper.
Code availability
Code used for the analyses and data visualization is publicly available on GitHub: https://github.com/Zandawala-lab/Synaptic-connectome-of-the-Drosophila-circadian-clock_Reinhard-and-Fukuda-et-al.-2024.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nils Reinhard, Ayumi Fukuda.
Contributor Information
Charlotte Helfrich-Förster, Email: charlotte.foerster@uni-wuerzburg.de.
Meet Zandawala, Email: mzandawala@unr.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54694-0.
References
- 1.Dunlap, J. C. Molecular bases for circadian clocks. Cell96, 271–290 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Takahashi, J. S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet.18, 164–179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohawk, J. A., Green, C. B. & Takahashi, J. S. Central and peripheral circadian clocks in mammals. Annu Rev. Neurosci.35, 445–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu, A. C. et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell129, 605–616 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maywood, E. S. et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr. Biol.16, 599–605 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Panda, S., Hogenesch, J. B. & Kay, S. A. Circadian rhythms from flies to human. Nature417, 329–335 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Helfrich-Förster, C. The circadian clock in the brain: a structural and functional comparison between mammals and insects. J. Comp. Physiol. A Neuroethol. Sens Neural Behav. Physiol.190, 601–613 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Zheng, Z. et al. A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell174, 730–743.e722 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlegel, P. et al. Whole-brain annotation and multi-connectome cell typing of Drosophila. Nature634, 139–152 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorkenwald, S. et al. Neuronal wiring diagram of an adult brain. Nature634, 124–138 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubowy, C. & Sehgal, A. Circadian rhythms and sleep in Drosophila melanogaster. Genetics205, 1373–1397 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abruzzi, K. C. et al. RNA-seq analysis of Drosophila clock and non-clock neurons reveals neuron-specific cycling and novel candidate neuropeptides. PLoS Genet.13, e1006613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang, X., Holy, T. E. & Taghert, P. H. Synchronous Drosophila circadian pacemakers display nonsynchronous Ca(2)(+) rhythms in vivo. Science351, 976–981 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma D., et al. A transcriptomic taxonomy of Drosophila circadian neurons around the clock. Elife10, (2021). [DOI] [PMC free article] [PubMed]
- 15.Ma, D. et al. Neural connectivity molecules best identify the heterogeneous clock and dopaminergic cell types in the Drosophila adult brain. Sci. Adv.9, eade8500 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy, D. et al. Peptidergic signaling from clock neurons regulates reproductive dormancy in Drosophila melanogaster. PLoS Genet.15, e1008158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinhard, N. et al. The lateral posterior clock neurons of Drosophila melanogaster express three neuropeptides and have multiple connections within the circadian clock network and beyond. J. Comp. Neurol.530, 1507–1529 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Reinhard, N. et al. The neuronal circuit of the dorsal circadian clock neurons in Drosophila melanogaster. Front. Physiol.13, 886432 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer O. T., et al. Connectomic analysis of the Drosophila lateral neuron clock cells reveals the synaptic basis of functional pacemaker classes. Elife11, (2022). [DOI] [PMC free article] [PubMed]
- 20.Barber A. F., Fong S. Y., Kolesnik A., Fetchko M., Sehgal A. Drosophila clock cells use multiple mechanisms to transmit time-of-day signals in the brain. Proc. Natl Acad. Sci. USA118, (2021). [DOI] [PMC free article] [PubMed]
- 21.Hidalgo, S., Anguiano, M., Tabuloc, C. A. & Chiu, J. C. Seasonal cues act through the circadian clock and pigment-dispersing factor to control EYES ABSENT and downstream physiological changes. Curr. Biol.33, 675–687.e675 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen, S. et al. Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat. Neurosci.23, 456–467 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Nässel, D. R. & Zandawala, M. Hormonal axes in Drosophila: regulation of hormone release and multiplicity of actions. Cell Tissue Res.382, 233–266 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKim T. H., et al. Synaptic connectome of a neurosecretory network in the Drosophila brain. bioRxiv, 2024.2008.2028.609616 (2024).
- 25.Shafer, O. T., Helfrich-Forster, C., Renn, S. C. & Taghert, P. H. Reevaluation of Drosophila melanogaster’s neuronal circadian pacemakers reveals new neuronal classes. J. Comp. Neurol.498, 180–193 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun, L., Jiang, R. H., Ye, W. J., Rosbash, M. & Guo, F. Recurrent circadian circuitry regulates central brain activity to maintain sleep. Neuron110, 2139–2154.e2135 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Li, M. T. et al. Hub-organized parallel circuits of central circadian pacemaker neurons for visual photoentrainment in Drosophila. Nat. Commun.9, 4247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshii, T., Rieger, D. & Helfrich-Förster, C. Two clocks in the brain: an update of the morning and evening oscillator model in Drosophila. Prog. Brain Res.199, 59–82 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Rieger, D., Shafer, O. T., Tomioka, K. & Helfrich-Förster, C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J. Neurosci.26, 2531–2543 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schubert, F. K., Hagedorn, N., Yoshii, T., Helfrich-Förster, C. & Rieger, D. Neuroanatomical details of the lateral neurons of Drosophila melanogaster support their functional role in the circadian system. J. Comp. Neurol.526, 1209–1231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, D. et al. Novel clock neuron subtypes regulate temporal aspects of sleep. bioRxiv2024, 592478 (2024). 2005.2003. [Google Scholar]
- 32.Lamaze, A., Kratschmer, P., Chen, K. F., Lowe, S. & Jepson, J. E. C. A wake-promoting circadian output circuit in Drosophila. Curr. Biol.28, 3098–3105 e3093 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Scheffer L. K., et al. A connectome and analysis of the adult Drosophila central brain. Elife9, (2020). [DOI] [PMC free article] [PubMed]
- 34.Talay, M. et al. Transsynaptic mapping of second-order taste neurons in flies by trans-Tango. Neuron96, 783–795.e784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selcho, M. et al. Anatomical characterization of PDF-tri neurons and peptidergic neurons associated with eclosion behavior in Drosophila. J. Comp. Neurol.526, 1307–1328 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Song B. J., Sharp S. J., Rogulja D. Daily rewiring of a neural circuit generates a predictive model of environmental light. Sci. Adv.7, (2021). [DOI] [PMC free article] [PubMed]
- 37.Fernandez, M. P., Berni, J. & Ceriani, M. F. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol.6, e69 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alpert, M. H., Gil, H., Para, A. & Gallio, M. A thermometer circuit for hot temperature adjusts Drosophila behavior to persistent heat. Curr. Biol.32, 4079–4087.e4074 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin, X. et al. A subset of DN1p neurons integrates thermosensory inputs to promote wakefulness via CNMa signaling. Curr. Biol.31, 2075–2087.e2076 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Marin, E. C. et al. Connectomics analysis reveals first-, second-, and third-order thermosensory and hygrosensory neurons in the adult Drosophila brain. Curr. Biol.30, 3167–3182.e3164 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang, M. et al. An extra-clock ultradian brain oscillator sustains circadian timekeeping. Sci. Adv.8, eabo5506 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Helfrich-Förster, C. Light input pathways to the circadian clock of insects with an emphasis on the fruit fly Drosophila melanogaster. J. Comp. Physiol. A Neuroethol. Sens Neural Behav. Physiol.206, 259–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alejevski, F. et al. The HisCl1 histamine receptor acts in photoreceptors to synchronize Drosophila behavioral rhythms with light-dark cycles. Nat. Commun.10, 252 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kind E., et al. Synaptic targets of photoreceptors specialized to detect color and skylight polarization in Drosophila. Elife10, (2021). [DOI] [PMC free article] [PubMed]
- 45.Homberg, U. In search of the sky compass in the insect brain. Naturwissenschaften91, 199–208 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Chen, D. M., Christianson, J. S., Sapp, R. J. & Stark, W. S. Visual receptor cycle in normal and period mutant Drosophila: microspectrophotometry, electrophysiology, and ultrastructural morphometry. Vis. Neurosci.9, 125–135 (1992). [DOI] [PubMed] [Google Scholar]
- 47.Pyza, E. & Meinertzhagen, I. A. Circadian rhythms in screening pigment and invaginating organelles in photoreceptor terminals of the housefly’s first optic neuropile. J. Neurobiol.32, 517–529 (1997). [PubMed] [Google Scholar]
- 48.Hulse B. K., et al. A connectome of the Drosophila central complex reveals network motifs suitable for flexible navigation and context-dependent action selection. Elife10, (2021). [DOI] [PMC free article] [PubMed]
- 49.Beer, K. & Helfrich-Förster, C. Model and non-model insects in chronobiology. Front Behav. Neurosci.14, 601676 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flyer-Adams, J. G. et al. Regulation of Olfactory associative memory by the circadian clock output signal Pigment-Dispersing Factor (PDF). J. Neurosci.40, 9066–9077 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nässel, D. R. & Zandawala, M. Endocrine cybernetics: neuropeptides as molecular switches in behavioural decisions. Open Biol.12, 220174 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckstein, N. et al. Neurotransmitter classification from electron microscopy images at synaptic sites in Drosophila melanogaster. Cell187, 2574–2594.e2523 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johard, H. A. et al. Peptidergic clock neurons in Drosophila: ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J. Comp. Neurol.516, 59–73 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Hamasaka, Y. et al. Glutamate and its metabotropic receptor in Drosophila clock neuron circuits. J. Comp. Neurol.505, 32–45 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Frenkel, L. et al. Organization of circadian behavior relies on glycinergic transmission. Cell Rep.19, 72–85 (2017). [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez Segarra A. J., Pontes G., Jourjine N., Del Toro A., Scott K. Hunger- and thirst-sensing neurons modulate a neuroendocrine network to coordinate sugar and water ingestion. Elife12, (2023). [DOI] [PMC free article] [PubMed]
- 57.Landayan D., Wang B. P., Zhou J., Wolf F. W. Thirst interneurons that promote water seeking and limit feeding behavior in Drosophila. Elife10, (2021). [DOI] [PMC free article] [PubMed]
- 58.Wang, F. et al. Neural circuitry linking mating and egg laying in Drosophila females. Nature579, 101–105 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, K. et al. Neural circuit mechanisms of sexual receptivity in Drosophila females. Nature589, 577–581 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Jiang, H. et al. Natalisin, a tachykinin-like signaling system, regulates sexual activity and fecundity in insects. Proc. Natl Acad. Sci. USA110, E3526–E3534 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riva, S., Ceriani, M. F., Risau-Gusman, S. & Franco, D. L. Circadian control of a sex-specific behaviour in Drosophila. bioRxiv2024, 586991 (2024). 2003.2027. [Google Scholar]
- 62.Diaz, M. M., Schlichting, M., Abruzzi, K. C., Long, X. & Rosbash, M. Allatostatin-C/AstC-R2 is a novel pathway to modulate the circadian activity pattern in Drosophila. Curr. Biol.29, 13–22.e13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davie, K. et al. A single-cell transcriptome atlas of the aging Drosophila Brain. Cell174, 982–998.e920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kapan, N., Lushchak, O. V., Luo, J. & Nässel, D. R. Identified peptidergic neurons in the Drosophila brain regulate insulin-producing cells, stress responses and metabolism by coexpressed short neuropeptide F and corazonin. Cell Mol. Life Sci.69, 4051–4066 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oh, Y. et al. A glucose-sensing neuron pair regulates insulin and glucagon in Drosophila. Nature574, 559–564 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nässel, D. R. & Vanden Broeck, J. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol. Life Sci.73, 271–290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Held M., et al. Aminergic and peptidergic modulation of Insulin-Producing Cells in Drosophila. bioRxiv, 2023.2009.2014.557555 (2023).
- 68.Im, S. H. & Taghert, P. H. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J. Comp. Neurol.518, 1925–1945 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ripoll-Sanchez, L. et al. The neuropeptidergic connectome of C. elegans. Neuron111, 3570–3589.e3575 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nässel, D. R. & Zandawala, M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog. Neurobiol.179, 101607 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Meissner G. W., et al. A searchable image resource of Drosophila GAL4 driver expression patterns with single neuron resolution. Elife12, (2023). [DOI] [PMC free article] [PubMed]
- 72.Morris, E. L. et al. Single-cell transcriptomics of suprachiasmatic nuclei reveal a Prokineticin-driven circadian network. EMBO J.40, e108614 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stengl, M. & Arendt, A. Peptidergic circadian clock circuits in the Madeira cockroach. Curr. Opin. Neurobiol.41, 44–52 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Helfrich-Förster, C. From neurogenetic studies in the fly brain to a concept in circadian biology. J. Neurogenet.28, 329–347 (2014). [DOI] [PubMed] [Google Scholar]
- 75.Liang, X., Holy, T. E. & Taghert, P. H. A Series of Suppressive Signals within the Drosophila Circadian Neural Circuit Generates Sequential Daily Outputs. Neuron94, 1173–1189.e1174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaze, K. M. & Helfrich-Förster, C. The Neuropeptide PDF Is Crucial for Delaying the Phase of Drosophila’s Evening Neurons Under Long Zeitgeber Periods. J. Biol. Rhythms36, 442–460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wulbeck, C., Grieshaber, E. & Helfrich-Förster, C. Pigment-dispersing factor (PDF) has different effects on Drosophila’s circadian clocks in the accessory medulla and in the dorsal brain. J. Biol. Rhythms23, 409–424 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Starnes A. N., Jones J. R. Inputs and Outputs of the Mammalian Circadian Clock. Biology (Basel)12, (2023). [DOI] [PMC free article] [PubMed]
- 79.Kim, H. J. & Harrington, M. E. Neuropeptide Y-deficient mice show altered circadian response to simulated natural photoperiod. Brain Res1246, 96–100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Helfrich-Förster, C., Winter, C., Hofbauer, A., Hall, J. C. & Stanewsky, R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron30, 249–261 (2001). [DOI] [PubMed] [Google Scholar]
- 81.Foster R. G., Hughes S., Peirson S. N. Circadian Photoentrainment in Mice and Humans. Biology (Basel)9, (2020). [DOI] [PMC free article] [PubMed]
- 82.Ramakrishnan, A. & Sheeba, V. Gap junction protein Innexin2 modulates the period of free-running rhythms in Drosophila melanogaster. iScience24, 103011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deng, B. et al. Chemoconnectomics: Mapping Chemical Transmission in Drosophila. Neuron101, 876–893.e874 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Kondo, S. et al. Neurochemical Organization of the Drosophila Brain Visualized by Endogenously Tagged Neurotransmitter Receptors. Cell Rep.30, 284–297.e285 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Yoshii, T. et al. Cryptochrome-dependent and -independent circadian entrainment circuits in Drosophila. J. Neurosci.35, 6131–6141 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zerr, D. M., Hall, J. C., Rosbash, M. & Siwicki, K. K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J. Neurosci.10, 2749–2762 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nern, A., Pfeiffer, B. D. & Rubin, G. M. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc. Natl Acad. Sci. USA112, E2967–E2976 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mu, S. et al. 3D reconstruction of cell nuclei in a full Drosophila brain. bioRxiv2021, 467197 (2021). 2011.2004. [Google Scholar]
- 89.Eccles JC, Jones RV, Paton WDM. From electrical to chemical transmission in the central nervous system: The closing address of the Sir Henry Dale Centennial Symposium Cambridge, 19 September 1975. Notes Rec. R. Soc. Lond.30, 219–230 (1976). [DOI] [PubMed] [Google Scholar]
- 90.Lacin H., et al Neurotransmitter identity is acquired in a lineage-restricted manner in the Drosophila CNS. Elife8, (2019). [DOI] [PMC free article] [PubMed]
- 91.Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics30, 2811–2812 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Dorkenwald, S. et al. FlyWire: online community for whole-brain connectomics. Nat. Methods19, 119–128 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bates A. S., et al. The natverse, a versatile toolbox for combining and analysing neuroanatomical data. Elife9, (2020). [DOI] [PMC free article] [PubMed]
- 94.McKinney W. Data Structures for Statistical Computing in Python. In: SciPy) (2010).
- 95.Yang, Z. et al. A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res28, 1013–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miyamoto, T. & Amrein, H. Diverse roles for the Drosophila fructose sensor Gr43a. Fly. (Austin)8, 19–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kahsai, L., Kapan, N., Dircksen, H., Winther, A. M. & Nässel, D. R. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. PLoS One5, e11480 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cannell, E. et al. The corticotropin-releasing factor-like diuretic hormone 44 (DH44) and kinin neuropeptides modulate desiccation and starvation tolerance in Drosophila melanogaster. Peptides80, 96–107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gera, J. et al. Anti-diuretic hormone ITP signals via a guanylate cyclase receptor to modulate systemic homeostasis in Drosophila. bioRxiv2024, 579245 (2024). [Google Scholar]
- 100.Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell184, 3573–3587.e3529 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Glossop, N. R. et al. VRILLE feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron37, 249–261 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Connectivity analyses can be performed using the cell IDs provided at https://codex.flywire.ai/ and with the scripts provided (see below). Source data for plots are provided with this paper. Source data are provided with this paper.
Code used for the analyses and data visualization is publicly available on GitHub: https://github.com/Zandawala-lab/Synaptic-connectome-of-the-Drosophila-circadian-clock_Reinhard-and-Fukuda-et-al.-2024.