Abstract
Background
Botulinum toxin is commonly used for cosmetic enhancements in various applications. However, the pain experienced during the injection process remains a significant concern.
Objective
This study aimed to evaluate the effectiveness and safety of a needle-free microjet drug injector, powered by an Er:YAG laser, for the injection of botulinum toxin to treat crow’s feet wrinkles.
Methods
Botulinum toxin injections were randomly administered using a microjet injector on one side and a conventional needle injection on the other. The results were evaluated by two dermatologists, who were blinded to the treatment method. They used a 5-point scale to assess the severity of both static and dynamic crow’s feet before and after the treatment. The participants’ pain levels during the procedure were measured using a visual analog scale, and the physician/subject global aesthetic improvement scale (GAIS) was used to assess overall aesthetic improvement.
Results
Ten Korean women (mean age, 50.7) participated in the study. Both sides exhibited significant improvement in crow’s feet wrinkles compared to the baseline, with no noticeable differences between the two sides. The microjet injector side showed a significantly lower mean pain score, while there was no difference between the sides in terms of P/SGAIS scores. The evaluation of the wrinkle scale demonstrated high reliability.
Conclusion
The needle-free microjet drug injector, which utilizes an Er:YAG laser, may be a useful option for treating crow’s feet wrinkles with botulinum toxin due to its ability to reduce pain.
Keywords: Botulinum toxins, Jet Injection, Periorbital wrinkles, Skin aging
INTRODUCTION
Botulinum toxin is used for various cosmetic purposes, including treating crow’s feet and forehead wrinkles, reducing masseter and calf mass, addressing facial erythema, and achieving overall facial rejuvenation1,2,3,4. Although botulinum toxin is recognized for its ability to reduce pain through nociceptive pathways, it does not mitigate the pain experienced during the injection process. Consequently, this often leads to discomfort and anxiety among patients undergoing the treatment5. While methods like anesthetic creams, vibrators, and similar approaches have been employed to alleviate pain, many patients continue to report discomfort during the injections6,7. While topical products containing lidocaine are crucial for minimizing procedural pain, their application can be time-consuming and may occasionally result in complications, such as contact dermatitis8. General anesthesia is an alternative, but its use is limited due to potential systemic side effects9. This study aimed to evaluate the effectiveness and safety of a novel needle-free microjet drug injector powered by an Er:YAG laser, specifically designed for administering botulinum toxin injections to improve crow’s feet wrinkles.
MATERIALS AND METHODS
Participants
We recruited 10 healthy participants, aged 18 to 65 years, exhibiting symmetrical crow’s feet wrinkles with scores ranging from 1 to 4 on the 5-point scale at both static and dynamic facial expressions (0, no wrinkles; 1, mild; 2, moderate; 3, severe; 4, very severe)10,11. We excluded individuals 1) who had undergone cosmetic facial treatments, including topicals, oral medications, lasers, and peels, within the past 2 months; 2) those who had received botulinum toxin treatment in the periorbital region within the last 6 months; 3) those with dermatitis or infectious skin diseases in the periorbital region; 4) who were pregnant or lactating; and 5) those deemed unfit for the study by the investigator. All participants voluntarily agreed to participate in the study, provided written informed consent, and had the freedom to withdraw at any time and for any reason.
Treatment protocols
Participants were administered 3 to 7 units of prabotulinum toxin A (NABOTA®; Daewoong Pharmaceutical Co. Ltd., Seoul, Korea) bilaterally, depending on the severity of wrinkles. Different injection techniques were used randomly on each side of face with an online random sequence generator. Prabotulinum toxin A was prepared at a concentration of 20 units per ml by diluting 50 units with 2.5 ml of saline. On one side, intradermal botulinum toxin injections were given to target the crow’s feet (1.5 cm lateral to the outer canthus with a range of 1 cm up and down). The injections were performed using a needle-free microjet drug injector powered by the Er:YAG laser (Mirajet, JSK Biomed Inc, Daejeon, Korea). The laser was set to a 4-mm spot size, 40-µs pulse width, 10-Hz frequency, and fluence between 1.3 and 1.6 J/cm2 for a single pass. On the opposite side, 1 to 2 units were injected intradermally at three specific points (1.5 cm lateral to the outer canthus and 1 cm points both up and down)1. To minimize discomfort, a topical anesthetic cream containing 2.5% lidocaine and 2.5% prilocaine was applied to the treatment areas 30 minutes before the procedure.
Assessment
Photographs capturing crow’s feet wrinkles during both static and dynamic facial expressions were taken using a digital camera at baseline and 2–4 weeks after treatment. Blinded two dermatologists, who were unaware of the treatment details, examined the pre-treatment and post-treatment photos and rated them on the 5-point scale10. The differences in glossiness and pore size between the two treatment sides were measured using Mark-Vu® (PSI PLUS, Suwon, Korea). Additionally, participants rated their pain levels using a visual analog scale (VAS; 0, no pain; 10, intolerable pain) after treatment and during follow-up. They also evaluated the overall aesthetic improvement using the Physician/Subject Global Aesthetic Improvement Scale (PGAIS and SGAIS; 1, much worse; 2, worse; 3, no change; 4, improved; 5, much improved). For safety, all adverse events including pain, bruising, bleeding, paresthesia, and muscle weakness were investigated.
Statistical analysis
Statistical analyses were performed using SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, NY, USA). The 5-point scale ratings for crow’s feet wrinkles, mean PGAIS, pain VAS, and SGAIS were analyzed using paired t-tests. Inter-rater reliability for the 5-point scale was assessed using intraclass correlation coefficients (ICC). The data were presented as the mean ± standard deviation. Differences with p-values below 0.05 were considered statistically significant.
Ethics statement
The study was conducted from December 2022 to November 2023 and adhered to the guidelines set forth in the Declaration of Helsinki. This study’s protocol was approved by the Institutional Review Board (IRB) of Chung-Ang University Hospital (IRB Approval No. 2209-023-524). All participants signed a photo release consent form authorizing the reproduction and distribution of any images collected during the study.
RESULTS
In the study, 10 Korean women successfully completed the entire protocol without any withdrawals. The participants, who had a mean age of 50.7±7.8 years (range, 38–59), exhibited no facial asymmetry and reported no significant medical histories.
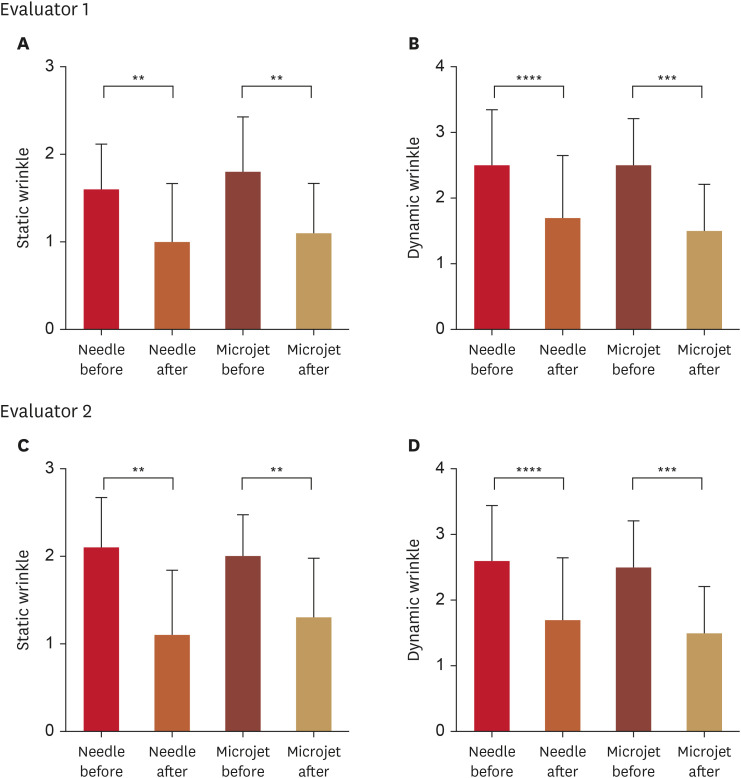
There was a significant improvement in the crow’s feet wrinkle scale in relation to both static and dynamic facial expressions when comparing both treatment methods to the baseline. However, no statistically significant difference was observed between the two sides (Fig. 1, Table 1). The ICC for the crow’s feet wrinkle scale, as evaluated by the two dermatologists for both static and dynamic facial expressions, exceeded 0.8 (Table 1). Additionally, no discernible variations were identified in pore size and glossiness between the two sides.
Fig. 1. Assessment of the 5-point scale for crow’s feet associated with static and dynamic facial expressions. Two dermatologists, who were blinded to the study, conducted the evaluation. Panels (A-D) illustrate the evaluation process.
**p<0.01; ***p<0.001; ****p<0.0001, as determined using a paired t-test.
Table 1. Assessment of alterations in the mean 5-point scale scores for crow’s feet, comparing static and dynamic facial expressions before and after treatment (n=10).
| Variables | Evaluator 1 | Evaluator 2 | ICC | |||||
|---|---|---|---|---|---|---|---|---|
| Needle | Microjet | p-value | Needle | Microjet | p-value | |||
| Static | 0.88 | |||||||
| Before | 1.60±0.52 | 1.80±0.63 | 0.1679 | 2.10±0.57 | 2.00±0.47 | 0.3434 | ||
| After | 1.00±0.67 | 1.10±0.57 | 0.5911 | 1.10±0.74 | 1.30±0.67 | 0.4433 | ||
| p-value | 0.0051 | 0.0095 | 0.0011 | 0.0013 | ||||
| Dynamic | 0.83 | |||||||
| Before | 2.50±0.85 | 2.50±0.71 | >0.9999 | 2.60±0.84 | 2.50±0.71 | 0.3434 | ||
| After | 1.70±0.95 | 1.50±0.71 | 0.3434 | 1.70±0.95 | 1.50±0.71 | 0.3434 | ||
| p-value | 0.0002 | <0.0001 | 0.0007 | <0.0001 | ||||
Values are presented as mean ± standard deviation.
This evaluation was conducted by two dermatologists, who were blinded to the treatment.
ICC: intraclass correlation coefficients.
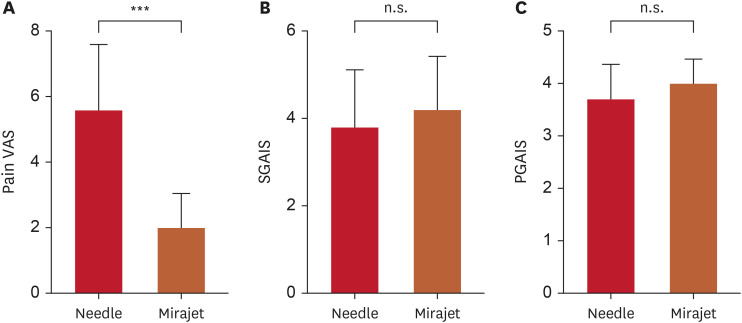
The mean pain VAS was significantly lower for the side that received treatment with the needle-free microjet injector. Furthermore, there was no significant difference in the mean PGAIS and SGAIS scores during the follow-up between the two sides (Fig. 2, Table 2). All participants reported experiencing mild to moderate pain during the procedure, but no other discomfort, such as muscle weakness, was reported.
Fig. 2. Evaluations of (A) the pain VAS during treatment, (B) the SGAIS, and (C) the PGAIS at follow-up.
VAS: visual analog scale, S/PGAIS: subject/physician global aesthetic improvement scale, n.s.: not significant.
***p<0.001; paired t-test.
Table 2. Evaluations capturing (A) the mean pain VAS scores during the procedure, as well as (B) the PGAIS, and (C) SGAIS ratings obtained during the subsequent follow-up (n=10).
| Variables | Needle | Microjet | p-value |
|---|---|---|---|
| Pain VAS | 5.60±2.01 | 2.00±1.05 | 0.0001 |
| PGAIS | 3.70±0.67 | 4.0±0.47 | 0.1934 |
| SGAIS | 3.80±13.2 | 4.20±1.23 | 0.2229 |
Values are presented as mean ± standard deviation.
VAS: visual analog scale, P/SGAIS: physician/subject global aesthetic improvement scale.
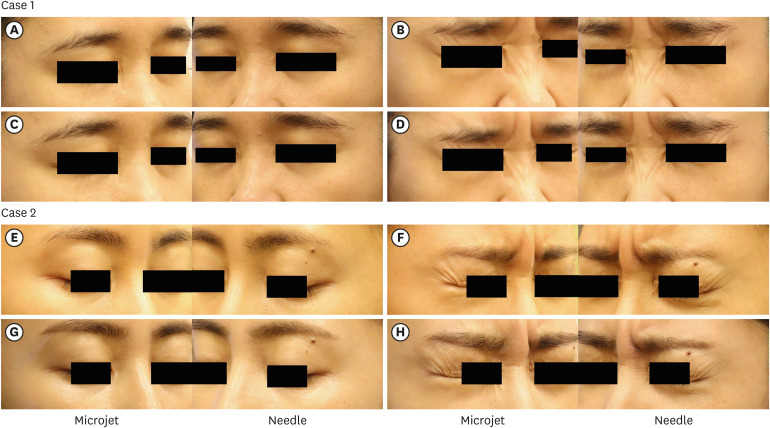
Representative photographs of participants are provided in Fig. 3.
Fig. 3. Clinical photographs of two representative cases, illustrating the improvements in crow’s feet wrinkles. (A, E) Static and (B, F) dynamic wrinkles before the treatment. (C, G) Static and (D, H) dynamic wrinkles after the treatment.
DISCUSSION
Botulinum toxin is administered at various skin depths depending on the indication1. For the treatment of crow’s feet, intradermal injections are more effective for static wrinkles, whereas intramuscular injections are preferred for dynamic wrinkles12. The use of intradermal injection of botulinum toxin is becoming increasingly popular because it has been shown to result in a statistically significant improvement of wrinkles and a high level of patient satisfaction13. However, individuals receiving intradermal injections may experience more pain compared to those receiving intramuscular injections14. While nanomicroneedles have been explored to minimize pain and deliver botulinum toxin to the dermis, they are reportedly less effective than conventional needle injections. This difference is presumably due to a reduced absorption rate when botulinum toxin is applied topically, despite the notable advantage in pain alleviation12. Although drug loss was not accurately measured, at least in this study, where small amounts of botulinum toxin were injected locally, no difference was felt compared to injection procedures.
Needleless jet injectors were designed to ensure adequate drug delivery to the dermis while minimizing needle-induced pain. Conventional macrojet injectors do not offer much pain relief and have inconsistent injection depths15. The needle-free microjet drug injector, which utilizes the Er:YAG laser, consistently delivers the drug to the dermis. It functions by irradiating the Er:YAG beam, subsequently forming a vapor bubble. The microjet drug injector with laser does not overheat the drug and does not cause degradation15. Given its potential to produce uniform results and reduce pain, the microjet holds promise for future scar treatments and skin rejuvenation applications16.
Our study revealed that microjet injections of botulinum toxin are equally effective in ameliorating crow’s feet wrinkles compared to traditional needle injections. However, the microjet technique had the added advantage of significantly reducing pain. This is presumed to be due to the consistent injection depth and less tissue damage of the microjet technique15,17. This pain mitigation can also be applied to other treatments, including the use of skin boosters such as hyaluronic acid, poly-l-lactic acid, poly-d-lactic acid, growth factors, and exosomes18,19. Considering that needle phobia affects 3.5%–20% of the general population, transdermal drug delivery using needle-free microjet technology may also offer psychological benefits and improve future dermal injection techniques20. However, it is not such a convenient to form a jet for injection, which requires a learning curve. It would be more useful if it could be made mechanically easier.
Some limitations of our study include the lack of double-blinding due to distinct methods, a small sample size, female-only participants, and a short follow-up duration, which prevent effective period comparisons. Since microjet injection is a method of intradermal injection technique, the duration of effect is probably shorter than intramuscular injection based on previous research21. The study was also confined to specific indications. However, the wrinkle scale was assessed by two blinded raters, resulting in a good ICC (0.88 and 0.83), indicating its validity19. Future research should incorporate larger sample sizes, extended follow-up durations, and various indications, such as intradermal procedures for treating forehead wrinkles, oily skin, and facial erythema.
In conclusion, microjet method may be a more favorable option for treating crow's feet wrinkles with botulinum toxin due to its ability to reduce pain.
ACKNOWLEDGMENT
We thank Hongseok Kim (VOS Dermatology Clinic, Seoul, Korea) and Daewoong Pharmaceutical Co., LTD., for their experimental design advice and Nabota® provision, respectively.
Footnotes
FUNDING SOURCE: This work was funded by JSK Biomed Inc.
CONFLICTS OF INTEREST: The authors have nothing to disclose.
DATA SHARING STATEMENT: The data that support the findings of this study are available from the corresponding author, PKY, upon reasonable request.
References
- 1.Ahn BK, Kim YS, Kim HJ, Rho NK, Kim HS. Consensus recommendations on the aesthetic usage of botulinum toxin type A in Asians. Dermatol Surg. 2013;39:1843–1860. doi: 10.1111/dsu.12317. [DOI] [PubMed] [Google Scholar]
- 2.Hanna E, Xing L, Taylor JH, Bertucci V. Role of botulinum toxin A in improving facial erythema and skin quality. Arch Dermatol Res. 2022;314:729–738. doi: 10.1007/s00403-021-02277-0. [DOI] [PubMed] [Google Scholar]
- 3.Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg. 2013;39:443–448. doi: 10.1111/dsu.12097. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Ji X, Xu Y, Liu J, Miao YY, Zhang JA, et al. The efficacy of intradermal injection of type A botulinum toxin for facial rejuvenation. Dermatol Ther. 2017;30:e12433. doi: 10.1111/dth.12433. [DOI] [PubMed] [Google Scholar]
- 5.Matak I, Bölcskei K, Bach-Rojecky L, Helyes Z. Mechanisms of botulinum toxin type A action on pain. Toxins (Basel) 2019;11:459. doi: 10.3390/toxins11080459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sami MS, Soparkar CN, Patrinely JR, Hollier LM, Hollier LH. Efficacy of botulinum toxin type a after topical anesthesia. Ophthal Plast Reconstr Surg. 2006;22:448–452. doi: 10.1097/01.iop.0000248989.33572.3c. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Czyz CN, Wulc AE. Investigating the efficacy of vibration anesthesia to reduce pain from cosmetic botulinum toxin injections. Aesthet Surg J. 2011;31:966–971. doi: 10.1177/1090820X11422809. [DOI] [PubMed] [Google Scholar]
- 8.van den Hove J, Decroix J, Tennstedt D, Lachapelle JM. Allergic contact dermatitis from prilocaine, one of the local anaesthetics in EMLA cream. Contact Dermat. 1994;30:239. doi: 10.1111/j.1600-0536.1994.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 9.Louer R, McKinney RC, Abu-Sultaneh S, Lutfi R, Abulebda K. Safety and efficacy of a propofol and ketamine based procedural sedation protocol in children with cerebral palsy undergoing botulinum toxin A injections. PM R. 2019;11:1320–1325. doi: 10.1002/pmrj.12146. [DOI] [PubMed] [Google Scholar]
- 10.Flynn TC, Carruthers A, Carruthers J, Geister TL, Görtelmeyer R, Hardas B, et al. Validated assessment scales for the upper face. Dermatol Surg. 2012;38:309–319. doi: 10.1111/j.1524-4725.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- 11.Rouvrais C, Bacqueville D, Bogdanowicz P, Haure MJ, Duprat L, Coutanceau C, et al. A new dermocosmetic containing retinaldehyde, delta-tocopherol glucoside and glycylglycine oleamide for managing naturally aged skin: results from in vitro to clinical studies. Clin Cosmet Investig Dermatol. 2017;10:35–42. doi: 10.2147/CCID.S123575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Yang JP, Zhu XG, Zhu J, Chang HQ, Guo SH, et al. A comparative in vivo study on three treatment approaches to applying topical botulinum toxin A for crow’s feet. BioMed Res Int. 2018;2018:6235742. doi: 10.1155/2018/6235742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon IK, Chang SE, Park GH, Roh MR. Comparison of microneedle fractional radiofrequency therapy with intradermal botulinum toxin A injection for periorbital rejuvenation. Dermatology. 2013;227:367–372. doi: 10.1159/000356162. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Lim OK, Choi WJ. Are there differences between intradermal and intramuscular injections of botulinum toxin on the forehead? Dermatol Surg. 2020;46:e126–e131. doi: 10.1097/DSS.0000000000002379. [DOI] [PubMed] [Google Scholar]
- 15.Schoppink J, Fernandez Rivas D. Jet injectors: perspectives for small volume delivery with lasers. Adv Drug Deliv Rev. 2022;182:114109. doi: 10.1016/j.addr.2021.114109. [DOI] [PubMed] [Google Scholar]
- 16.Jang HJ, Yu H, Lee S, Hur E, Kim Y, Lee SH, et al. Towards clinical use of a laser-induced microjet system aimed at reliable and safe drug delivery. J Biomed Opt. 2014;19:058001. doi: 10.1117/1.JBO.19.5.058001. [DOI] [PubMed] [Google Scholar]
- 17.Cu K, Bansal R, Mitragotri S, Fernandez Rivas D. Delivery strategies for skin: comparison of nanoliter jets, needles and topical solutions. Ann Biomed Eng. 2020;48:2028–2039. doi: 10.1007/s10439-019-02383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi KH, Park MS, Ree YS, Kim HM. A review on “skin boosters”: hyaluronic acid, poly-L-lactic acid and pol-D-lactic acid, polydeoxyribonucleotide, polynucleotides, growth factor, and exosome. Aesthetics. 2023;4:1–5. [Google Scholar]
- 19.Lee JJ, Yi KH, Kim HS, An MH, Seo KK, Huh CH, et al. A novel needle-free microjet drug injector using Er:YAG LASER: a completely new concept of transdermal drug delivery system. Clin Anat. 2022;35:682–685. doi: 10.1002/ca.23892. [DOI] [PubMed] [Google Scholar]
- 20.Love AS, Love RJ. Considering needle phobia among adult patients during mass COVID-19 vaccinations. J Prim Care Community Health. 2021;12:21501327211007393. doi: 10.1177/21501327211007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vachiramon V, Subpayasarn U, Triyangkulsri K, Jurairattanaporn N, Rattananukrom T. Different injection patterns of incobotulinumtoxinA for crow’s feet: a split-face comparative study. J Eur Acad Dermatol Venereol. 2021;35:256–262. doi: 10.1111/jdv.16997. [DOI] [PubMed] [Google Scholar]