Graphical abstract
Keywords: Osteonecrosis, Jaw, Bisphosphonates, Denosumab, Practice guidelines, Consensus
Highlights
-
•
Wound care and antibiotics should be considered in all stages of MRONJ patients.
-
•
Consider surgery for symptomatic patients with sequestrum or bone abnormalities accompanied by recurrent infections.
-
•
He’s classification stages patients based on radiographic and clinical traits, tailoring surgical procedures to each stage.
-
•
Propose multidisciplinary comprehensive treatment for optimal outcomes.
Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is a side effect that occurs after treatment for systemic diseases. However, most institutions currently rely on empirical methods to make diagnosis and treatment plans, and there is a lack of consensus or guidelines for the classification, staging and treatment of MRONJ in China. To address this gap and improve prognosis, an expert panel representing 11 renowned domestic medical colleges and affiliated hospitals in China was convened. The panel made a comprehensive literature review of previous treatment experiences and research findings to address issues of definitions, etiology and risk factors, diagnosis, treatment and prevention methods. The panel concluded that the diagnosis of MRONJ can be made on the basis of a history of related medications and typical clinical manifestations, with either typical radiographic manifestations or histopathological manifestations, after excluding jaw metastasis. Surgical treatment should be considered for symptomatic patients with sequestrum or bone abnormalities accompanied by recurrent infections, and He’s classification was considered a practical clinical MRONJ staging system. Multidisciplinary comprehensive treatment should be proposed to achieve optimal treatment outcomes.
1. Introduction
With advancement in cancer treatment, the survival rate of cancer patients has improved significantly [1], [2]. The skeleton is a common site for metastases from several solid tumors, notably breast, lung and prostate cancers. Patients with bone metastasis often experience reduced survival rates, impaired quality of life and increased risk of skeletal related events (SREs), including pathological fractures [3]. Likewise, as population ageing becomes one of the key challenges in 21st century, aging-related diseases such as osteopenia and osteoporosis have become increasingly prevalent [4]. These conditions can also lead to SREs. As a result, bone-modifying agents are widely used to prevent or mitigate these complications.
Bone is a highly dynamic tissue that undergoes constant remodelling to maintain health, achieved through a delicate balance between osteoblast and osteoclast activity. However, bone can be particularly susceptible to cancer treatments. Beyond the direct impact of bone-modifying agents, other drugs—such as immunomodulators, targeted drugs and anti-angiogenic agents—may disrupt bone homeostasis, potentially leading to secondary bone diseases, such as osteonecrosis [5].
Medication-related osteonecrosis of the jaw (MRONJ) is defined as dysregulation and bone destruction in the jaw, primarily caused by bone-modifying agents, anti-angiogenic agents, targeted drugs or immunomodulators for systematic diseases, including bone metastasis and osteoporosis, without the history of radiotherapy. Osteoporosis patients treated with oral medications have a MRONJ occurrence rate of approximately 0.02 %–0.04 %, which increases significantly to 0.7 %–9% in cancer patients receiving intravenous injections [6]. The clinical manifestations include local redness, swelling, pain, fistula formation, osteonecrosis, bone exposure and even pathological fractures, leading to severe oral dysfunction [7]. In recent years, with the increasing use of bone-modifying agents and the increased incidence rate of MRONJ, the understanding of its pathophysiology, risk factors and therapeutic approaches has increased [8]. In 2007, 2009 and 2014, the American Association of Oral and Maxillofacial Surgeons (AAOMS) proposed management strategies in position papers for MRONJ patients or those who are at risk of developing MRONJ [9], [10], [11]. In 2022, updated research on pathogenesis and treatment was published [12]. Currently, the classification, staging, diagnosis and treatment of MRONJ in China mainly refer to AAOMS standards. However, the classification and staging of MRONJ in the AAOMS position paper, which is widely used currently, focus only on clinical manifestations, ignoring the importance of imaging findings. In addition, there are differences between Asian and Western populations, and medical conditions and levels vary across China. These limitations reduce the applicability of treatment principles in China. To further standardize the diagnosis and treatment of MRONJ and improve treatment efficiency, experts specializing in MRONJ across China convened a seminar. The diagnosis and treatment opinions of experts from 11 renowned medical colleges and affiliated hospitals were collected. Further revisions have been made on the basis of recent research findings and experiences both domestically and overseas. Finally, this consensus is presented and suggested as a valuable reference for clinical practice.
2. Discussion
2.1. Definition
In 2003, Marx first reported 36 cases of necrosis and bone exposure of the jaw caused by BPs. To distinguish them from osteoradionecrosis of the jaw (ORNJ), those cases were defined as bisphosphonate-related osteonecrosis of the jaw (BRONJ) [13]. Four years later, the AAOMS established the diagnostic criteria for BRONJ: BP treatment history; continuous exposed necrotic bone in the oral and maxillofacial region for over 8 weeks; and no radiotherapy in the head and neck region [9]. A Canadian expert consensus suggests that 8 weeks of clinical observation is needed because complete soft tissue repair after bone exposure requires 8 weeks [14]. In 2009, the AAOMS made adjustments to the definition of BRONJ. They changed the word “exposed necrotic bone” to “exposed bone” and added stage 0 to the classification [10]. There are no significant changes to the other definitions. However, this definition has several limitations because it relies solely on clinical manifestations and has no histological basis.
In recent years, continuous reports about osteonecrosis of the jaw caused by non-BPs, such as other bone-modifying agents and anti-angiogenic agents, have been published. In 2014, the AAOMS renamed and recast BRONJ as MRONJ [11]: medication history of anti-resorptive or anti-angiogenic medications; bone exposure in the jaw or fistula in the affected area persisting for more than 8 weeks; and no history of jaw bone metastatic cancer or head and neck radiotherapy. Compared with the definition from the 2009 position paper, the 2014 version changed the description of “exposed bone” to “bone exposure or intraoral/extraoral fistula” and excluded patients with other cancerous jaw bone metastases.
On the basis of the 2022 update of the AAOMS position paper and the opinions of relevant domestic experts, this consensus defines MRONJ as follows: (1) previous or current treatment with bone-modifying agents, alone or in combination with targeted drugs, immunomodulators or anti-angiogenic agents; (2) presence of exposed necrotic bone in the maxillofacial region or continuous access of the bone surface through intraoral/extraoral fistula for more than 8 weeks; and (3) no history of radiotherapy or metastatic tumor disease in the jaw. Common medications that cause MRONJ are listed in Table 1.
Table 1.
Common medications that cause MRONJ.
| Chemical name | Product name | Category | Mechanism | Route of administration |
|---|---|---|---|---|
| Zoledronate | Aclasta/Tianqingyitai/Zometa | Bone-modifying agent | Osteoclast inhibition | Intravenous injection |
| Alendronate | Gubon | Bone-modifying agent | Osteoclast inhibition | Oral administration |
| Denosumab | Prolia/Xgeva | RANKL inhibitor | RANKL inhibitor | Subcutaneous injection |
| Bevacizumab | Avastin | Anti-angiogenic agent | anti-VEGF | Intravenous injection |
| Pazopanib | Votrient | Anti-TKI medication | anti-VEGFR | Oral administration |
| Apatinib | Aitan | Anti-TKI medication | anti-VEGFR | Oral administration |
| Imatinib | Glivec | Anti-TKI medication | Multiple targets | Oral administration |
| Sunitinib | Sutent | Anti-TKI medication | Multiple targets | Oral administration |
| Methotrexate | Maoxiang | Immunosuppressant | Immune suppression at low doses |
Intravenous injection/ Oral administration |
| Temsirolimus | Temsirolimus | mTOR inhibitor | Anti-mTOR | Intravenous injection |
| Everolimus | Afinitor | mTOR inhibitor | Anti-mTOR | Oral administration |
2.2. Etiology and risk factors
While bone-modifying agents play a crucial role for controlling diseases such as osteoporosis and bone metastasis, they can also result in complications such as MRONJ. Additionally, recent studies have linked other medications—anti-angiogenic agents, immunomodulators and targeted drugs—with MRONJ development [15], [16]. Understanding the anatomical characteristics in jaw will benefit the understanding of MRONJ pathogenesis. Compared with other skeletal tissues, the jawbone is more susceptible to MRONJ because of its anatomical structure, physiological functions and oral microbial environment. The bone remodelling rate in the jawbone is faster than that in other skeletal tissues and is approximately 20 times greater than that in the ilium [17], leading to more drug accumulation in this area. Additionally, although the jawbone has an abundant blood supply, drugs such as BPs and other anti-angiogenic agents can inhibit blood flow and vascular growth, leading to local ischemia and an increased risk of bone necrosis [17]. Furthermore, the oral cavity communicates with the external environment, hosting a complex microbial ecosystem. Dental infections such as periodontitis, pulpitis and periapical periodontitis are common in the oral cavity. Trauma such as tooth extraction can result in local infection by oral flora, potentially triggering MRONJ. The incidence of MRONJ varies among countries, but with the recent widespread clinical usage of MRONJ-related medications, the overall incidence is increasing. MRONJ is difficult to treat and can severely affect patients’ quality of life, which has attracted increasing attention.
Currently, there are several hypotheses regarding the pathogenesis of MRONJ, including the bone remodelling inhibition theory, angiogenesis inhibition theory, microbial infection theory and immune dysfunction theory.
The bone remodelling inhibition theory acts as the central hypothesis among all theories. The bone is a highly dynamic system, which is very vulnerable to stimulations [5]. The balance between osteogenesis and bone resorption relies on the functional coupling between osteoblasts and osteoclasts. BPs inhibits the function of osteoclasts directly [18], which can be proved by the giant nonfunctional osteoclasts after long-time BPs usage [19]. Denosumab directly binds to RANKL, a mediator for osteoclast formation, and inhibit both osteoclast differentiation and the function of osteoclasts [18]. Some treatments targeting bone homeostasis have been found effective in MRONJ treatment, such as teriparatide and bone morphogenetic protein-2 (BMP-2) [20], [21], [22], [23], [24]. These results further prove the central role of bone remodelling inhibition theory in MRONJ occurrence.
MRONJ was firstly defined as avascular necrosis, which might be caused by the anti-angiogenic effects attributed to BPs [25]. The angiogenesis inhibition theory proposes that BPs can inhibit jawbone angiogenesis, causing local bone ischemia. A special type of vessel cells called type H endothelial cells links osteogenesis and angiogenesis [26]. Animal study has found the impaired skeletal angiogenesis after zoledronate usage, together with reduced osteoprogenitors [27]. Drugs that target vascular endothelial growth factor (VEGF) and related pathways, such as bevacizumab, can also induce bone necrosis [28], providing more support to this theory.
The theory of microbial infection is still under debate [29], [30]. Although tooth extraction and implant surgery are related to more incidences of MRONJ, infections including periodontitis, apical periodontitis and peri-implantitis seem to be more important than invasive surgeries themselves [31], [32], [33]. Besides, poor hygiene and oral health maintenance are correlated with higher incidence of MRONJ [34]. The jawbone is separated from various oral microbes only by a thin layer of mucoperiosteum. Therefore, it is susceptible to odontogenic infection due to minor trauma and subsequently causes MRONJ. There is no specific pathogenic microbe to be found related with MRONJ yet, although nearly 82.18 % MRONJ lesions are presented with Actinomyces [35]. The indigenous microbiota, however, might prevent MRONJ from happening [36].
Most MRONJ patients experience immune suppression, due to a history of long-term corticosteroid usage, malignancy or chemotherapy. This state may be induced either by the primary disease or by its associated treatments. Besides, patients with immune-related systematic conditions, such as diabetes, are at an increased risk of developing MRONJ [37]. Evidence of immune dysfunction in MRONJ patients includes elevated levels of inflammatory cytokines in serum, as well as abnormal distribution of T cells observed in both peripheral blood and affected lesions [29], [38]. This disrupted immune homeostasis leads to an imbalance in bone turnover and reduced bone remodelling, ultimately contributing to MRONJ development [39].
High-risk factors associated with the above hypotheses include medication-related factors, local factors and systemic factors, which act alone or in combination [12]. Potential risk factors are summarized in Table 2.
Table 2.
Risk factors for MRONJ.
| Risk factors | Contents |
|---|---|
| Medication-related factors | Type of drugs: anti-resorptive medications, anti-angiogenic medications, immunosuppressants, targeted drugs, etc. |
| Routes of administration: intravenous injection, oral administration, subcutaneous injection | |
| Duration of medication usage | |
| Cumulative usage | |
| Oral-related factors | Dental treatment history: tooth extraction, dental implant surgery, periodontal surgery |
| Dental trauma, defective prosthesis | |
| Odontogenic infection | |
| Poor oral hygiene environment | |
| Systemic factors | Gender, age |
| Primary diseases: multiple myeloma, lung cancer, breast cancer, prostate cancer, osteoporosis | |
| Other treatments: chemotherapy | |
| Poor lifestyle: smoking, obesity, malnutrition | |
| Other systemic diseases: diabetes mellitus, hyperparathyroidism, chronic kidney | |
| diseases, vitamin D deficiency, low calcium |
2.2.1. Medication-related factors
The types of medications used to inhibit bone resorption and metastasis in malignant tumors are closely related to the progression of MRONJ. Among all cases of distant metastasis of solid tumors, the bone metastasis rates are 70 %–88.74 % for prostate cancer, 50 %–70 % for breast cancer and 34.56 %–40 % for lung cancer [40], [41], [42], [43]. Different mechanisms of diverse therapeutic medications lead to different incidence rates and onset times. For example, the incidence rate of nitrogen-containing BPs is higher than that of non-nitrogen-containing BPs. Zoledronates are the most potent BPs for inducing MRONJ. The incidence of MRONJ induced by zoledronate is approximately five times greater than that induced by pamidronate or ibandronate [44], [45]. Denosumab, an antibody specifically targeting RANKL, is associated with a higher incidence of MRONJ than bisphosphonates alone, with a recent study reporting an incidence rate of 11.6 %. Additionally, patients treated with denosumab alone exhibited an earlier onset of MRONJ [46]. Drug combinations can also increase the incidence rate. Studies have shown that the combination of BPs and denosumab raised the incidence rate by an additional 0.7 %–4.7 % over the baseline rate [46], [47], [48]. The combination of bisphosphonates and anti-angiogenic agents increased the incidence rate to 16 %, compared to 0.3 %–0.4 % with anti-angiogenic agents alone [49], [50].
Different routes of administration can also lead to different incidence rates of MRONJ. Patients receiving intravenous BPs have a significantly higher incidence rate than those with oral administration. The former is 0.3 %–5% [51], [52], [53], and the latter is (0.1–0.7)/10000 [54], [55], [56]. The latency period in osteoporosis patients taking BPs through oral administration is longer than that in cancer patients receiving intravenous infusion [57].
The duration of medication is also related to MRONJ. The median time to MRONJ occurrence was 17 months to 8.4 years for cancer patients after the usage of bone-modifying agents [46], [58]. For osteoporosis patients, the occurrence time was much longer, with the median of 5.3 years of BP therapy [59]. For cancer patients receiving intravenous zoledronate for more than 2 years (4 mg/time, once/month), the incidence rate is 3.8 %–18 %, which is significantly higher than that of patients treated within 2 years (1.6 %–4%). For patients receiving denosumab, the incidence rates are 1.9 % (less than 2 years) and 6.9 % (more than 2 years)[60]. The MRONJ recurrence rate after treatment is also related to the duration of medication use. The use of zoledronates over 18 months increases the risk of postoperative recurrence [61]. Furthermore, concurrent treatment with chemotherapy medications or corticosteroids and the combined use of corticosteroids and bone-modifying agents also increase the risk of MRONJ [62], [63], [64], [65].
2.2.2. Local factors
Dental surgery is the most common risk factor for MRONJ. In MRONJ patients, tooth extraction is considered an event of susceptibility, with a rate of 62 %–82 % [62], [66], [67]. In osteoporosis patients receiving bone-modifying agents, the incidence of MRONJ after tooth extraction is approximately 1 % [68], whereas it increases to 25 %–28 % in patients receiving bone-modifying agents for malignant tumors [69], [70]. Notably, prior infection may play a more significant role than extraction itself, as demonstrated by results from animal experiments [31], [32]. Other dental surgeries, such as implant surgery, are also considered risk factors for MRONJ. For example, the incidence rate of MRONJ following implant surgery in osteoporosis patients treated with denosumab is 0.5 % [68]. Peri-implantitis might be associated with the occurrence of the disease [33]. For cancer patients receiving bone-modifying agents, more caution should be taken when performing dental surgeries.
MRONJ can develop in both the maxilla and mandible. The incidence rate in the mandible is 75 %, whereas it is 25 % in the maxilla and 4.5 % in both jaws [62], [66]. Wearing dentures, especially the ill-fitting removable dentures, is related to an increased risk of MRONJ in cancer patients receiving zoledronate [71]. In cancer patients receiving intravenous zoledronate, ibandronate or pamidronate, the risk of MRONJ development is twice that of the control group [72]. Existing infectious oral diseases, such as periapical or periodontal diseases, are also considered risk factors for MRONJ [63], [66]. Half of the cancer patients with MRONJ have existing infectious oral diseases [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73].
2.2.3. Systemic factors
The dose of drugs used for the primary disease, such as malignant tumors and osteoporosis, is an important parameter for assessing the risk of MRONJ. Owing to the larger doses used in malignant tumors, the incidence rate of MRONJ (<5%) is much higher than that in osteoporosis (<0.05 %). Certain types of cancer also affect the risk of MRONJ [72], [73], [74]. For example, increased risk was observed in patients with prostate cancer who were injected with denosumab [74].
In addition to primary disease, age and gender are also risk factors for MRONJ. There are more MRONJ patients in the female population than in the male population, probably due to the higher incidence rates of primary diseases such as breast cancer and osteoporosis. The average age of MRONJ patients is 65.2 years, although some patients are aged 40 years or younger. In patients under 24 years of age who receive bone-modifying agents for benign bone diseases, there is no increasing risk for MRONJ even with prolonged treatment [62], [64], [71], [72], [73]. The presence of other systemic diseases, including hypertension, diabetes and anaemia (haemoglobin < 10 g/dL), may also increase the risk of MRONJ [62], [64].
2.3. Diagnosis
2.3.1. Clinical manifestations
The main clinical manifestations of MRONJ include the following [75]: ① grayish-yellow or grayish-black exposed bone with smooth or irregular edges; ② extraoral or intraoral fistulas; and ③ soft tissues around the rough bone surface that may exhibit redness, swelling, pain, ulcers or detachment. As the disease progresses, a series of typical signs may appear, such as pain in the affected area, numbness, space infection, restricted mouth opening, loosening or loss of teeth in the affected area, and pathological fractures, and may further lead to systemic symptoms. Early diagnosis of MRONJ is rather difficult. Patients often lack obvious and specific clinical manifestations, but some nonspecific symptoms and radiographic changes [76], such as maxillary sinusitis and temporomandibular joint arthritis, may occur.
2.3.2. Radiographic features
Compared with ORNJ lesions, MRONJ lesions have clearer borders on radiographic examination. There are various radiographic features at different stages, including different levels of osteosclerosis. At first, it involves the alveolar ridge and hard palate, but extensive sclerosis can be observed in the late stage. Persistent loss of alveolar ridges also occurs, with disorganized trabecular bone structures. Diffuse lytic destruction of bone and sequestration are also common. Signs such as soft tissue masses and periosteal reactions are relatively rare and are usually associated with concurrent infection.
The latest guidelines from the AAOMS summarize the radiographic features of MRONJ. The features of X-rays include the following: ① Absent or delayed bone remodelling at extraction sites; ② alveolar bone defects and resorption unrelated to periodontal disease; ③ regional sclerosis of the alveolar bone and surrounding bone; ④ blurring and thickening of the periodontal ligament; ⑤ irregular bone destruction, changes in the trabecular pattern or sequestrum formation; and ⑥ Oroantral fistula. Increased radiodensity of cancellous bone on CT is a good indicator for detecting early bone necrosis. Magnetic resonance imaging (MRI) can be utilized to assess bone marrow changes and identify bone changes before the acute stage of osteomyelitis [77].
This consensus suggests that comprehensive preoperative radiographic examination is essential and offers important reference value for assessing the extent of jaw necrosis. The characteristic imaging features and practice recommendations in MRONJ patients are summarized in Table 3.
Table 3.
Usage recommendations of radiographic examinations for MRONJ.
| Examination | Recommendations and characteristics |
|---|---|
| Panoramic radiograph | More structures for diagnosis compared with periapical film. Manifestations include increased density of trabecular bone, incomplete healing of extraction socket, sequestrum formation and thickening of the cortical bone around the inferior alveolar canal or the floor of the maxillary sinus. Can be considered as one of the initial imaging screening options. |
| Spiral CT/CBCT | Typical pathological changes include diffuse osteosclerosis, osteolytic area formation, destruction of cortical bone, periosteal proliferation, fistula formation and incomplete healing of extraction sockets. Generally, the area displayed by imaging exceeds the clinically exposed region. CBCT is superior to spiral CT in terms of observing bone changes, as it provides clearer visualization of trabecular bone and has a lower radiation dose, although it cannot measure CT values. Spiral CT can be used to assess maxillary sinus inflammation and changes in the muscle spaces around the mandibular ramus. |
| MRI | Signal changes in bone marrow: low signal intensity on T1WI and high signal intensity on T2WI. Surrounding soft tissue lesion changes: low signal on T1WI and high signal on T2WI and fat-suppressed images. MRI has an advantage in evaluating early bone marrow changes and the soft tissue changes around the area of bone necrosis. Signal intensity signal decrease in marrow may be observed before clinical symptoms. |
| PET-CT | There is early radionuclide accumulation. After sequestrum formation, there is a characteristic decrease in radionuclide uptake. It is highly sensitive for detecting early-stage disease. |
2.3.3. Histopathological features
The pathology of MRONJ mainly manifests as bone necrosis and osteomyelitis. Large volumes of sequestrum formation are usually observed with skeletal cell disappearance and empty lacunae. Occasionally, osteoclasts are visible on the sequestrum surface. The sequestrum is often surrounded by microbial colonies, inflammatory granulation tissue or squamous epithelium. The bone tissue adjacent to the sequestrum shows obvious manifestations of osteomyelitis without typical necrosis. Osteoclasts and osteoblasts are active, and alkaline blue reversal lines can be observed on the bone surface. Fibrosis and vascular proliferation occur between trabeculae, tissue edema and lymphocyte infiltration. Reactive new bone is formed on the cortical surface. Pathologically, MRONJ lesions can be divided into inflammatory zones, sclerotic zones and reactive bone zones. There is free sequestration in inflammatory zones. Sclerotic zones, which surround inflammatory zones, contain varying degrees of empty lacunae and have poor or absent vascularity. Reactive bone zones are located between sclerotic zones and normal bone tissues, which have active osteogenesis and osteoclastic activity [77]. Histopathological examination is an important reference to support the clinical diagnosis and differentiation of diseases such as ORNJ. Considering that MRONJ patients have poor postoperative wound healing ability, biopsy is generally not recommended. However, if any indication of malignancy is detected during comprehensive clinical and radiographic evaluations, biopsy should be promptly performed.
2.3.4. Diagnostic criteria
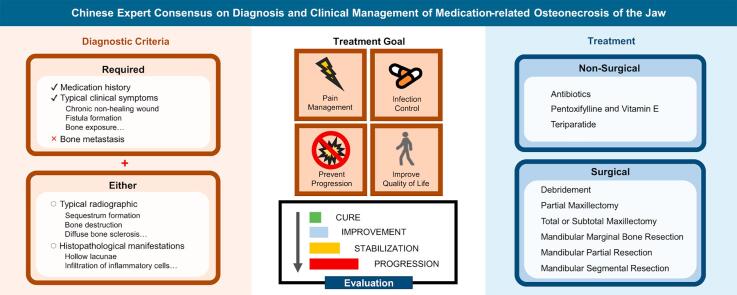
① History of bone-modifying agents, anti-angiogenic agents, targeted drugs or immunomodulators, without a history of head and neck radiotherapy. ② Persistent presence of the following typical clinical symptoms and signs for more than 8 weeks: inflammatory swelling of maxillofacial soft tissue, local purulent discharge, pain, numbness of the lower lip, chronic nonhealing wound, fistula formation, bone exposure, sequestrum formation, restricted mouth opening, etc. ③ No bone metastasis was detected, as confirmed by clinical and radiographic assessments before surgery and histopathological examinations afterward, with biopsy considered if needed. ④ Typical radiographic manifestations of MRONJ: X-ray reveals osteopenia, bone destruction, defects, sequestrum formation, or pathological fractures. CT scans revealed diffuse bone sclerosis, patchy and moth-eaten bone absorption areas, cortical bone destruction, periosteal proliferation or pathological fractures. Inflammatory central areas of the mandible mainly manifest as bone resorption, with or without sequestrum separation, peripheral bone proliferation, increased bone marrow cavity density, unclear mandibular canal image, etc. In the maxilla, sequestrum and soft tissue inflammation within the maxillary sinus are more common. ⑤ Histopathological manifestations: Hollow lacunae and different degrees of fibrosis and inflammatory cell infiltration within the bone marrow cavity of the sequestrum center, around which there are abundant osteoclasts and osteoblasts. Electron microscopy revealed osteocyte shrinkage, organelle disappearance, and chromatin condensation in the cell nucleus as well as collagen fibre dissolution and degeneration in the bone matrix. The diagnosis of MRONJ can be made with criteria ①, ②, and ③, along with either ④ or ⑤.
2.3.5. Differential diagnosis
In clinical practice, it is often necessary to differentiate MRONJ from pyogenic osteomyelitis and ORNJ. Some cases in the mandible also need to be differentiated from jaw metastatic carcinoma. Differential diagnosis can be made on the basis of medical history, imaging, clinical manifestations and histopathological features, with medical history being the key point. Patients with MRONJ all have a clear history of relevant drug usage, so maxillofacial surgeons should ask carefully about their past medical history and medication history. The differential points between MRONJ, pyogenic osteomyelitis of the jaw, ORNJ and jaw metastatic carcinoma are shown in Table 4A, Table 4B.
Table 4A.
Differential diagnosis of pyogenic osteomyelitis of the jaw, ORNJ, MRONJ and metastatic carcinoma.
| Objects | Pyogenic osteomyelitis of the jaw | ORNJ | MRONJ | Metastatic carcinoma |
|---|---|---|---|---|
| Medical history | Odontogenic or injury-inducing infection | Radiotherapy for head and neck cancer | Related medication treated for bone metastasis of malignant cancer, multiple myeloma, rheumatoid diseases, osteoporosis | Malignant cancer in other organs |
| Clinical manifestations | Swelling and congestion in buccal and facial soft tissues | Redness, stiffening and desquamation in skin | Pain and swelling around the lesion | Mucosal ulcer or soft tissue mass |
| Severe throbbing pain | Ulcer and necrosis of the oral mucosa with chronic purulent discharge | Bone exposure, fistula or purulent discharge | Pain | |
| Affected tooth | Possible formation of sequestered bone | Loose or lost teeth within the lesion region | Loose tooth | |
| Fistula formation and purulent discharge | Skin numbness | Lower lip numbness | ||
| Possible formation of sequestered bone | Interstitial infection | Trismus | ||
| Mouth opening limitation | Facial paralysis | |||
| Possible formation of sequestered bone | Temporomandibular symptoms occur in those who have metastasis in temporomandibular joints [78], [79]. | |||
| Pathological fracture | ||||
| Radiographic characteristics | Affected teeth with significant periosteal reaction | Bone resorption and destruction within irradiated region | Different levels of bone sclerosis in early stage, with low density of bone destruction lesion concurrently or subsequently. Sequestrum formation. | Periosteal reaction, bone structure changes and soft tissue lump formation. |
| Diffused bone destruction | CT manifestation can be divided into 3 categories: osteolytic, osteogenic and mixed types. |
Table 4B.
Differential diagnosis of pyogenic osteomyelitis of the jaw, ORNJ, MRONJ and metastatic carcinoma.
| Objects | Pyogenic osteomyelitis of the jaw | ORNJ | MRONJ | Metastatic carcinoma |
|---|---|---|---|---|
| Pathological characteristics | Purulent foci with bone destruction: Moth-eaten pattern of bone resorption | Degeneration and necrosis of the bone: | Osteonecrosis, inflammation and infection: | Consistent with the primary tumor |
| Sequestrum formation | Rough, disappeared or fractured lamellar bone structure | Diffused bone destruction | ||
| Inflammatory granulation tissue around the bone | Absence of osteocytes | Sequestrum formation with alkaline blue reversal lines Inflammatory granulation tissue is formed within trabecular bone | ||
| Infiltration of inflammatory cells | Sequestrum formation | Inflammatory cell infiltration | ||
| Inflammatory cell infiltration | Flora formation | |||
| Significant fibrosis can be seen | ||||
| Infection flora | Staphylococcus aureus, Hemolytic streptococci, Streptococcus pneumoniae, Escherichia coli, Proteus, etc. [17] | Klebsiella, Pseudomonas aeruginosa, Staphylococcus aureus, etc. [80] | Bacteroidetes, Spirochaetes, Synergistetes, Tenericutes, etc. [81] |
2.4. Classification and staging
To facilitate the severity grading of MRONJ, the classic four-stage classification method was proposed by the AAOMS in 2009 [10]. It was revised in 2014 [11] to guide clinical diagnosis and treatment more accurately: Stage 0, no apparent clinical manifestation of osteonecrosis, without specific symptoms or radiographic abnormalities; Stage I, exposed bone or skin fistula that can be probed to bone without apparent infection or other clinical symptoms; Stage II, exposed bone or skin fistula through which can be probed to bone, with local infection or corresponding clinical symptoms; Stage III, exposed necrotic bone or skin fistula that can be probed to bone with symptoms such as pain and infection, accompanied by at least one of the following manifestations: necrotic bone extending beyond the alveolar bone, bone lesion reaching the mandibular inferior border or maxillary sinus, pathological fracture, extraoral skin fistula or intraoral communicating fistula. After the concept of Stage 0 was proposed by the AAOMS in 2009, some organizations subsequently added Stage 0 to the MRONJ clinical guidelines [82], [83], [84]. However, the definition of Stage 0 has been a controversial issue in academia. Therefore, the 2022 update from the AAOMS clearly states that “Stage 0″ refers only to suspected bone necrosis in high-risk MRONJ patients and is not a diagnostic criterion for MRONJ. Therefore, the AAOMS still retains the existing (2014 AAOMS) classification system [11] without any modifications. Currently, the most common staging systems for MRONJ include the AAOMS staging system [12], the Common Terminology Criteria for Adverse Events (CTCAE) 5.0, the ONJ staging system developed by the International Task Force in 2017, and Italian position paper developed by the Italian Societies of Oral Pathology and Medicine and of Maxillofacial Surgery (SIPMO-SICMF) [85]. With increasing reports of various clinical manifestations, radiographic features and treatment modalities of MRONJ, the limitations of the classic AAOMS four-stage grading system have gradually been revealed. The position paper from SIPMO-SICMF adopted a clinical-radiological staging system, which combines both the radiological and clinical manifestations, revealing clearer standards for clinicians.
This expert panel highlights the importance of early surgery for any MRONJ patients if condition permits. Therefore, this panel attached great importance to imaging tests, which manifest the border and range of the lesion. In 2020, He Yue et al. proposed a new clinical staging system for MRONJ (He's classification) based on the basis of the imaging findings, clinical characteristics and surgical treatment experience of 74 MRONJ patients, combined the AAMOS staging system and previous case reports domestically and overseas, and provided corresponding treatment strategies at the same time [86]. This study demonstrated the feasibility and effectiveness of debridement for Stage IA and IIA patients. However, it was not applicable for Stage IB and IIB patients without clear boundaries. The definition of Stage 0 in He’s classification differs from that in AAOMS staging. This clinical staging system accurately and objectively describes the clinical characteristics and severity of MRONJ patients, providing guidance for treatment plans. After discussion with experts, this consensus adopts this type of clinical staging (Table 5).
Table 5.
Classification and treatment strategy for MRONJ.
| Classification and staging | Definitions | Treatment strategy |
|---|---|---|
| Stage 0 | No significant bone abnormalities in radiographic examination. Clinical manifestations include unhealing extraction sockets, loose teeth or bone exposure in the premise of excluding periodontal diseases. | Conservative therapies or local debridement |
| Stage Ⅰ | Radiographic manifestations are as follows: bone abnormalities above mandibular nerve tube or the line connecting two mental holes in the mandible; bone abnormalities not penetrating into maxillary floor or nasal floor in the maxilla | |
| Stage ⅠA | Localized lesion with clear boundaries between sequestrum and normal bone | Debridement |
| Stage ⅠB | Diffused lesion without clear boundaries between sequestrum and normal bone | Mandibular marginal bone resection/partial maxillectomy |
| Stage Ⅱ | Radiographic manifestations are as follows: bone abnormalities extending to mandibular nerve tube or the line connecting two mental holes in the mandible; bone abnormalities penetrating into maxillary floor or nasal floor in the maxilla, but not reaching infraorbital foramen | |
| Stage ⅡA | Localized lesion with clear boundaries between sequestrum and normal bone, without maxillary sinus inflammation in the maxillary lesion | Debridement |
| Stage ⅡB | Diffused lesion without clear boundaries between sequestrum and normal bone, with significant maxillary sinus inflammation in the maxillary lesion | Mandibular segmental resection + reconstructive surgery/ partial maxillectomy + mucosal extirpation |
| Stage Ⅲ | Radiographic manifestations are as follows: bone abnormalities extending to the inferior border of the mandible; bone abnormalities penetrating infraorbital foramen, with or without reaching orbital floor or skull base, including maxillary sinus inflammation or not. | Mandibular segmental resection + reconstructive surgery/ partial, subtotal or total maxillectomy + mucosal extirpation |
2.5. Treatment
2.5.1. Treatment Goals
The objectives of treating MRONJ include relieving pain, managing infections in both soft and hard tissues, preventing lesion enlargement, and collaborating with the treatment of primary diseases such as cancer, ultimately improving patients' quality of life. Once diagnosed with MRONJ, oral maxillofacial doctors need to collaborate with specialists in internal medicine, oncology and infectious diseases to develop a treatment plan together.
2.5.2. Nonsurgical treatment
2.5.2.1. Antibiotics
Systemic antimicrobial therapy for MRONJ can be divided into prophylactic and therapeutic types. Therapeutic treatment mainly targets severe local infection symptoms and systemic infection symptoms. Ideally, antibiotics should specifically target bacteria without side effects and high costs [87]. They should not only have bactericidal effects on residual bacteria but also inhibit the development of mutant bacteria due to prolonged wound exposure. The recommended therapeutic antibiotics for MRONJ patients include penicillin, amoxicillin, metronidazole, quinolones, clindamycin, doxycycline, erythromycin, ciprofloxacin and sitafloxacin [88], [89]. Monotherapy with antibiotics can effectively reduce the microbial population at the lesion site, but polypharmacy may sometimes be necessary [90]. Owing to differences in the infectious microbial flora and individual variations, effective antibiotic selection should be based on bacterial culture, susceptibility test results and local bacterial epidemiology. The oral microbiome also includes fungi, most of which are Candida albicans, in addition to bacteria. Disruption of the oral microbiome balance can lead to secondary fungal infections and exacerbate bone tissue damage. Therefore, combined antifungal therapy may be necessary. Therapeutic antibiotic use should continue until relevant signs and symptoms are relieved or eliminated. The suggestions of antibiotic usage are discussed later in 2.8.3.
2.5.2.2. Hyperbaric oxygen therapy (HBOT)
Hyperbaric oxygen therapy (HBOT) promotes capillary proliferation, increases effective tissue oxygen levels, accelerates collateral circulation formation and enhances leukocyte activity. In recent years, HBOT has been used as an adjuvant therapy for osteomyelitis, ORNJ and other diseases. HBOT provides reactive oxygen and nitrogen by producing substrates for nitric oxide synthase (O2 and L-arginine) and producing superoxide radicals. These reactive oxygen and nitrogen species are signals for osteoclast activation and differentiation [91], [92], [93], [94]. Therefore, HBOT is considered a potential treatment modality for MRONJ [95]. HBOT twice a day at 2 atm for 2 h per session can shorten the MRONJ healing time, alleviate pain and improve quality of life [[96]. However, the use of HBOT still remains controversial. Although 68 % patients improved their symptoms with the average time of 39.7 weeks, only 52 % patients archived complete gingival healing [96]. Doctors should weigh the high costs against the limited therapeutic benefits before recommending this treatment.
2.5.2.3. Pentoxifylline and vitamin E
The combination of pentoxifylline (PEN) and vitamin E (tocopherol, TO) (PENTO) has been used for many years to treat osteoradionecrosis. Given its significant efficacy, some scholars have also explored its use in treating MRONJ. In a case series, it was reported that 74 % in area of bony exposure decreased after treatment [97]. The recommended doses are PEN 400 mg, bid, and TO 400–500 IU, bid [97], [98].
2.5.2.4. Teriparatide
Teriparatide is a synthetic peptide of parathyroid hormone commonly used to treat refractory osteoporosis. In recent years, it has been used to treat MRONJ. It regulates osteoblast and osteoclast differentiation through the Wnt and RANK/RANKL signalling pathways and modulates bone immunity by influencing T cells [20]. Eight weeks of continuous teriparatide application can improve symptoms of bone necrosis and reduce lesion size [21], [99]. In a controlled randomized study, 45.4 % lesions were resolved by 52 weeks, compared with 33.3 % in the placebo group [22].The use of these methods successively for 6 months to one year can lower the clinical stage [100], [101]. The recommended dosage is 20 μg per day for at least 8 weeks through subcutaneous injection, with adjustments based on bone healing conditions [21], [101]. However, teriparatide can have a tumor-promoting effect, so it should be avoided in MRONJ caused by tumor therapy.
2.5.2.5. Ozone and low-level laser therapy
Ozone and laser therapy were both promising adjuvant therapies in the medical field. As a strong oxidizing agent, once after application, ozone can produce multiple biological effects, such as infection control and pain management. There was a controlled randomized clinical trial about the preventive effect after tooth extraction in patients treated with MRONJ-related drugs. After intra-mucosal perialveolar injections of oxygen-ozone mixture, a significant improvement in wound healing was detected both in the inflammatory phase and proliferative phase [102]. In a case series, after 20 application ozone infiltration therapy together with debridement, 81 % lesions archived total remission [103]. However, while case series and animal studies has shown the potential of ozone therapy, there still lacks solid evidence of the positive therapeutic effect of ozone [103], [104].
Low-level laser therapy (LLLT) uses lasers to produce reactive oxygen species (ROS), modulate the inflammatory level within the tissue, inducing microbial death and promoting healing [105]. Different types of laser were used in previous studies, such as diode laser, GaAs diode laser and Nd:YAG laser. Sessions between 2 and 10 were adapted with the frequency of every 2 days, weekly or monthly [106]. LLLT can alleviate pain, edema and reduce lesion size. Together with antibiotics and surgical therapy, 89 % lesions achieved clinical improvement [107], [108]. However, there still lacks high quality study evaluating the therapeutic effects of LLLT.
2.5.3. Surgical treatment
2.5.3.1. Indications and Contraindications for surgical treatment
This consensus holds the view that surgical treatment should be considered for symptomatic MRONJ patients with sequestrum or bone abnormalities accompanied by recurrent infections. However, surgery should not be performed in patients with the following conditions: ① unstable cancerous bone metastasis and ② complicated systemic diseases for which anaesthesia is not feasible.
2.5.3.2. Debridement
For patients with clear boundaries between the lesions and surrounding bone tissues or with free sequestration from normal bone tissues by mucosal soft tissues, removing the sequestrum only is sufficient for surgical intervention. Debridement precautions include removal of abnormal bone and soft tissues as well as tight suturing of soft tissues or packing with iodophor gauze strips. Platelet concentrate products can be used concurrently during surgery to improve the efficacy of surgery (80 %–100 %) [109], [110], [111], [112], [113]. Debridement is suitable for patients classified as Stage IA or IIA according to He's classification.
2.5.3.3. Partial maxillectomy
Partial maxillectomy is necessary for cases in which the maxilla has no clear boundary between abnormal and normal bone tissue because the lesion cannot be removed by simple debridement. MRONJ in the posterior maxillary area often leads to maxillary sinusitis due to lesion penetration into the maxillary sinus floor [114]. For patients with maxillary sinusitis, mucosal extirpation can be performed concurrently to reduce local inflammation, promote wound healing and prevent lesion progression. For patients without maxillary sinusitis, partial maxillectomy alone is sufficient. Partial maxillectomy is suitable for patients classified as stage IB or IIB, and some patients classified as stage III according to He's classification are selected.
2.5.3.4. Total or subtotal maxillectomy
For patients with diffuse maxillary sequestration accompanied by significant maxillary sinusitis or maxillary bone changes penetrating the infraorbital foramen, orbital floor or skull base, subtotal or total maxillectomy may be chosen on the basis of the extent of the lesion. For stage III maxillary MRONJ patients, due to the unique anatomical morphology of the maxilla and the possible involvement of the zygomatic bone, maxillary sinus and nasal cavity, complete lesion resection may cause facial collapse and seriously affect quality of life. Local pedicled soft tissue flaps, free vascularized flaps and prostheses can be used to repair defects and improve oral-nasal fistula, speech intelligibility and aesthetic outcomes [115], [116]. Subtotal or total maxillectomy is suitable for patients classified as Stage III according to He's classification.
2.5.3.5. Mandibular marginal bone resection and partial resection
For patients without clear boundaries between abnormal and normal bone tissues in the mandible, marginal resection or partial resection is needed. In addition to removing the lesion, a portion of healthy bone is scraped off until fresh blood is observed. The following points should be noted: ① Tension-free tight sutures of the wound should be used. ② Every effort was made to protect the inferior alveolar neurovascular bundle. Mandibular marginal bone resection and partial resection are suitable for patients classified as stage IB according to He’s classification.
2.5.3.6. Mandibular segmental resection
For patients with lesions extending beyond the inferior alveolar nerve canal, mandibular segmental resection is preferable to partial mandibular resection. For patients who are in poor condition and not suitable for bone or soft tissue flap reconstruction, a simple option of bone resection is followed by the use of a titanium plate to connect the ends [117]. For patients in relatively good condition, reconstructive surgery may be performed. Vascularized bone tissue flaps are preferred for mandibular reconstruction because they not only restore the shape of the mandible but also offer the possibility for subsequent dental restoration. For patients who are not suitable for bone flap repair, soft tissue flap repair may be performed. If there is a deficiency in soft tissue volume, a combination of a genioglossus flap or submandibular gland transposition may serve as a supplement [118]. For complex defects with significant deficiencies in both soft and hard tissues, a combined approach involving both a vascularized bone tissue flap and a soft tissue flap may be performed when a single flap is insufficient. Mandibular segmental resection with or without reconstruction is applicable to patients classified as Stage IIB or III according to He’s classification.
2.5.3.7. Determination of surgical resection margins
Determining the surgical resection margins during surgery has always been a challenge in the treatment of MRONJ. Incomplete removal of lesions may lead to aggravation or recurrence [119]. Although radiographic examinations can reveal the extent of the lesion, the actual condition is often more severe than indicated by imaging studies [120]. For patients with localized lesions, the boundary can usually be determined after removing the necrotic bone. For patients with diffuse lesions, the boundary of normal bone needs to be judged on the basis of the blood supply.
2.6. Multidisciplinary comprehensive treatment
MRONJ patients often have complex systemic diseases such as malignancies, so a multidisciplinary comprehensive treatment plan should be proposed during treatment. In 2019, the Multinational Association of Supportive Care in Cancer (MASCC), the International Society of Oral Oncology (ISOO) and the American Society of Clinical Oncology (ASCO) developed the “MASCC/ISOO/ASCO Clinical Practice Guidelines for Medication-Related Osteonecrosis of the Jaw”, which emphasizes the necessity of multidisciplinary treatment (MDT) during the management of MRONJ. The guidelines describe the MDT approach for MRONJ. When cancer patients visit general hospitals, oncologists and oral maxillofacial surgeons can hold consultations, communicate about the patient's condition and develop treatment plans collaboratively [8], [121]. This consensus further proposes recommendations on the basis of the previous guidelines. For cancer patients with MRONJ, an MDT clinical management system, including oncologists, oral maxillofacial surgeons, nutritionists, infectious disease specialists, radiologists and rehabilitation physicians, should be established. To decrease the risk of MRONJ development, oncologists should contact oral maxillofacial surgeons to conduct comprehensive oral examinations and treatments before prescribing medication. For cancer patients diagnosed with MRONJ, oral maxillofacial surgeons should communicate with oncologists to understand the patient's tumor progression, overall health status and medication history. Moreover, oncologists should assess the necessity of continuing medication. Finally, physicians from various departments within the system should collaborate to develop treatment plans and rehabilitation protocols to achieve optimal treatment outcomes.
The optimal duration of drug holiday around surgery has been a longstanding concern for multidisciplinary physicians. This consensus suggests that relevant medication should be discontinued for at least one month after the confirmation of well-controlled systemic diseases in consultation with specialties and ensuring all blood test indicators are within normal ranges before the operation.
2.7. Evaluation of therapeutic effects
After carefully discussion between previous studies, local conditions and expert experiences, the consensus have decided the evaluation schedule and standard [85]. Patients with MRONJ should all undergo regular follow-up after surgery, with evaluations every 3 months within the first year and every 6 months thereafter. This consensus suggests the use of “cure”, “improvement”, “stabilization” or “progression” to evaluate therapeutic effects. Preliminary evaluation of therapeutic effects should be conducted three months after surgery, followed by a stability assessment one year after surgery (Table 6) [8].
Table 6.
Terms used to evaluate the therapeutic effect of MRONJ.
| Effect | Wound healing | Pain | Infection | Radiographic manifestation |
|---|---|---|---|---|
| Cured | Complete healing | No pain | No inflammation or infection | Normal trabecular bone structure without sequestrum |
| Improved | Significant improvement | Significant alleviation | No inflammation or infection | Improved trabecular bone structure without sequestrum |
| Stable | Mild improvement | Mild alleviation | Mild inflammation or infection | No improvement in trabecular bone structure without sequestrum |
| Progression | Deterioration or no improvement | Deterioration or no improvement | No improvement | Decreasing trabecular bone with sequestrum or osteolytic changes |
2.8. Prevention
2.8.1. Oral maintenance and prevention before the use of related medications
Dentists should conduct a thorough oral check-up and provide any necessary treatment before starting medication therapy. By using clinical and imaging examinations, the conditions of the periodontium, dental pulp, periapical region, denture wear and presence of soft tissue inflammation should be assessed. Caries should be filled, and teeth that cannot be retained should be extracted. Periodontal treatment, sharp bone spur trimming and denture adjustment should also be performed. Additionally, dental professionals should assess patients’ enthusiasm for oral prevention and their knowledge of oral care, providing guidance on effective oral hygiene practices such as proper tooth brushing as well as flossing and mouthwash use [122].
2.8.2. Oral maintenance and prevention during medication usage
After initiating MRONJ-related medications, patients should maintain good oral hygiene habits and undergo regular follow-up to detect and treat oral diseases as early as possible, thereby preventing the occurrence of MRONJ. Although clinicians should carefully consider invasive surgeries such as tooth extraction, currently, there is no evidence to suggest that discontinuing medication therapy will alter the risk of MRONJ after tooth extraction. Therefore, a history of MRONJ-related medication usage is not an absolute contraindication for tooth extraction. Physicians need to communicate with patients fully about the risks and benefits of surgery and decide whether to suspend medication use [123].
2.8.3. Prophylactic use of antibiotics
Prophylactic antibiotic use is primarily aimed at patients undergoing MRONJ surgical treatment to prevent postoperative wound infections and ensure normal wound healing. The recommended antibiotics include cephalosporins or amoxicillin/sulbactam combined with oral metronidazole. Azithromycin can be used as an alternative for those allergic to cephalosporins [124], [125]. Prophylactic antibiotic use in the perioperative period of tooth extraction significantly reduces the occurrence of MRONJ in patients receiving BP treatment [126]. Bacteria cultured from infected soft tissues around MRONJ lesions and treated with sensitive antibiotics can prevent MRONJ recurrence and reduce the number of surgical interventions [127].
3. Conclusion
This consensus gathers the opinions of experts from 11 medical colleges and affiliated hospitals in China. After discussions, consensus has been reached on seven aspects of MRONJ, including its history and definition, etiology and risk factors, diagnosis, classification and staging, treatment, and evaluation of therapeutic effects and prevention. It is hoped that these viewpoints can be widely used in clinical practice and guide clinicians during treatment. As research progresses, the understanding of MRONJ will become deeper, and new treatment methods will be widely applied. This consensus will also be revised in a timely manner to effectively guide clinicians in standardized diagnosis and treatment.
CRediT authorship contribution statement
Han-Jin Ruan: Writing – original draft, Visualization. Heng Chen: Writing – original draft, Investigation. Jin-Song Hou: Writing – review & editing. Jin-Gang An: Writing – review & editing. Yu-Xing Guo: Writing – review & editing. Bing Liu: Writing – review & editing. Lei Tian: Writing – review & editing. Jian Pan: Writing – review & editing. Jin-Song Li: Writing – review & editing. Can-Hua Jiang: Writing – review & editing. Zhen Tian: Writing – review & editing. Jie Xu: Writing – review & editing. Ling Zhu: Writing – review & editing. Chang-Fu Sun: Writing – review & editing. Ke-Qian Zhi: Writing – review & editing. Qing Qu: Writing – review & editing. Chun-Lin Zong: Writing – review & editing. Meng-Yu Li: Writing – review & editing. Zhi-Yuan Zhang: Writing – review & editing. Yue He: Writing – review & editing, Supervision, Conceptualization.
Funding
This work was funded by Shanghai Science and Technology Commission Science and Technology Innovation Action Plan, Grand/Award Number: 21Y11903700.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by Shanghai Science and Technology Commission Science and Technology Innovation Action Plan, Grand/Award Number: 21Y11903700. This research did not receive any other specific grant from funding agencies in the commercial or not-for-profit sectors.
Contributor Information
Han-Jin Ruan, Email: ruanhj_cn@outlook.com.
Heng Chen, Email: ivychann@126.com.
Jin-Song Hou, Email: houjs@mail.sysu.edu.cn.
Jin-Gang An, Email: anjingang@126.com.
Yu-Xing Guo, Email: gladiater1984@163.com.
Bing Liu, Email: liubing9909@whu.edu.cn.
Lei Tian, Email: tianleison@163.com.
Jian Pan, Email: jianpancn@scu.edu.cn.
Jin-Song Li, Email: lijinsong1967@163.com.
Can-Hua Jiang, Email: canhuaj@csu.edu.cn.
Zhen Tian, Email: tian0304_cn@163.com.
Jie Xu, Email: xujie@shsmu.edu.cn.
Ling Zhu, Email: puxuke12@126.com.
Chang-Fu Sun, Email: changfusun@hotmail.com.
Ke-Qian Zhi, Email: zhikeqian@sina.com.
Qing Qu, Email: qq11478@rjh.com.cn.
Chun-Lin Zong, Email: mujiezi23@163.com.
Meng-Yu Li, Email: mengyuli0463@163.com.
Zhi-Yuan Zhang, Email: zhzhy0502@163.com.
Yue He, Email: william5218@126.com.
References
- 1.Lu Z., Chen Y., Liu D., Jiao X., Liu C., Wang Y., Zhang Z., Jia K., Gong J., Yang Z., Shen L. The landscape of cancer research and cancer care in China. Nat. Med. 2023;29:3022–3032. doi: 10.1038/s41591-023-02655-3. [DOI] [PubMed] [Google Scholar]
- 2.Hiensch A.E., Depenbusch J., Schmidt M.E., Monninkhof E.M., Pelaez M., Clauss D., Gunasekara N., Zimmer P., Belloso J., Trevaskis M., Rundqvist H., Wiskemann J., Müller J., Sweegers M.G., Fremd C., Altena R., Gorecki M., Bijlsma R., van Leeuwen-Snoeks L., Huinink D. ten B., Sonke G., Lahuerta A., Mann G.B., Francis P.A., Richardson G., Malter W., van der Wall E., Aaronson N.K., Senkus E., Urruticoechea A., Zopf E.M., Bloch W., Stuiver M.M., Wengstrom Y., Steindorf K., May A.M. Supervised, structured and individualized exercise in metastatic breast cancer: a randomized controlled trial. Nat. Med. 2024;30:2957. doi: 10.1038/s41591-024-03143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez R.K., Wade S.W., Reich A., Pirolli M., Liede A., Lyman G.H. Incidence of bone metastases in patients with solid tumors: analysis of oncology electronic medical records in the United States. BMC Cancer. 2018;18:44. doi: 10.1186/s12885-017-3922-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reid I.R., McClung M.R. Osteopenia: a key target for fracture prevention. Lancet Diabetes Endocrinol. 2024;12:856–864. doi: 10.1016/S2213-8587(24)00225-0. [DOI] [PubMed] [Google Scholar]
- 5.Zhu N., Ni H., Guo S., Shen Y.-Q., Chen Q. Bone complications of cancer treatment. Cancer Treat. Rev. 2024;130 doi: 10.1016/j.ctrv.2024.102828. [DOI] [PubMed] [Google Scholar]
- 6.Tenore G., Mohsen A., Rossi A.F., Palaia G., Rocchetti F., Cassoni A., Valentini V., Ottolenghi L., Polimeni A., Romeo U. Does Medication-Related Osteonecrosis of the Jaw Influence the Quality of Life of Cancer Patients? Biomedicines. 2020;8:95. doi: 10.3390/biomedicines8040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J.J., He Y. Research progress on treatment of medication-related osteonecrosis of the jaw. China Journal of Oral and Maxillofacial Surgery. 2020;18:474–477. [Google Scholar]
- 8.Yarom N., Shapiro C.L., Peterson D.E., Van Poznak C.H., Bohlke K., Ruggiero S.L., Migliorati C.A., Khan A., Morrison A., Anderson H., Murphy B.A., Alston-Johnson D., Mendes R.A., Beadle B.M., Jensen S.B., Saunders D.P. Medication-Related Osteonecrosis of the Jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. JCO. 2019;37:2270–2290. doi: 10.1200/JCO.19.01186. [DOI] [PubMed] [Google Scholar]
- 9.Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws, American Association of Oral and Maxillofacial Surgeons, American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws, J Oral Maxillofac Surg 65 (2007) 369–376. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed]
- 10.Ruggiero S.L., Dodson T.B., Assael L.A., Landesberg R., Marx R.E., Mehrotra B. American Association of Oral and Maxillofacial Surgeons, American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J. Oral Maxillofac. Surg. 2009;67:2–12. doi: 10.1016/j.joms.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., O’Ryan F. American Association of Oral and Maxillofacial Surgeons, American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J. Oral Maxillofac. Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Ruggiero S.L., Dodson T.B., Aghaloo T., Carlson E.R., Ward B.B., Kademani D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022;80:920–943. doi: 10.1016/j.joms.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Marx R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J. Oral Maxillofac. Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 14.Khan A.A., Sándor G.K., Dore E., Morrison A.D., Alsahli M., Amin F., Peters E., Hanley D.A., Chaudry S.R., Dempster D.W., Glorieux F.H., Neville A.J., Talwar R.M., Clokie C.M., Al Mardini M., Paul T., Khosla S., Josse R.G., Sutherland S., Lam D.K., Carmichael R.P., Blanas N., Kendler D., Petak S., St-Marie L.G., Brown J., Evans A.W., Rios L., Compston J.E. Canadian Association of Oral and Maxillofacial Surgeons, Canadian consensus practice guidelines for bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2008;35:1391–1397. [PubMed] [Google Scholar]
- 15.Raje N., Terpos E., Willenbacher W., Shimizu K., García-Sanz R., Durie B., Legieć W., Krejčí M., Laribi K., Zhu L., Cheng P., Warner D., Roodman G.D. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 16.Bone H.G., Wagman R.B., Brandi M.L., Brown J.P., Chapurlat R., Cummings S.R., Czerwiński E., Fahrleitner-Pammer A., Kendler D.L., Lippuner K., Reginster J.-Y., Roux C., Malouf J., Bradley M.N., Daizadeh N.S., Wang A., Dakin P., Pannacciulli N., Dempster D.W., Papapoulos S. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 17.Z. Zhang, Oral and Maxillofacial Surgery, in: People’s Medical Publishing House, 2020: p. 130.
- 18.Brown J.E., Coleman R.E. Denosumab in patients with cancer-a surgical strike against the osteoclast. Nat. Rev. Clin. Oncol. 2012;9:110–118. doi: 10.1038/nrclinonc.2011.197. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein R.S., Roberson P.K., Manolagas S.C. Giant osteoclast formation and long-term oral bisphosphonate therapy. N. Engl. J. Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T., Wang Y., Hao Z., Hu Y., Li J. Parathyroid hormone and its related peptides in bone metabolism. Biochem. Pharmacol. 2021;192 doi: 10.1016/j.bcp.2021.114669. [DOI] [PubMed] [Google Scholar]
- 21.Cheung A., Seeman E. Teriparatide therapy for alendronate-associated osteonecrosis of the jaw. N. Engl. J. Med. 2010;363:2473–2474. doi: 10.1056/NEJMc1002684. [DOI] [PubMed] [Google Scholar]
- 22.Sim I.W., Borromeo G.L., Tsao C., Hardiman R., Hofman M.S., Papatziamos Hjelle C., Siddique M., Cook G.J.R., Seymour J.F., Ebeling P.R. Teriparatide Promotes Bone Healing in Medication-Related Osteonecrosis of the Jaw: A Placebo-Controlled, Randomized Trial. J. Clin. Oncol. 2020;38:2971–2980. doi: 10.1200/JCO.19.02192. [DOI] [PubMed] [Google Scholar]
- 23.Dang A.T., Ono M., Wang Z., Tosa I., Hara E.S., Mikai A., Kitagawa W., Yonezawa T., Kuboki T., Oohashi T. Local E-rhBMP-2/β-TCP Application Rescues Osteocyte Dendritic Integrity and Reduces Microstructural Damage in Alveolar Bone Post-Extraction in MRONJ-like Mouse Model. International Journal of Molecular Sciences. 2024;25:6648. doi: 10.3390/ijms25126648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung J., Yoo H.Y., Kim G.T., Lee J.W., Lee Y.A., Kim D.Y., Kwon Y.D. Short-Term Teriparatide and Recombinant Human Bone Morphogenetic Protein-2 for Regenerative Approach to Medication-Related Osteonecrosis of the Jaw: A Preliminary Study. J. Bone Miner. Res. 2017;32:2445–2452. doi: 10.1002/jbmr.3237. [DOI] [PubMed] [Google Scholar]
- 25.Sanna G., Zampino M.G., Pelosi G., Nolè F., Goldhirsch A. Jaw avascular bone necrosis associated with long-term use of biphosphonates. Ann. Oncol. 2005;16:1207–1208. doi: 10.1093/annonc/mdi206. [DOI] [PubMed] [Google Scholar]
- 26.Salhotra A., Shah H.N., Levi B., Longaker M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020;21:696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.X. Shen, W. Zhu, P. Zhang, Y. Fu, J. Cheng, L. Liu, R. Xu, H. Jiang, Macrophage miR-149-5p induction is a key driver and therapeutic target for BRONJ, JCI Insight 7 (n.d.) e159865. doi: 10.1172/jci.insight.159865. [DOI] [PMC free article] [PubMed]
- 28.Allegra A., Innao V., Pulvirenti N., Musolino C. Antiresorptive Agents and Anti-Angiogenesis Drugs in the Development of Osteonecrosis of the Jaw. Tohoku J. Exp. Med. 2019;248:27–29. doi: 10.1620/tjem.248.27. [DOI] [PubMed] [Google Scholar]
- 29.Kalyan S., Quabius E.S., Wiltfang J., Mönig H., Kabelitz D. Can peripheral blood γδ T cells predict osteonecrosis of the jaw? An Immunological Perspective on the Adverse Drug Effects of Aminobisphosphonate Therapy. J Bone Miner Res. 2013;28:728–735. doi: 10.1002/jbmr.1769. [DOI] [PubMed] [Google Scholar]
- 30.Silveira F.M., Etges A., Correa M.B., Vasconcelos A.C.U. Microscopic Evaluation of the Effect of Oral Microbiota on the Development of Bisphosphonate-Related Osteonecrosis of the Jaws in Rats. J Oral Maxillofac Res. 2016;7:e3. doi: 10.5037/jomr.2016.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soundia A., Hadaya D., Esfandi N., Gkouveris I., Christensen R., Dry S.M., Bezouglaia O., Pirih F., Nikitakis N., Aghaloo T., Tetradis S. Zoledronate Impairs Socket Healing after Extraction of Teeth with Experimental Periodontitis. J. Dent. Res. 2018;97:312–320. doi: 10.1177/0022034517732770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadaya D., Soundia A., Gkouveris I., Dry S.M., Aghaloo T.L., Tetradis S. Development of Medication-Related Osteonecrosis of the Jaw After Extraction of Teeth With Experimental Periapical Disease. J. Oral Maxillofac. Surg. 2019;77:71–86. doi: 10.1016/j.joms.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troeltzsch M., Cagna D., Stähler P., Probst F., Kaeppler G., Troeltzsch M., Ehrenfeld M., Otto S. Clinical features of peri-implant medication-related osteonecrosis of the jaw: Is there an association to peri-implantitis? J Craniomaxillofac Surg. 2016;44:1945–1951. doi: 10.1016/j.jcms.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Vandone A.M., Donadio M., Mozzati M., Ardine M., Polimeni M.A., Beatrice S., Ciuffreda L., Scoletta M. Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: a single-center clinical experience. Ann. Oncol. 2012;23:193–200. doi: 10.1093/annonc/mdr039. [DOI] [PubMed] [Google Scholar]
- 35.Cerrato A., Zanette G., Boccuto M., Angelini A., Valente M., Bacci C. Actinomyces and MRONJ: A retrospective study and a literature review. J Stomatol Oral Maxillofac Surg. 2021;122:499–504. doi: 10.1016/j.jormas.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Williams D.W., Vuong H.E., Kim S., Lenon A., Ho K., Hsiao E.Y., Sung E.C., Kim R.H. Indigenous microbiota protects against inflammation-induced osteonecrosis. J. Dent. Res. 2020;99:676–684. doi: 10.1177/0022034520908594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peer A., Khamaisi M. Diabetes as a risk factor for medication-related osteonecrosis of the jaw. J. Dent. Res. 2015;94:252–260. doi: 10.1177/0022034514560768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu X., Wang Z., Zhou T., Shan L. Determination of the molecular mechanism by which macrophages and γδ-T cells contribute to ZOL-induced ONJ. Aging (Albany NY) 2020;12:20743–20752. doi: 10.18632/aging.104006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W., Gao L., Ren W., Li S., Zheng J., Li S., Jiang C., Yang S., Zhi K. The Role of the Immune Response in the Development of Medication-Related Osteonecrosis of the Jaw. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.606043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang W., Rixiati Y., Zhao B., Li Y., Tang C., Liu J. Incidence, prevalence, and outcomes of systemic malignancy with bone metastases. J. Orthop. Surg. (Hong Kong) 2020;28 doi: 10.1177/2309499020915989. [DOI] [PubMed] [Google Scholar]
- 41.Coleman R.E., Croucher P.I., Padhani A.R., Clézardin P., Chow E., Fallon M., Guise T., Colangeli S., Capanna R., Costa L. Bone metastases. Nat. Rev. Dis. Primers. 2020;6:83. doi: 10.1038/s41572-020-00216-3. [DOI] [PubMed] [Google Scholar]
- 42.Fornetti J., Welm A.L., Stewart S.A. Understanding the Bone in Cancer Metastasis. J. Bone Miner. Res. 2018;33:2099–2113. doi: 10.1002/jbmr.3618. [DOI] [PubMed] [Google Scholar]
- 43.Huang J.F., Shen J., Li X., Rengan R., Silvestris N., Wang M., Derosa L., Zheng X., Belli A., Zhang X.L., Li Y.M., Wu A. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: a large population-based study. Ann Transl Med. 2020;8:482. doi: 10.21037/atm.2020.03.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelaz A., Junquera L., Gallego L., García-Consuegra L., García-Martínez L., Cutilli T., Olay S. Epidemiology, pharmacology and clinical characterization of bisphosphonate-related osteonecrosis of the jaw. A retrospective study of 70 cases. Acta Otorrinolaringol. Esp. 2015;66:139–147. doi: 10.1016/j.otorri.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi O., Kokuryo S., Tsurushima H., Tanaka J., Habu M., Uehara M., Nishihara T., Tominaga K. Lipopolysaccharide aggravates bisphosphonate-induced osteonecrosis in rats. Int. J. Oral Maxillofac. Surg. 2015;44:528–534. doi: 10.1016/j.ijom.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Brunner C., Arvandi M., Marth C., Egle D., Baumgart F., Emmelheinz M., Walch B., Lercher J., Iannetti C., Wöll E., Pechlaner A., Zabernigg A., Volgger B., Castellan M., Andraschofsky O.T., Markl A., Hubalek M., Schnallinger M., Puntscher S., Siebert U., Schönherr S., Forer L., Bruckmoser E., Laimer J. Incidence of Medication-Related Osteonecrosis of the Jaw in Patients With Breast Cancer During a 20-Year Follow-Up: A Population-Based Multicenter Retrospective Study. J. Clin. Oncol. 2024:JCO2400171. doi: 10.1200/JCO.24.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boquete-Castro A., Gómez-Moreno G., Calvo-Guirado J.L., Aguilar-Salvatierra A., Delgado-Ruiz R.A. Denosumab and osteonecrosis of the jaw. A Systematic Analysis of Events Reported in Clinical Trials. Clin Oral Implants Res. 2016;27:367–375. doi: 10.1111/clr.12556. [DOI] [PubMed] [Google Scholar]
- 48.Hernandez R.K., Quigley J., Pirolli M., Quach D., Chen K.S., Arellano J., Liede A. Patients with bone metastases from solid tumors initiating treatment with a bone-targeted agent in 2011: a descriptive analysis using oncology clinic data in the US. Support. Care Cancer. 2014;22:2697–2705. doi: 10.1007/s00520-014-2251-y. [DOI] [PubMed] [Google Scholar]
- 49.Christodoulou C., Pervena A., Klouvas G., Galani E., Falagas M.E., Tsakalos G., Visvikis A., Nikolakopoulou A., Acholos V., Karapanagiotidis G., Batziou E., Skarlos D.V. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 50.Guarneri V., Miles D., Robert N., Diéras V., Glaspy J., Smith I., Thomssen C., Biganzoli L., Taran T., Conte P. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res. Treat. 2010;122:181–188. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 51.Coleman R., Woodward E., Brown J., Cameron D., Bell R., Dodwell D., Keane M., Gil M., Davies C., Burkinshaw R., Houston S.J., Grieve R.J., Barrett-Lee P.J., Thorpe H. Safety of zoledronic acid and incidence of osteonecrosis of the jaw (ONJ) during adjuvant therapy in a randomised phase III trial (AZURE: BIG 01–04) for women with stage II/III breast cancer. Breast cancer research and treatment. 2011;127:429–438. doi: 10.1007/s10549-011-1429-y. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Olivo M.A., Shah N.A., Pratt G., Risser J.M., Symanski E., Suarez-Almazor M.E. Bisphosphonates in the treatment of patients with lung cancer and metastatic bone disease: a systematic review and meta-analysis. Supportive Care Cancer. 2012;20:2985–2998. doi: 10.1007/s00520-012-1563-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mauri D, Valachis A, Polyzos I.P, Polyzos N.P., Kamposioras K, Pesce L.L. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat. 2009;116:433–439. doi: 10.1007/s10549-009-0432-z. [DOI] [PubMed] [Google Scholar]
- 54.Nh B.T., B M., H H., O Z., Ju P. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2022;7 doi: 10.1002/14651858.CD012432.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beth-Tasdogan N.H., Mayer B., Hussein H., Zolk O. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2017;10:CD012432. doi: 10.1002/14651858.CD012432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grbic J.T., Black D.M., Lyles K.W., Reid D.M., Orwoll E., McClung M., Bucci-Rechtweg C., Su G. The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J. Am. Dent. Assoc. 2010;141:1365–1370. doi: 10.14219/jada.archive.2010.0082. [DOI] [PubMed] [Google Scholar]
- 57.Feng Z., An J., He Y., Zhang Y. A comparative study of the clinical characteristics of patients with medication-related osteonecrosis of the jaw and osteoporosis or malignancy, Oral Surg Oral Med Oral Pathol. Oral Radiol. 2022;134:543–547. doi: 10.1016/j.oooo.2022.04.049. [DOI] [PubMed] [Google Scholar]
- 58.Hata H., Imamachi K., Ueda M., Matsuzaka M., Hiraga H., Osanai T., Harabayashi T., Fujimoto K., Oizumi S., Takahashi M., Yoshikawa K., Sato J., Yamazaki Y., Kitagawa Y. Prognosis by cancer type and incidence of zoledronic acid-related osteonecrosis of the jaw: a single-center retrospective study. Support. Care Cancer. 2022;30:4505–4514. doi: 10.1007/s00520-022-06839-4. [DOI] [PubMed] [Google Scholar]
- 59.Fung P., Bedogni G., Bedogni A., Petrie A., Porter S., Campisi G., Bagan J., Fusco V., Saia G., Acham S., Musto P., Petrucci M.T., Diz P., Colella G., Mignogna M.D., Pentenero M., Arduino P., Lodi G., Maiorana C., Manfredi M., Hallberg P., Wadelius M., Takaoka K., Leung Y.Y., Bonacina R., Schiødt M., Lakatos P., Taylor T., De Riu G., Favini G., Rogers S.N., Pirmohamed M., Nicoletti P. GENVABO Consortium, S. Fedele, Time to Onset of Bisphosphonate-Related Osteonecrosis of the Jaws: a Multicentre Retrospective Cohort Study. Oral Dis. 2017;23:477–483. doi: 10.1111/odi.12632. [DOI] [PubMed] [Google Scholar]
- 60.Ng T.L., Tu M.M., Ibrahim M.F.K., Basulaiman B., McGee S.F., Srikanthan A., Fernandes R., Vandermeer L., Stober C., Sienkiewicz M., Jeong A., Saunders D., Awan A.A., Hutton B., Clemons M.J. Long-term impact of bone-modifying agents for the treatment of bone metastases: a systematic review. Support Care Cancer. 2021;29:925–943. doi: 10.1007/s00520-020-05556-0. [DOI] [PubMed] [Google Scholar]
- 61.Chen S., Ren H., He Y., An J., Zhang Y. Recurrence-Related Factors of Medication-Related Osteonecrosis of the Jaw: A Five-Year Experience. J Oral Maxillofac Surg. 2021;79:2472–2481. doi: 10.1016/j.joms.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 62.Saad F., Brown J.E., Van Poznak C., Ibrahim T., Stemmer S.M., Stopeck A.T., Diel I.J., Takahashi S., Shore N., Henry D.H., Barrios C.H., Facon T., Senecal F., Fizazi K., Zhou L., Daniels A., Carrière P., Dansey R. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 2012;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 63.McGowan K., McGowan T., Ivanovski S. Risk factors for medication-related osteonecrosis of the jaws: A systematic review. Oral Dis. 2018;24:527–536. doi: 10.1111/odi.12708. [DOI] [PubMed] [Google Scholar]
- 64.Tsao C., Darby I., Ebeling P.R., Walsh K., O’Brien-Simpson N., Reynolds E., Borromeo G. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J. Oral Maxillofac. Surg. 2013;71:1360–1366. doi: 10.1016/j.joms.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Feng Z., An J., Zhang Y. Factors Influencing Severity of Medication-Related Osteonecrosis of the Jaw: a Retrospective Study. J. Oral Maxillofac. Surg. 2021;79:1683–1688. doi: 10.1016/j.joms.2020.12.045. [DOI] [PubMed] [Google Scholar]
- 66.Hallmer F., Andersson G., Götrick B., Warfvinge G., Anderud J., Bjørnland T. Prevalence, initiating factor, and treatment outcome of medication-related osteonecrosis of the jaw-a 4-year prospective study, Oral Surg Oral Med Oral Pathol. Oral Radiol. 2018;126:477–485. doi: 10.1016/j.oooo.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Aljohani S., Fliefel R., Ihbe J., Kühnisch J., Ehrenfeld M., Otto S. What is the effect of anti-resorptive drugs (ARDs) on the development of medication-related osteonecrosis of the jaw (MRONJ) in osteoporosis patients: a systematic review. J. Craniomaxillofac. Surg. 2017;45:1493–1502. doi: 10.1016/j.jcms.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 68.Watts N.B., Grbic J.T., Binkley N., Papapoulos S., Butler P.W., Yin X., Tierney A., Wagman R.B., McClung M. Invasive Oral Procedures and Events in Postmenopausal Women With Osteoporosis Treated With Denosumab for Up to 10 Years. J. Clin. Endocrinol. Metab. 2019;104:2443–2452. doi: 10.1210/jc.2018-01965. [DOI] [PubMed] [Google Scholar]
- 69.Avishai G., Muchnik D., Masri D., Zlotogorski-Hurvitz A., Chaushu L. Minimizing MRONJ after Tooth Extraction in Cancer Patients Receiving Bone-Modifying Agents. J. Clin. Med. 2022;11:1807. doi: 10.3390/jcm11071807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasegawa T., Hayashida S., Kondo E., Takeda Y., Miyamoto H., Kawaoka Y., Ueda N., Iwata E., Nakahara H., Kobayashi M., Soutome S., Yamada S.I., Tojyo I., Kojima Y., Umeda M., Fujita S., Kurita H., Shibuya Y., Kirita T., Komori T. Japanese Study Group of Co-operative Dentistry with Medicine (JCDM), Medication-related osteonecrosis of the jaw after tooth extraction in cancer patients: a multicenter retrospective study. Osteoporos Int. 2019;30:231–239. doi: 10.1007/s00198-018-4746-8. [DOI] [PubMed] [Google Scholar]
- 71.Kyrgidis A., Vahtsevanos K., Koloutsos G., Andreadis C., Boukovinas I., Teleioudis Z., Patrikidou A., Triaridis S. Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients. J. Clin. Oncol. 2008;26:4634–4638. doi: 10.1200/JCO.2008.16.2768. [DOI] [PubMed] [Google Scholar]
- 72.Vahtsevanos K., Kyrgidis A., Verrou E., Katodritou E., Triaridis S., Andreadis C.G., Boukovinas I., Koloutsos G.E., Teleioudis Z., Kitikidou K., Paraskevopoulos P., Zervas K., Antoniades K. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J. Clin. Oncol. 2009;27:5356–5362. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 73.Yamazaki T., Yamori M., Ishizaki T., Asai K., Goto K., Takahashi K., Nakayama T., Bessho K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: a cohort study. Int. J. Oral Maxillofac. Surg. 2012;41:1397–1403. doi: 10.1016/j.ijom.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 74.Qi W.X., Tang L.N., He A.N., Yao Y., Shen Z. Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int. J. Clin. Oncol. 2014;19:403–410. doi: 10.1007/s10147-013-0561-6. [DOI] [PubMed] [Google Scholar]
- 75.Zhou G.L., Tian L., Shao X.X., Zong C.L., Liu Y.P. Research Progress on Medication-Related Osteonecrosis of the Jaw. Journal of Modern Stomatology. 2019;33:237–242. [Google Scholar]
- 76.Gupta S., Gupta H., Mandhyan D., Srivastava S. Bisphophonates related osteonecrosis of the jaw, Natl. J. Maxillofac. Surg. 2013;4:151–158. doi: 10.4103/0975-5950.127643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arce K., Assael L.A., Weissman J.L., Markiewicz M.R. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J. Oral Maxillofac. Surg. 2009;67:75–84. doi: 10.1016/j.joms.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Owosho A.A., Xu B., Kadempour A., Yom S.K., Randazzo J., Ghossein R.A., Huryn J.M., Estilo C.L. Metastatic solid tumors to the jaw and oral soft tissue: A retrospective clinical analysis of 44 patients from a single institution. J Craniomaxillofac Surg. 2016;44(8):1047–1053. doi: 10.1016/j.jcms.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He H.B., Gong M., Zhang Z.W. Clinical analysis of metastatic carcinoma of the jaws China Continuing. China Continuing Medical Education. 2016;8:104–105. doi: 10.3969/j.issn.1674-9308.2016.34.056. [DOI] [Google Scholar]
- 80.Zhu Y., Liang J., Wang F., Li J., Wang C., Hou J. Bacterial spectrum analysis and antimicrobial susceptibility study of osteoradionecrosis of the jaw in Southern China. Oral Dis. 2022;28:2015–2025. doi: 10.1111/odi.13968. [DOI] [PubMed] [Google Scholar]
- 81.Li Q., Pu Y., Lu H., Zhao N., Wang Y., Guo Y., Guo C., Porphyromonas Treponema, and Mogibacterium promote IL8/IFNγ/TNFα-based pro-inflammation in patients with medication-related osteonecrosis of the jaw. J Oral Microbiol. 2020;13:1851112. doi: 10.1080/20002297.2020.1851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim K.M., Rhee Y., Kwon Y.D., Kwon T.G., Lee J.K., Kim D.Y. Medication Related Osteonecrosis of the Jaw: 2015 Position Statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons. J Bone Metab. 2015;22:151–165. doi: 10.11005/jbm.2015.22.4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Japanese Allied Committee on Osteonecrosis of the Jaw, T. Yoneda, H. Hagino, T. Sugimoto, H. Ohta, S. Takahashi, S. Soen, A. Taguchi, T. Nagata, M. Urade, T. Shibahara, S. Toyosawa, Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw, J Bone Miner Metab 35 (2017) 6–19. doi: 10.1007/s00774-016-0810-7. [DOI] [PubMed]
- 84.Chalem M., Medina A., Sarmiento A.K., Gonzalez D., Olarte C., Pinilla E., Paz J., Casas N., Vega M.P., Diaz E. Therapeutic approach and management algorithms in medication-related osteonecrosis of the jaw (MONJ): recommendations of a multidisciplinary group of experts. Arch. Osteoporos. 2020;15 doi: 10.1007/s11657-020-00761-0. [DOI] [PubMed] [Google Scholar]
- 85.Bedogni A., Mauceri R., Fusco V., Bertoldo F., Bettini G., Di Fede O., Lo Casto A., Marchetti C., Panzarella V., Saia G., Vescovi P., Campisi G. Italian position paper (SIPMO-SICMF) on medication-related osteonecrosis of the jaw (MRONJ) Oral Dis. 2024;30:3679–3709. doi: 10.1111/odi.14887. [DOI] [PubMed] [Google Scholar]
- 86.Liu Z.L., Jiang J.J., Li X.G., Zhu F.S., Tang X., Zhu L., He Y. A New Clinical Staging and Treatment Strategy for Medication-Related Osteonecrosis of the Jaw, Chinese. J. Oral Maxillofac. Surg. 2020;18:501–507. [Google Scholar]
- 87.Ji X., Pushalkar S., Li Y., Glickman R., Fleisher K., Saxena D. Antibiotic effects on bacterial profile in osteonecrosis of the jaw. Oral Dis. 2012;18:85–95. doi: 10.1111/j.1601-0825.2011.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vescovi P., Nammour S. Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) therapy. A Critical Review. Minerva Stomatol. 2010;59(181–203):204–213. [PubMed] [Google Scholar]
- 89.Hoefert S., Eufinger H. Relevance of a prolonged preoperative antibiotic regime in the treatment of bisphosphonate-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2011;69:362–380. doi: 10.1016/j.joms.2010.06.200. [DOI] [PubMed] [Google Scholar]
- 90.Damm D.D., Jones D.M. Bisphosphonate-related osteonecrosis of the jaws: a potential alternative to drug holidays. Gen. Dent. 2013;61:33–38. [PubMed] [Google Scholar]
- 91.Allen B.W., Demchenko I.T., Piantadosi C.A. Two faces of nitric oxide: implications for cellular mechanisms of oxygen toxicity. J. Appl. Physiol. 1985;106(2009):662–667. doi: 10.1152/japplphysiol.91109.2008. [DOI] [PubMed] [Google Scholar]
- 92.Piantadosi C.A., Tatro L.G. Regional H2O2 concentration in rat brain after hyperoxic convulsions. J. Appl. Physiol. 1985;69(1990):1761–1766. doi: 10.1152/jappl.1990.69.5.1761. [DOI] [PubMed] [Google Scholar]
- 93.Demchenko I.T., Boso A.E., O’Neill T.J., Bennett P.B., Piantadosi C.A. Nitric oxide and cerebral blood flow responses to hyperbaric oxygen. J. Appl. Physiol. 1985;88(2000):1381–1389. doi: 10.1152/jappl.2000.88.4.1381. [DOI] [PubMed] [Google Scholar]
- 94.Demchenko I.T., Boso A.E., Whorton A.R., Piantadosi C.A. Nitric oxide production is enhanced in rat brain before oxygen-induced convulsions. Brain Res. 2001;917:253–261. doi: 10.1016/s0006-8993(01)03057-8. [DOI] [PubMed] [Google Scholar]
- 95.Freiberger J.J. Utility of hyperbaric oxygen in treatment of bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2009;67:96–106. doi: 10.1016/j.joms.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 96.Freiberger J.J., Padilla-Burgos R., McGraw T., Suliman H.B., Kraft K.H., Stolp B.W., Moon R.E., Piantadosi C.A. What is the role of hyperbaric oxygen in the management of bisphosphonate-related osteonecrosis of the jaw: a randomized controlled trial of hyperbaric oxygen as an adjunct to surgery and antibiotics. J. Oral Maxillofac. Surg. 2012;70:1573–1583. doi: 10.1016/j.joms.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Epstein M.S., Wicknick F.W., Epstein J.B., Berenson J.R., Gorsky M. Management of bisphosphonate-associated osteonecrosis: pentoxifylline and tocopherol in addition to antimicrobial therapy. An Initial Case Series. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:593–596. doi: 10.1016/j.tripleo.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 98.Magremanne M., Reychler H. Pentoxifylline and tocopherol in the treatment of yearly zoledronic acid-related osteonecrosis of the jaw in a corticosteroid-induced osteoporosis. J. Oral Maxillofac. Surg. 2014;72:334–337. doi: 10.1016/j.joms.2013.06.188. [DOI] [PubMed] [Google Scholar]
- 99.Dayisoylu E.H., Şenel F.Ç., Üngör C., Tosun E., Çankaya M., Ersöz S., Taskesen F. The effects of adjunctive parathyroid hormone injection on bisphosphonate-related osteonecrosis of the jaws: an animal study. Int. J. Oral Maxillofac. Surg. 2013;42:1475–1480. doi: 10.1016/j.ijom.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Kim K.M., Park W., Oh S.Y., Kim H.J., Nam W., Lim S.K., Rhee Y., Cha I.H. Distinctive role of 6-month teriparatide treatment on intractable bisphosphonate-related osteonecrosis of the jaw. Osteoporos Int. 2014;25:1625–1632. doi: 10.1007/s00198-014-2622-8. [DOI] [PubMed] [Google Scholar]
- 101.Kakehashi H., Ando T., Minamizato T., Nakatani Y., Kawasaki T., Ikeda H., Kuroshima S., Kawakami A., Asahina I. Administration of teriparatide improves the symptoms of advanced bisphosphonate-related osteonecrosis of the jaw: preliminary findings. Int. J. Oral Maxillofac. Surg. 2015;44:1558–1564. doi: 10.1016/j.ijom.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 102.Di Fede O., La Mantia G., Del Gaizo C., Mauceri R., Matranga D., Campisi G. Reduction of MRONJ risk after exodontia by virtue of ozone infiltration: A randomized clinical trial. Oral Dis. 2024 doi: 10.1111/odi.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goker F., Donati G., Gallo F., Sparaco A., Rania V., Busa A., Grecchi F., Grecchi E., Colapinto G., Del Fabbro M. Ozone infiltration as an adjunctive treatment to piezoelectric surgery in the management of medication-related osteonecrosis of the jaws: case series of 29 patients. Oral Maxillofac. Surg. 2024;28:1197–1207. doi: 10.1007/s10006-024-01246-x. [DOI] [PubMed] [Google Scholar]
- 104.Pereira-Silva M., Hadad H., de Jesus L.K., de Freitas Santana M.E., de Oliveira J.M., Almeida H.H., Nímia O.M., Filho R., Okamoto S.B., Macedo C.F.P., Junior F.Á.S. Ozone therapy effect in medication-related osteonecrosis of the jaw as prevention or treatment: microtomographic, confocal laser microscopy and histomorphometric analysis. Clin Oral Investig. 2024;28:151. doi: 10.1007/s00784-024-05547-z. [DOI] [PubMed] [Google Scholar]
- 105.Tartaroti N.C., Marques M.M., Naclério-Homem M. da G., Migliorati C.A., Zindel Deboni M.C. Antimicrobial photodynamic and photobiomodulation adjuvant therapies for prevention and treatment of medication-related osteonecrosis of the jaws: Case series and long-term follow-up. Photodiagn. Photodyn. Ther. 2020;29 doi: 10.1016/j.pdpdt.2020.101651. [DOI] [PubMed] [Google Scholar]
- 106.Martins M.A.T., Martins M.D., Lascala C.A., Curi M.M., Migliorati C.A., Tenis C.A., Marques M.M. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: a preliminary study. Oral Oncol. 2012;48:79–84. doi: 10.1016/j.oraloncology.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 107.Scoletta M., Arduino P.G., Reggio L., Dalmasso P., Mozzati M. Effect of low-level laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: preliminary results of a prospective study. Photomed. Laser Surg. 2010;28:179–184. doi: 10.1089/pho.2009.2501. [DOI] [PubMed] [Google Scholar]
- 108.Vescovi P., Manfredi M., Merigo E., Guidotti R., Meleti M., Pedrazzi G., Fornaini C., Bonanini M., Ferri T., Nammour S. Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): a retrospective analysis of 101 treated sites with long-term follow-up. Photomed. Laser Surg. 2012;30:5–13. doi: 10.1089/pho.2010.2955. [DOI] [PubMed] [Google Scholar]
- 109.Mozzati M., Gallesio G., Arata V., Pol R., Scoletta M. Platelet-rich therapies in the treatment of intravenous bisphosphonate-related osteonecrosis of the jaw: a report of 32 cases. Oral Oncol. 2012;48:469–474. doi: 10.1016/j.oraloncology.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 110.Kim J.W., Kim S.J., Kim M.R. Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: a prospective feasibility study. Br J Oral Maxillofac Surg. 2014;52:854–859. doi: 10.1016/j.bjoms.2014.07.256. [DOI] [PubMed] [Google Scholar]
- 111.Dincă O., Zurac S., Stăniceanu F., Bucur M.B., Bodnar D.C., Vlădan C., Bucur A. Clinical and histopathological studies using fibrin-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Rom. J Morphol. Embryol. 2014;55:961–964. [PubMed] [Google Scholar]
- 112.Nørholt S.E., Hartlev J. Surgical treatment of osteonecrosis of the jaw with the use of platelet-rich fibrin: a prospective study of 15 patients. Int. J Oral Maxillofac. Surg. 2016;45:1256–1260. doi: 10.1016/j.ijom.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Park J.H., Kim J.W., Kim S.J. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J Oral Maxillofac Surg. 2017;75:1176–1184. doi: 10.1016/j.joms.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 114.Baba A., Goto T.K., Ojiri H., Takagiwa M., Hiraga C., Okamura M., Hasegawa S., Okuyama Y., Ogino N., Yamauchi H., Kobashi Y., Yamazoe S., Munetomo Y., Mogami T., Nomura T. CT imaging features of antiresorptive agent-related osteonecrosis of the jaw/medication-related osteonecrosis of the jaw. Dentomaxillofac. Radiol. 2018;47:20170323. doi: 10.1259/dmfr.20170323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melville J.C., Tursun R., Shum J.W., Young S., Hanna I.A., Marx R.E. A technique for the treatment of oral-antral fistulas resulting from medication-related osteonecrosis of the maxilla: the combined buccal fat pad flap and radical sinusotomy, Oral Surg Oral Med Oral Pathol. Oral Radiol. 2016;122:287–291. doi: 10.1016/j.oooo.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 116.Aljohani S., Troeltzsch M., Hafner S., Kaeppler G., Mast G., Otto S. Surgical treatment of medication-related osteonecrosis of the upper jaw: Case series. Oral Dis. 2019;25:497–507. doi: 10.1111/odi.12992. [DOI] [PubMed] [Google Scholar]
- 117.Tian M., Wang D.N., Luo S.Y., Zhai Y.W., Sun G.W. Effect of the surgical treatment of maxillary medication-related osteonecrosis of the jaw. Chinese Journal of Stomatology. 2021;56:447–451. doi: 10.3760/cma.j.cn112144-20210119-00030. [DOI] [PubMed] [Google Scholar]
- 118.An J.G., Lyu X.M., Jia K.K. Application of the submandibular gland for management of stage 3 medication-related osteonecrosis of the mandible. Zhonghua Kou Qiang Yi Xue Za Zhi. 2021;56:441–446. doi: 10.3760/cma.j.cn112144-20210203-00058. [DOI] [PubMed] [Google Scholar]
- 119.Carlson E.R., Basile J.D. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2009;67:85–95. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 120.Klingelhöffer C., Klingelhöffer M., Müller S., Ettl T., Wahlmann U. Can dental panoramic radiographic findings serve as indicators for the development of medication-related osteonecrosis of the jaw? Dentomaxillofac. Radiol. 2016;45:20160065. doi: 10.1259/dmfr.20160065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen H., He Y. Interpretation of medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO clinical practice guideline. Zhonghua Kou Qiang Yi Xue Za Zhi. 2022;57:128–135. doi: 10.3760/cma.j.cn112144-20211028-00477. [DOI] [PubMed] [Google Scholar]
- 122.Bacci C., Cerrato A., Bardhi E., Frigo A.C., Djaballah S.A., Sivolella S. A retrospective study on the incidence of medication-related osteonecrosis of the jaws (MRONJ) associated with different preventive dental care modalities. Support. Care Cancer. 2022;30:1723–1729. doi: 10.1007/s00520-021-06587-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mauceri R., Coniglio R., Abbinante A., Carcieri P., Tomassi D., Panzarella V., Di Fede O., Bertoldo F., Fusco V., Bedogni A., Campisi G. The preventive care of medication-related osteonecrosis of the jaw (MRONJ): a position paper by Italian experts for dental hygienists. Support. Care Cancer. 2022;30:6429–6440. doi: 10.1007/s00520-022-06940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guo Y., Zhao N., Wang D., Wang Y., Guo C. Clinical application of double-layer soft tissue closure technology based on pedicled buccal fat pad in repairing maxillary defects after medication-related osteonecrosis of jaw surgery. Hua Xi Kou Qiang Yi Xue Za Zhi. 2022;40:61–67. doi: 10.7518/hxkq.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Fede O., Panzarella V., Mauceri R., Fusco V., Bedogni A., Lo Muzio L., null Sipmo Onj Board, Campisi G. The Dental Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. Biomed Res. Int. 2018 (2018):2684924. doi: 10.1155/2018/2684924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Poxleitner P., Engelhardt M., Schmelzeisen R., Voss P. The Prevention of Medication-related Osteonecrosis of the Jaw. Dtsch. Arztebl. Int. 2017;114:63–69. doi: 10.3238/arztebl.2017.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zirk M., Wenzel C., Buller J., Zöller J.E., Zinser M., Peters F. Microbial diversity in infections of patients with medication-related osteonecrosis of the jaw. Clin. Oral Invest. 2019;23:2143–2151. doi: 10.1007/s00784-018-2655-z. [DOI] [PubMed] [Google Scholar]