Abstract
Arthritis is one of the most common symptoms of Behcet’s Disease (BD) observed in 57% of Japanese patients with BD. The relationship between arthritis and other clinical symptoms of BD and the impact of arthritis on the quality of life (QOL) of patients with BD are still unclear. Therefore, the current study aimed to clarify the differences in clinical symptoms depending on the presence or absence of arthritis and evaluate the impact of arthritis on QOL in these patients. Fifty-three Japanese patients diagnosed with BD and being treated for more than 6 months were included in this study. Patients were divided based on the presence of arthritis symptoms into an arthritis and a non-arthritis group. Clinical symptoms, disease activity, and QOL of both groups were compared using the Patient’s global assessment (PGA), Evaluator’s global assessment (EGA), and Behcet‘s disease current activity form (BDCAF) as indices of disease activity. To evaluate disease activity related to lesions other than arthritis, BDCAF excluding arthritis items was also calculated. The Behcet’s disease quality of life (BDQOL) scale was used to assess patients’ QOL. Oral ulcers and skin lesions were significantly more frequent in the arthritis group than in the non-arthritis group (p = 0.009 and 0.048, respectively). Among skin domains, papulopustular lesions tended to be more frequent in the arthritis group. EGA and BDCAF (both including and excluding arthritis-related items) scores were significantly higher in the arthritis group (p = 0.019, < 0.0001, and 0.0004 respectively). Although PGA and BDQOL tended to be higher in the arthritis group, the difference between the two groups was not statistically significant. The disease course in Japanese BD patients with arthritis was more frequently complicated by oral ulcers and skin lesions than in those without arthritis. In addition, BD patients with arthritis tended to have generally higher disease activity and low QOL than their non-arthritis counterparts. Further research is required to confirm these results.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82102-6.
Keywords: Behcet’s disease, Arthritis, Quality of life, Behcet‘s disease current activity form
Subject terms: Quality of life, Vasculitis syndromes
Introduction
Behcet’s disease (BD) is a systemic inflammatory disease characterized by recurrent oral ulcers, skin and eye lesions, and genital ulcers. BD is considered as one type of vasculitis. Since there are no disease-specific physical or laboratory findings for BD, the diagnosis is made based on the combination of symptoms that appear over the course of the disease. The prevalence of BD is known to be high in the Mediterranean region, East Asia, and Japan, and low in North America and northern Europe. The frequency of each symptom of BD also tends to vary from country to country, and it has been reported that the frequency of gastrointestinal lesions is high in Japan1. Recurrent arthritis, one of the most common symptoms of BD (observed in 57% of patients with BD in Japan and about 30–60% of patients with BD in the world)1, primarily involves large joints such as the knees and ankles but is generally not associated with deformity or erosion. BD-related arthritis is effectively managed by colchicine and non-steroidal anti-inflammatory drugs (NSAIDs)2–4; however, azathioprine, interferon-alpha, or tumor necrosis factor-alpha (TNF-α) inhibitors are recommended for recurrent or refractory arthritis4. In addition, a few studies have reported the beneficial effects of apremilast (phosphodiesterase 4 inhibitor) on BD-related arthritis5,6.
Although patients with BD present with a variety of lesions, these lesions do not appear randomly. Previous cluster analyses have suggested that there are several subsets of BD9. Several studies have reported an association between arthritis and papulopustular skin lesions and mucosal lesions in BD, but the association between arthritis and other lesions in BD is still not fully elucidated7–10. Arthritis in BD is not only associated with other lesions but may also affect overall disease activity. Although Behcet’s disease current activity form (BDCAF) has been frequently used as an index of overall disease activity in BD, there have been no reports evaluating the impact of arthritis in BD on overall disease activity using the BDCAF11. Furthermore, arthritis in BD often affects large joints and is expected to affect patients’ quality of life (QOL); however, only a few studies have explored the relationship between arthritis and QOL in patients with BD12–14. The Behcet’s disease quality of life (BDQOL) scale is well known as an index of QOL specific to BD, but these previous reports used general QOL indicators such as health assessment questionnaire and short form-36 QOL scale, and there have been no reports evaluating the impact of arthritis on QOL using BDQOL15.
Therefore, this study aimed to clarify the differences in clinical symptoms depending on the presence or absence of arthritis of BD in Japanese patients and the impact of arthritis on overall disease activity and patients’ QOL using indices specific to BD.
Results
Patient characteristics
Out of the 53 patients with BD enrolled in this study, 26 patients (49.1%) had symptoms of arthritis at the time of evaluation (arthritis group). There were no significant differences between the arthritis and non-arthritis groups in terms of age, sex, disease duration, HLA-A26 and HLA-B51 positivity rates, and medications, except for a higher use of 5-aminosalicylic acid use in the arthritis group. Regarding the lesions observed so far, no differences were found between the two groups, except for arthritis, which was more prevalent in the arthritis group. In terms of the severity of arthritis, the arthritis group had low to moderate arthritis activity (median [IQR]: SDAI: 9.7 [5.9–12.7]; CDAI: 9.4 [5.9–12.0]; DAS-28CRP 2.8 [2.3–3.4]; Table 1).
Table 1.
Characteristics of patients with Behcet’s disease (N = 53).
| Non-arthritis group | Arthritis group | p-value | |
|---|---|---|---|
| Number of cases | 27 | 26 | |
| Age, year | 49.7 ± 13.8 | 48.5 ± 12.9 | 0.7351 |
| Female, n(%) | 17 (63.0) | 21 (80.8) | 0.1471 |
| Disease duration, year | 14.3 ± 10.7 | 12.0 ± 7.6 | 0.5324 |
| HLA-B51 positive, n(%) | 9 (52.9), n = 17 | 6 (33.3), n = 18 | 0.2399 |
| HLA-A26 positive, n(%) | 4 (26.7), n = 15 | 8 (50.0), n = 16 | 0.1794 |
| Major lesions ever observed | |||
| Oral ulcer, n(%) | 27 (100) | 26 (100) | - |
| Genital ulcer, n(%) | 24 (88.9) | 23 (88.5) | 1.0000 |
| Skin lesion, n(%) | 23 (85.2) | 24 (92.3) | 0.6687 |
| Erythema nodosum, n(%) | 17 (63.0) | 12 (46.2) | 0.2729 |
| Thrombophlebitis, n(%) | 2 (7.4) | 2 (7.7) | 1.0000 |
| Papulopustular skin lesions, n(%) | 20 (74.1) | 23 (88.5) | 0.2935 |
| Arthritis, n(%) | 18 (66.7) | 26 (100) | 0.0018* |
| Eye involvement, n(%) | 9 (33.3) | 7 (26.9) | 0.7664 |
| Epididymitis, n(%) | 3 (11.1) | 1 (3.9) | 0.6104 |
| Gastrointestinal involvement, n(%) | 3 (11.1) | 4 (15.4) | 0.7040 |
| Nervous system involvement, n(%) | 5 (18.5) | 4 (16.0) | 1.0000 |
| Vascular involvement, n(%) | 0 (0) | 2 (7.7) | 0.2358 |
| Medication, n(%) | |||
| NSAIDs | 1 (3.7) | 5 (19.2) | 0.0642 |
| Colchicine | 19 (70.4) | 17 (65.4) | 0.6974 |
| Glucocorticoids | 8 (29.6) | 10 (38.5) | 0.4970 |
| Dose, median(IQR), mg/d | 2.5 (2.1, 5.0) | 3.5 (2.0, 5.0) | 0.8906 |
| Methotrexate | 2 (7.4) | 7 (7.7) | 0.9687 |
| Azathioprine | 1 (3.7) | 0 (0) | 0.2423 |
| Cyclosporine | 1 (3.7) | 0 (0) | 0.2423 |
| Tumor necrosis factor inhibitors | 3 (11.1) | 4 (15.4) | 0.6456 |
| Apremilast | 6 (22.2) | 4 (15.4) | 0.5234 |
| 5-aminosalicylic acid | 0 (0) | 3 (11.5) | 0.0347* |
| Duration of current treatment, month | 51.7 ± 70.2 | 36.8 ± 48.5 | 0.9708 |
| Arthritis activity | |||
| SDAI, median(IQR) | - | 9.7 [5.9–12.7] | - |
| CDAI, median(IQR) | - | 9.4 [5.9–12.0] | - |
| DAS28-CRP, median(IQR) | - | 2.8 [2.3–3.4] | - |
SDAI: Simplified disease activity index, CDAI: Clinical disease activity index, DAS: Disease activity score.
p-values were determined by the Mann-Whitney U test, Fisher’s exact test, or chi-square test.
*Data were considered significant at p < 0.05.
Differences in the frequency of active major lesions of BD depending on the presence or absence of arthritis at the time of evaluation
Oral ulcers and skin lesions were significantly more frequent in the arthritis group than in the non-arthritis group (p = 0.009 and 0.048, respectively). While papulopustular lesions were more common in the arthritis group, this difference did not reach statistical significance. There was no significant difference regarding the incidence of other lesions between the two groups (Table 2).
Table 2.
Differences in the frequency of active major lesions of BD depending on the presence or absence of arthritis at the time of evaluation.
| Non-arthritis group | Arthritis group | p-value | |
|---|---|---|---|
| n = 27 | n = 26 | ||
| Oral ulcer | 14 (51.9%) | 22 (84.6%) | 0.0092* |
| Genital ulcer | 6 (22.2%) | 8 (30.8%) | 0.4800 |
| Skin lesion | 4 (14.8%) | 10 (38.5%) | 0.0483* |
| Erythema nodosum | 3 (11.1%) | 4 (15.4%) | 0.6456 |
| Thrombophlebitis | 0 (0%) | 0 (0%) | - |
| Papulopustular skin lesions | 2 (7.4%) | 7 (26.9%) | 0.0530 |
| Eye involvement | 0 (0%) | 1 (3.9%) | 0.2294 |
| Epididymitis | 1 (3.7%) | 0 (0%) | 0.2423 |
| Gastrointestinal involvement | 1 (3.7%) | 0 (0%) | 0.2423 |
| Nervous system involvement | 0 (0%) | 0 (0%) | - |
| Vascular involvement | 0 (0%) | 0 (0%) | - |
p-values were determined by Fisher’s exact test or chi-square test.
*Data were considered significant at p < 0.05.
Impact of arthritis on BD disease activity and patients’ QOL
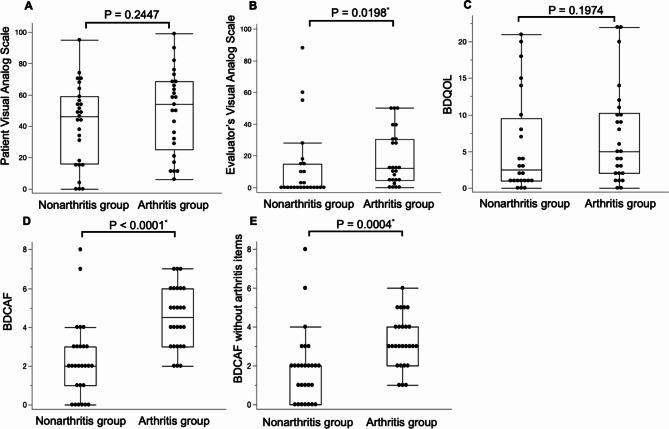
EGA was significantly higher in the arthritis group compared to the non-arthritis group (p = 0.019). BDCAF scores, including and excluding arthritis items, were significantly higher in the arthritis group (p = < 0.0001 and 0.0004, respectively). Although the median PGA and BDQOL scores were higher in the arthritis group, there was no statistically significant difference between the two groups (Fig. 1).
Fig. 1.
Comparison of disease assessment indices for Behcet’s disease with and without arthritis. (A) Patients visual analog scale (B) evaluator’s visual analog scale (C) Behcet’s disease quality of life scale (BDQOL) (D) Behcet’s disease current activity form (BDCAF) (E) BDCAF without items related to arthritis. *Data were considered significant at p < 0.05.
Discussion
In this study, 49.1% of BD patients had arthritis at the time of evaluation and 83.0% of BD patients had a history of arthritis, which is higher than that described in the existing literature1. Notably, it has been reported that Japanese patients with BD who are HLA-A26-positive have significantly fewer complications of arthritis than the HLA-A26-negative patients16. In contrast, we observed that 75.0% of our patients whose HLA-A26 were positive (n = 12) had a history of arthritis compared to 73.7% whose HLA-A26 were negative (n = 19). The possible reasons for this discrepancy from the previous report include a small number of patients in this study and the use of different Japanese diagnostic criteria in the previous report17.
We also noted a statistically significant difference in the number of patients receiving 5-aminosalicylic acid in the arthritis versus the non-arthritis group. In all cases, the drug, 5-aminosalicylic acid, was administered for intestinal lesions. Seven of the fifty-three patients (four and three in the arthritis and non-arthritis groups) reported a history of gastrointestinal lesions. In the arthritis group, three of the four patients received 5-aminosalicylic acid and had no gastrointestinal symptoms at the time of examination. In the non-arthritis group, no patient received this drug, and only one patient had gastrointestinal symptoms at the time of examination (Table 2). In other words, although there was a difference in the administration rate of 5-aminosalicylic acid between the arthritis group and the non-arthritis group, there was no significant difference in the rate of gastrointestinal symptoms.
Several reports have explored the relationship between arthritis and other lesions in BD. Diri et al. reported a significant association between joint symptoms and skin symptoms, particularly papulopustular skin lesions, in BD patients8. Tunc et al. also reported a similar association between papulopustular skin lesions and arthritis7. Soejima et al. indicated the presence of mucocutaneous clusters with arthritis in a cluster analysis of Japanese patients with BD9. Tono et al. reported high comorbidity rates for joint and skin symptoms in Japanese patients with BD10. In the present study, oral ulcers and skin lesions were significantly more common in the arthritis group than in the non-arthritis group; although the difference was not significant, papulopustular skin lesions were more frequent in the former. Thus, the results of this study seem to support previous findings that papulopustular skin lesions tend to occur in combination with arthritic symptoms in BD.
BDCAF is a measure of overall disease activity in BD and is based on patients’ reports of 12 symptoms in the 4 weeks prior to evaluation11. To our knowledge, there have been no reports evaluating the impact of arthritis in BD on overall disease activity using the BDCAF. These 12 items include symptoms of arthritis and arthralgia, and as expected, the arthritis group had higher scores than the non-arthritis group. To eliminate the effect of arthritis-related symptoms, we calculated the BDCAF score excluding arthritis and arthralgia items; however, the arthritis group still scored significantly higher than the non-arthritis group. These results suggest that patients with BD who have arthritis tend to have a generally higher disease activity in lesions other than those involving joints. However, it should be noted that arthritis was assessed based on the presence or absence of arthritis at the time of assessment, whereas the BDCAF assessed the condition over the past 4 weeks from the time of arthritis assessment and therefore were not assessed at strictly the same time points.
To date, only a few reports have compared the QOL of BD patients with or without arthritis. Gur et al. evaluated the health and QOL of BD patients using the Health Assessment Questionnaire (HAQ) and the Nottingham Health Profile (NHP) and reported significantly higher scores for both HAQ and NHP in patients with arthritis than in those without arthritis12. Unlike previous studies, our present study is the first to report the impact of arthritis on QOL using BDQOL, a QOL index specific to BD. In our study, the arthritis group tended to have higher BDQOL values than the non-arthritis group, but the differences were not significant. The relatively low arthritis activity in the arthritis group in our study may be responsible for the lack of statistically significant differences.
This study had several limitations. First, arthritis and other active lesions of BD were determined by different rheumatologists; therefore, we cannot rule out the potential bias caused by multiple evaluators. Second, all eligible patients were under treatment. Although there were no significant differences in the therapeutic agents other than 5-aminosalicylic acid between the arthritis group and non-arthritis groups, it is possible that the therapeutic agents had an impact on lesions, disease activity, and patients’ QOL. Therefore, it should be recognized that our results represent real-world patient data affected by treatment modifications. Third, most patient characteristics in this study did not show significant differences between the two groups, which may be because of the small sample size. Fourth, the extent of damage caused by both treatment and disease acquired during the course of BD, which is closely related to quality of life, was not examined in this study. Since no damage indices for BD have been established, it would be necessary to establish damage indices for BD in order to examine QOL in BD.
In conclusion, Japanese BD patients with arthritis experience a greater frequency of oral ulcers and skin lesions than those without arthritis. In addition, these patients tend to have high disease activity and low QOL, even when their arthritis activity is mild to moderate. Since this study was a small single-center retrospective exploration, further prospective research is needed to clarify the relationship between clinical characteristics and QOL in patients with BD.
Methods
Participants
In this study, we retrospectively investigated all patients in BD who were being treated for more than 6 months at the Kagawa University Hospital, Kagawa, Japan between January 2022 to February 2023. 53 patients aged ≥ 18 years who were diagnosed with BD according to the International Criteria for BD18 were included in this study. Patients with BD who did not meet this criteria were excluded from this study. Based on the presence or absence of arthritis at the time of evaluation, we divided the patients into an arthritis group and a non-arthritis group. The presence or absence of arthritis was assessed by physical examination by a rheumatologist as described below. We evaluated their clinical symptoms, disease activity, and QOL by reviewing the hospital’s records.
The study was approved by the ethics committee of Kagawa University, Kagawa, Japan (2023-057), and conducted in accordance with the principles of the Declaration of Helsinki. The Ethics Committee of the Faculty of Medicine at Kagawa University waived the need for written informed consent due to the retrospective nature of the study.
Evaluation of arthritis, disease activity, and patients’ QOL
The presence or absence of arthritis and other active lesions in BD was determined by the rheumatologist who examined the patients. Swelling of 66 joints and tenderness of 68 joints were assessed and those with at least one observed arthritis were classified as the arthritis group. The severity of arthritis (arthritis activity) was assessed using disease activity score 28 using C-reactive Protein (DAS28-CRP)19, clinical disease activity index (CDAI)20, and simplified disease activity index (SDAI)21. The indices–Patient’s global assessment (PGA), evaluator’s global assessment (EGA), and Behcet‘s disease current activity form (BDCAF)11 were used to assess BD disease activity. The BDCAF is a validated and effective tool for comprehensively evaluating the activity of Behcet’s disease22, and the BDCAF questionnaire was completed by a rheumatologist for the patient. To evaluate BD disease activity related to lesions other than arthritis, the BDCAF score was also calculated excluding arthritis items (arthralgia and arthritis). Additionally, Behcet’s disease quality of life (BDQOL) scale15 was used to assess patients’ QOL. BDQOL is a disease-specific evaluation scale that was developed and validated for use in patients with BD23,24. BDQOL comprises 30 items which measure disease-related restrictions on patients’ activities and emotional responses to these restrictions. Each item is scored 0 or 1 with a total scoring range of 0–30; a lower score indicates better QOL. These questionnaires used in this study were created based on those used in clinical trials25 and included as supplementary information (Supplementary file). All these measurements were made during treatment.
Statistical analyses
Continuous variables were expressed as median (interquartile range, IQR) unless otherwise mentioned, and the Mann–Whitney U test was used to compare continuous variables. Fisher’s exact test or chi-square test was used to compare categorical variables. A two-sided p-value of < 0.05 was considered statistically significant. All analyses were performed using the JMP Pro 16 software (SAS Institute, Cary, NC, USA).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to Enago for providing English language services for this manuscript.
Abbreviations
- BD
Behcet’s Disease
- BDCAF
Behcet’s disease current activity form
- BDQOL
Behcet’s disease quality of life
- EGA
Evaluator’s global assessment
- HAQ
Health Assessment Questionnaire
- NHP
Nottingham Health Profile
- PGA
Patient’s global assessment
- QOL
Quality of life
Author contributions
K.S., R.W., N.K. and H.D. designed the study. T.K., H.S., S.N., T.M., Y.U., R.M., M.M., K.C., R.K., H.Y. and N.M. assisted in collecting the data. K.S. and R.W. analyzed the data. K.S. prepared the first draft of the manuscript; R.W. and H.D. revised the manuscript. The final manuscript was reviewed and approved by all authors.
Funding
This study is partly supported by grants from the Behçet’s Disease Research Committee, Research on Specific Disease of the Health Science Research, the Ministry of Health, Labor, and Welfare (23FC1020).
Data availability
The data supporting the findings in this study can be obtained from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Faculty of Medicine, Kagawa University (2023-057) and conducted in accordance with the Declaration of Helsinki. The Ethics Committee of the Faculty of Medicine at Kagawa University waived the need for written informed consent due to the retrospective nature of the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sakane, T., Takeno, M., Suzuki, N. & Inaba, G. Behçet’s disease. N Engl. J. Med.341, 1284–1291 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Yurdakul, S., Yazici, H. & Tüzün, Y. The arthritis of Behçet’s disease: A prospective study. Ann. Rheum. Dis.42, 505–515 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatemi, G., Silman, A. & Bang, D. EULAR recommendations for the management of Behçet disease. Ann. Rheum. Dis.67, 1656–1662 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Hatemi, G., Christensen, R. & Bang, D. 2018 update of the EULAR recommendations for the management of Behçet’s syndrome. Ann. Rheum. Dis.77, 808–818 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Iizuka, Y., Takase-Minegishi, K. & Hirahara, L. Beneficial effects of apremilast on genital ulcers, skin lesions, and arthritis in patients with Behçet’s disease: A systematic review and meta-analysis. Mod. Rheumatol.32, 1153–1162 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Wakiya, R., Ushio, Y. & Ueeda, K. Efficacy and safety of apremilast and its impact on serum cytokine levels in patients with Behçet’s disease. Dermatol. Ther.35, e15616 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Tunc, R., Keyman, E., Melikoglu, M., Fresko, I. & Yaziciet, H. Target organ associations in Turkish patients with Behçet’s disease: A cross sectional study by exploratory factor analysis. J. Rheumatol.29, 2393–2396 (2002). [PubMed] [Google Scholar]
- 8.Diri, E. et al. Papulopustular skin lesions are seen more frequently in patients with Behçet’s syndrome who have arthritis: A controlled and masked study. Ann. Rheum. Dis.60, 1074–1076 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soejima, Y. et al. Changes in the proportion of clinical clusters contribute to the phenotypic evolution of Behçet’s disease in Japan. Arthritis Res. Ther.23, 49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tono, T., Kikuchi, H. & Sawada, T. Clinical features of Behçet’s disease patients with joint symptoms in Japan: A national multicenter study. Mod. Rheumatol.32, 1146–1152 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Lawton, G., Bhakta, B. B., Chamberlain, M. A. & Tennant, A. The Behcet’s disease activity index. Rheumatol. (Oxford)43, 73–78 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Gur, A., Sarac, A. J., Burkan, Y. K., Nas, K. & Cevik, R. Arthropathy, quality of life, depression, and anxiety in Behcet’s disease: Relationship between arthritis and these factors. Clin. Rheumatol.25, 524–531 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Canpolat, O. & Yurtsever, S. The quality of life in patients with behçet’s disease. Asian Nurs. Res. (Korean Soc. Nurs. Sci.)5, 229–235 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Nagano, A., Takeuchi, M. & Horita, N. Behçet’s disease and activities of daily living. Rheumatology (Oxford)61, 1133–1140 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Gilworth, G. et al. Development of the BD-QoL: A quality of life measure specific to Behçet’s disease. J. Rheumatol.31, 931–937 (2004). [PubMed] [Google Scholar]
- 16.Asako, K. Association of HLA-A26 with Behçet’s disease in Japanese patients (in Japanese). Clin. Rheumatol. Relat. Res. (Rinshoriumachi)23, 29–36 (2011). [Google Scholar]
- 17.Ideguchi, H. et al. Characteristics of vascular involvement in Behçet’s disease in Japan: A retrospective cohort study. Clin. Exp. Rheumatol.29, S47–53 (2011). [PubMed] [Google Scholar]
- 18.International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD). The international criteria for behçet’s disease (ICBD): A collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J. Eur. Acad. Dermatol. Venereol.28, 338–347 (2014). [DOI] [PubMed] [Google Scholar]
- 19.van der Heijde, D. M. et al. Judging disease activity in clinical practice in rheumatoid arthritis: First step in the development of a disease activity score. Ann. Rheum. Dis.49, 916–920 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aletaha, D. et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: Calidation of a clinical activity score. Arthritis Res. Ther.7, R796–806 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolen, J. S. et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford)42, 244–257 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Bhakta, B. B. et al. Behçet’s disease: Evaluation of a new instrument to measure clinical activity. Rheumatology38, 728–733 (1999). [DOI] [PubMed] [Google Scholar]
- 23.Vojdanian, M. et al. Validity and reliability of the Persian version of Behcet’s disease quality-of-life (BD-QoL) questionnaire: A cross-cultural adaptation. Rheumatol. Int.35, 677–684 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Touma, Z. et al. Cross-cultural adaptation and validation of Behçet’s disease quality of life questionnaire. BMC Med. Res. Methodol.11, 52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatemi, G. et al. Trial of apremilast for oral ulcers in Behçet’s syndrome. N. Engl. J. Med.381, 1918–1928 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings in this study can be obtained from the corresponding author upon reasonable request.