Abstract
Background
Omalizumab, a monoclonal antibody targeting immunoglobulin E (IgE), is approved for adults and adolescents (12 years or older) with chronic spontaneous urticaria (CSU) that does not respond to high-dose antihistamines. In Korea, there is limited research on predictive biomarkers for omalizumab response in CSU patients.
Objective
This retrospective, single-institution study aimed to identify clinical parameters predicting omalizumab response in Korean CSU patients.
Methods
We analyzed records of CSU patients aged 19 or older starting omalizumab between January 2017 and October 2019. Omalizumab efficacy was assessed using the Urticaria Activity Score summed over 7 days (UAS7), categorized as well-controlled, mild, moderate, or severe. Ninety CSU patients participated in this study.
Results
Among these, improvements in UAS7 categories from baseline to 12 weeks of treatment were observed in 78 patients, while 12 patients showed no significant efficacy. The present study identified potential biomarkers that could predict a patient’s response to omalizumab, including disease duration and total serum IgE levels (p=0.022, p=0.046). Notably, a significant correlation was observed between higher detection rates in multiple antigen simultaneous test (MAST) food testing and lower treatment responses (p=0.033).
Conclusion
Shorter disease duration of CSU and elevated initial serum total IgE level may serve as potential predictive biomarkers for the responsiveness to omalizumab. Furthermore, a higher MAST food detection rate seemed to correlate with a less favorable treatment response, suggesting patients with a high MAST food detection rate might benefit from a food evaluation in addition to omalizumab treatment.
Keywords: Biomarkers, Chronic urticaria, Omalizumab
INTRODUCTION
Chronic spontaneous urticaria (CSU) is a disease characterized by the presence of transient wheals, or swollen patches, with or without angioedema, for at least 6 weeks, and has been reported to greatly affect quality of life, causing symptoms such as severe itching and sleep disturbances1,2. Although second-generation antihistamines are the first-line treatment for CSU, up to 60% of patients do not adequately respond to this treatment, and oral corticosteroids may be added3,4. Omalizumab is the first biological agent prescribed for the treatment of CSU, and was approved by the US Food and Drug Administration (FDA) in 2014. It works by binding to the high-affinity immunoglobulin E (IgE) receptor (FcεRI) binding site, which prevents IgE from binding, and subsequently reduces the allergic response4,5. The efficacy of omalizumab in CSU is well documented, but its high cost is a hurdle to its widespread use6. As such, there have been many attempts to define possible biomarkers which could be used to predict the treatment efficacy of omalizumab. Interleukin (IL)-31 levels, serum total IgE, and basophil FcεRI expression, and basophil CD203c upregulating activity are reported to be related to the clinical response to omalizumab7,8,9,10. Previous studies, however, have focused primarily on Caucasian populations. In Korea, several studies have been reported focusing on the natural course, quality of life (QOL) assessment, and disease course of patients with CSU11,12, but studies on laboratory biomarkers that are easy to identify at the initial visit and which can be used to predict the treatment response to omalizumab are insufficient, especially in Asian populations13.
We retrospectively evaluated the electronic medical records of all enrolled patients with CSU treated with omalizumab at Yonsei University Severance Hospital to analyze and elucidate which clinical and laboratory biomarkers may be used to predict the response to omalizumab in Korean patients with CSU.
MATERIALS AND METHODS
Study population and patient selection
The protocol for the present study was approved by the Institutional Review Board of Yonsei University Severance Hospital (IRB No. 4-2022-1221). We retrospectively evaluated the electronic medical records of 107 patients with CSU who visited the dermatology and allergy-asthma center of Yonsei University Severance Hospital in Seoul and had a documented omalizumab prescription between January 2017 and October 2019. The exclusion criteria were as follows: <19 years of age; history of atopic dermatitis, atopic rhinitis, or asthma; and no information on laboratory tests, family history, or comorbidities in the medical record.
Clinical & laboratory factors
The following demographic/characteristic data and lab results were obtained from the patients’ electronic medical records: age; sex; duration of CSU; interval and dose of omalizumab treatment; history of thyroid disease, angioedema, allergic disease, or other comorbidities; history of other medications, such as non-steroidal anti-inflammatory drugs (NSAIDs); blood chemistry tests; anti-nuclear antibody (ANA); eosinophil cationic protein (ECP); serum total IgE; multiple antigen simultaneous test (MAST); C3; C4; CH50; erythrocyte sedimentation rate (ESR); C-reactive protein (CRP); thyroid function tests (TFTs) including T3, free T4, and thyroid stimulating hormone (TSH); and neutrophil, eosinophil, and basophil counts. TFTs were categorized as normal or abnormal, with the abnormal group consisting of patients for which the T3, free T4, and/or TSH were out of the normal range (T3, 0.60–1.81 ng/mL; free T4, 0.89–1.76 ng/dL; TSH, 0.55–4.78 μIU/mL). ANA was classified as positive or negative. The MAST inhalant panel consisted of 19 items: Dermatophagoides pteronyssinus; Dermatophagoides farina; storage mites; house dust; cockroach mix; Cladosporium; Aspergillus; Alternaria; yeast; Candida albicans; rye; short ragweed; mugwort; Japanese hop; alder; birch-alder mix; white oak; cat; and dog. The MAST food panel consisted of 42 items: pork; beef; chicken; silk cocoon; egg white; milk; cheddar cheese; cacao; peanut; walnut; chestnut; maize; sesame; soybean; potato; wheat flour; barley; rice; buckwheat; garlic; onion mix; celery; cucumber; mushroom; tomato; apple; peach; cross-reactive carbohydrate determinate (CCD) bromelain; kiwi; mango; banana; citrus mix; crab; shrimp; mackerel; codfish; mussel; tuna; salmon; clam; squid; and anchovy.
Evaluation of CSU severity and response of the treatment
The severity of urticaria was evaluated using the Urticaria Activity Score summed over 7 days (UAS7; 0–42), which was administered immediately before the first dose of omalizumab, and again 3 months after the first treatment. The patients were classified into four groups (well-controlled, mild, moderate, and severe) based on their total score14,15. The well-controlled group included those with a UAS7 score of 0 or more but less than 7 points (0≤UAS7<7); the mild group included those with a UAS7 score of 7 or more but less than 16 points (7≤UAS7<16); the moderate group, 16 or more but less than 28 (16≤UAS7<28); and the severe group, 28 or more but 42 or less (28≤UAS7≤42). The responder group included patients who had a decrease of at least one UAS7 classification (e.g., severe-to-moderate or moderate-to-mild) between their pre-treatment and 3-month scores, and the non-responder group included those who had no decrease.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics (version 26.0; IBM Corp., Armonk, NY, USA). Normality testing was performed to assess the suitability of the t-test or Mann-Whitney U tests. Furthermore, χ2 and Fisher’s exact tests, and logistic regression analysis were also performed. Statistical significance was set at p<0.05 for all analyses.
RESULTS
Demographics and clinical characteristics
From January 2017 to October 2019, a total of 107 patients diagnosed with CSU started treatment with omalizumab at our hospital. Of the 107 patients, 9 under the age of 19 years or with a history of comorbidities, such as atopic dermatitis, allergic rhinitis, and/or asthma were excluded from the present study. Of the remaining 98 patients, 8 were excluded due to loss to follow-up or lack of laboratory data (Table 1).
Table 1. Demographic and clinical characteristics of patients with CSU (n=90).
| Parameter | Value | |
|---|---|---|
| Age (yr) | 44.41±15.11 | |
| Sex | ||
| Male | 39 (43.3) | |
| Female | 51 (56.7) | |
| Duration of CSU (mo) | 12.0 (4.0–36.0) | |
| Interval of omalizumab | ||
| 4 wk | 89 (98.9) | |
| 8 wk | 1 (1.1) | |
| Dosage of omalizumab | ||
| 150 mg | 87 (96.7) | |
| 300 mg | 3 (3.3) | |
| Thyroid disease | 7 (7.8) | |
| Angioedema | 20 (22.2) | |
| NSAID exacerbation | 8 (8.9) | |
| Lab findings | ||
| Total IgE (IU/ml) | 154.00 (54.00–254.00) | |
| ANA positivity (≥1:80) | 20 (22.2) | |
| TFT abnormality | 11 (12.2) | |
| ECP (µg/L) | 17.80 (11.60–30.80) | |
| Severity (Initial) | ||
| Well-controlled (0≤UAS7<7) | 0 (0.0) | |
| Mild (7≤UAS7<16) | 3 (3.3) | |
| Moderate (16≤UAS7<28) | 41 (45.6) | |
| Severe (28≤UAS7≤42) | 46 (51.1) | |
| Response | ||
| Responder group | 78 (86.7) | |
| Non-responder group | 12 (13.3) | |
Values are presented as number only, number (%), mean ± standard deviation, or median (Q1–Q3).
CSU: chronic spontaneous urticaria, NSAID: non-steroidal anti-inflammatory drug, ANA: antinuclear antibody, TFT: thyroid function test, ECP: eosinophil cationic protein, UAS7: Urticaria Activity Score summed over 7 days.
A total of 90 patients were evaluated in the present study, 39 (43.3%) of whom were male and 51 (56.7%) of whom were female, with a male-to-female ratio of 1:1.3, and an average age of 44.41±15.11 years. The median value of duration of the CSU was 12.0 (4.0–36.0) months, with 89 patients (98.9%) treated with omalizumab every 4 weeks and 1 patient (1.1%) treated with omalizumab every 8 weeks. Omalizumab was administered at a dose of 150 mg to 87 patients (96.7%) and at a dose of 300 mg to 3 (3.3%). Throughout the study period, the dosage and administration interval of omalizumab remained consistent for each patient. Thyroid disease was present in 7 patients (7.8%), angioedema in 20 patients (22.2%), and a history of NSAID-induced exacerbation in 8 patients (8.9%).
Correlation analysis between the duration of CSU and the severity of the disease, as measured by the UAS7 score, showed a positive correlation with a coefficient of 0.374, indicating that longer disease duration tends to be associated with greater severity. However, the correlation between CSU patients’ total IgE levels and the severity of the disease measured by UAS7 was −0.010, suggesting no statistically significant relationship.
Response to omalizumab treatment
Of the 90 patients, no one was in the well-controlled group (0≤UAS7<7), 3 (3.3%) were in the mild group (7≤UAS7<16), 41 (45.6%) in the moderate group (16≤UAS7<28), and 46 (51.1%) in the severe group (28≤UAS7≤42) upon the initial assessment. After the 3-month assessment, 78 patients (86.7%, responders group) had a decrease of at least 1 category, but there was no change in 12 patients (13.3%, non-responders group) (Table 1).
Characteristics of laboratory findings and factors affecting treatment response
The 90 patients with CSU included in the present study had a median serum total IgE level of 154.00 (54.00–254.00) IU/mL, 20 (22.2%) of whom had positive ANA results, 11 (12.2%) had abnormal TFT results, and the median ECP level was 17.80 (11.60–30.80) (Table 1). The median age of the patients in the responder group was 45.0 (32.0–57.0) years, and that for the non-responder group was 37.0 (24.0–59.5) years, indicating that there was no significant difference between the two groups in age (p=0.386). However, for CSU duration, the responder group had a median duration of 11.5 (4.0–32.5) months, while the non-responder had a median duration of 37.5 (14.3–84.5) months, indicating a significant difference between the 2 groups (p=0.022) (Table 2).
Table 2. Comparisons of clinical characteristics and laboratory results between responders and non-responders.
| Characteristics | Responders (n=78) | Non-responders (n=12) | p-value* | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (yr)† | 45.0 (32.0–57.0) | 37.0 (24.0–59.5) | 0.386 | |
| Disease duration (mo)† | 11.5 (4.0–32.5) | 37.5 (14.3–84.5) | 0.022* | |
| Female‡ | 46 (59.0) | 5 (41.7) | 0.260 | |
| Thyroid disease‡ | 5 (6.4) | 2 (16.7) | 0.230 | |
| Angioedema‡ | 17 (21.8) | 3 (25.0) | 0.725 | |
| NSAID aggravation‡ | 7 (9.0) | 1 (8.3) | >0.999 | |
| Laboratory results† | ||||
| Neutrophil counts (103/μL) | 4.11 (3.15–5.98) | 4.17 (3.21–4.75) | 0.873 | |
| Basophil counts (103/μL) | 0.03 (0.02–0.04) | 0.05 (0.01–0.05) | 0.095 | |
| Eosinophil counts (103/μL) | 0.12 (0.05–0.19) | 0.19 (0.07–0.33) | 0.078 | |
| C3 (mg/dl) | 115.0 (99.7–125.6) | 114.4 (97.1–125.7) | 0.928 | |
| C4 (mg/dl) | 24.5 (20.9–29.3) | 24.1 (20.1–32.0) | 0.830 | |
| CH50 (U/ml) | 59.0 (56.0–67.5) | 73.5 (58.0–86.5) | 0.189 | |
| ESR (mm/hr) | 11.0 (5.5–27.0) | 9.0 (5.3–12.8) | 0.253 | |
| CRP (mg/dl) | 1.00 (0.60–2.78) | 1.10 (0.38–11.03) | 0.881 | |
| ECP (µg/L) | 17.20 (11.65–30.90) | 24.50 (10.67–33.65) | 0.931 | |
| Serum total IgE (IU/ml) | 185.00 (60.40–274.00) | 90.50 (36.38–164.50) | 0.046* | |
| MAST (Class 2 or above) | 2.0 (0.0–3.0) | 2.0 (0.0–5.0) | 0.164 | |
| MAST – Food§ | 0.0 (0.0–0.0) | 1.0 (0.0–1.5) | 0.033* | |
| MAST – Inhalant§ | 2.0 (0.0–3.0) | 0.0 (0.0–2.0) | 0.334 | |
| Abnormal thyroid function test∥¶ | 8 (10.3) | 3 (25.0) | 0.161 | |
| Anti-nuclear antibody positivity∥ | 18 (23.1) | 2 (16.7) | >0.999 | |
Values are presented as numbers only, number (%), or medians (Q1–Q3).
NSAID: non-steroidal anti-inflammatory drug, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, ECP: eosinophil cationic protein, IgE: immunoglobulin E, MAST: multiple antigen simultaneous test, TSH: thyroid stimulating hormone.
*p<0.05 indicates statistical significance.
†The p-values were calculated by Mann-Whitney U test.
‡The p-values was calculated by χ2 or Fisher’s exact test.
§This results are class 2 or above according to the MAST analysis.
∥The p-values were calculated using linear or Fisher’s exact test.
¶Thyroid function test includes T3, T4, free T4, TSH levels (abnormal, normal).
Of the 78 patients in the responder group, 46 (59.0%) were female, whereas 5 (41.7%) in the non-responder group were female. There were 5 patients (6.4%) with thyroid disease in the responder group and 2 (16.7%) in the non-responder group; 17 patients (21.8%) in the responder group and 3 (25.0%) in the non-responder group had a history of angioedema; and 7 patients (9.0%) in the responder group and 1 (8.3%) in the non-responder group had NSAID aggravation. There were no significant differences between the groups in terms of sex, thyroid disease, angioedema, allergic diseases, or NSAID-induced aggravation.
The serum total IgE levels were 185.00 (60.40–274.00) IU/mL in the responder group and 90.50 (36.38–164.50) IU/mL in the non-responder group, indicating a significant difference between the 2 groups (p=0.046). Neutrophil counts were 4.11×103/μL in the responder group and 4.17×103/μL in the non-responder group; eosinophil counts were 0.12×103/μL in the responder group and 0.19×103/μL in the non-responder group; basophil counts were 0.03×103/μL in the responder group and 0.05×103/μL in the non-responder group; complement hemolytic activity (CH50) was 59.0 in the responder group and 73.5 in the non-responder group; C3 was 115.0 in the responder group and 114.4 in the non-responder group; C4 was 24.5 in the responder group and 24.1 in the non-responder group; ESR was 11.0 in the responder group and 9.0 in the non-responder group; CRP was 1.00 in the responder group and 1.10 in the non-responder group; and ECP was 17.20 in the responder group and 24.50 in the non-responder group.
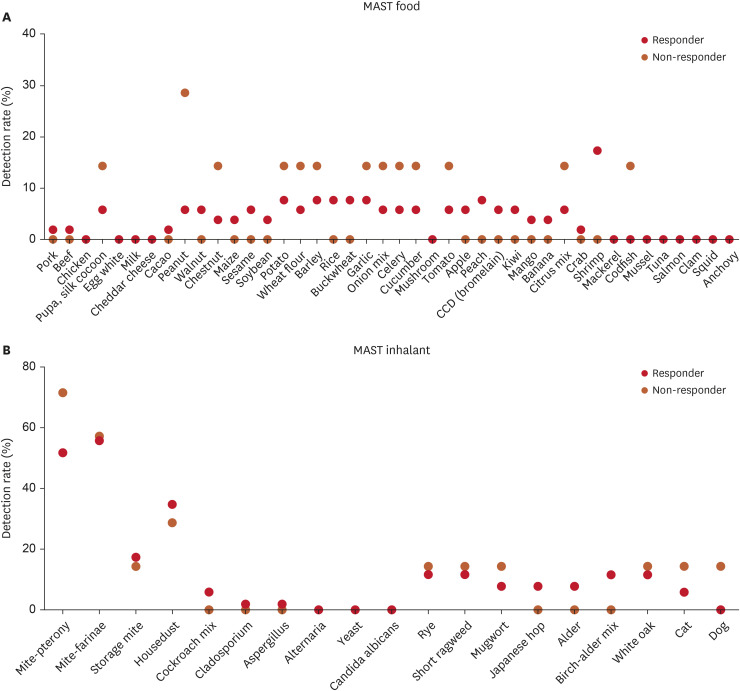
The responder group had lower eosinophil and basophil counts and CH50 values than the non-responder group; however, these differences were not statistically significant (p=0.078, p=0.095, and p=0.189, respectively) (Table 2). Other neutrophil counts, C3, C4, ESR, and CRP levels results also showed no significant differences between the two groups. For the MAST test, there was no difference between the two groups in the detection rate of Class 2 and above (p=0.164). However, while the detection rate of MAST inhalant showed no significant difference (p=0.334), the MAST food detection rate was notably higher in the non-responder group (p=0.033). Despite this, none of the individual items within the 19 MAST inhalant panels or the 42 MAST food panels showed statistical significance (Fig. 1).
Fig. 1. Comparison of MAST food and MAST inhalant detection rates between responder and non-responder groups.
MAST: multiple antigen simultaneous test.
TFT abnormalities were found in 8 patients (10.3%) in the responder group and 3 (25.0%) in the non-responder group, and the responder group included 18 patients (23.1%), while the non-responder group included 2 patients (16.7%) with positive ANA, although, this difference was not statistically significant (Table 2).
Exploring correlations among clinical variables in CSU: a heatmap analysis
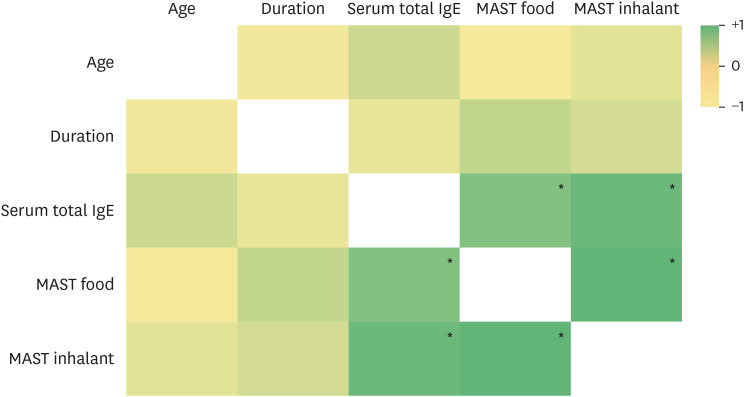
We conducted a correlation analysis of several variables and generated a heat map (Fig. 2). Although there was a negative correlation (−0.065) between disease duration and serum total IgE levels, the association was not statistically significant (p=0.577). In contrast, total serum IgE exhibited a statistically significant positive correlation with both MAST food (correlation coefficient=0.442) and MAST inhalant (correlation coefficient=0.542), with a p-value <0.05.
Fig. 2. Heatmap illustrating the correlation between variables in CSU patients.
CSU: chronic spontaneous urticaria, IgE: immunoglobulin E, MAST: multiple antigen simultaneous test.
*p<0.05.
DISCUSSION
In the present study, we retrospectively evaluated the electronic medical records of patients with CSU who were treated with omalizumab, in order to determine which biomarkers could be used to predict a good response to omalizumab. Shorter disease duration and higher initial total IgE levels were significantly associated with an increased response to omalizumab. Of note, an increased level of MAST food panel sensitivity was significantly associated with a higher probability of belonging to the non-responder group. Furthermore, our correlation analysis indicates that disease severity, as measured by UAS7 scores, may also serve as a key prognostic factor in omalizumab treatment.
The duration of CSU was longer in non-responders than in responders, with a mean duration of 11.5 months for the responder group, compared to 37.5 months for the non-responder group, meaning the duration of CSU in the non-responder group was almost 3 times longer than that in the responder group. This finding is consistent with those of previous studies. Marzano et al., in particular, reported that the duration of CSU before starting omalizumab was significantly longer in patients who experienced a first or second relapse16.
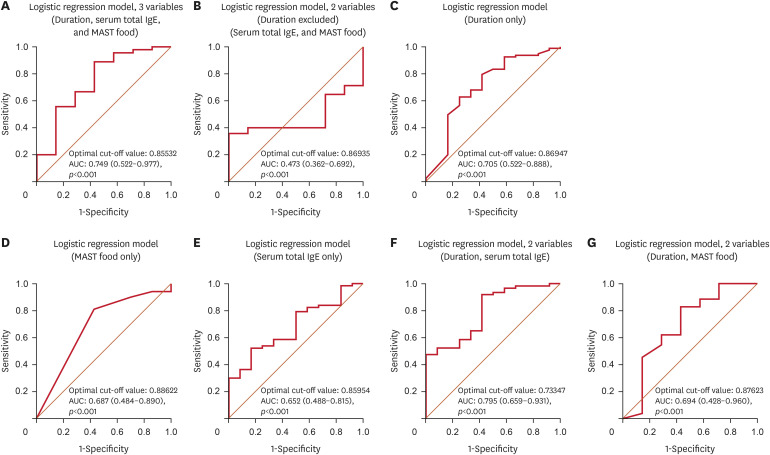
In the logistic regression analysis, we evaluated disease duration, serum total IgE, and MAST food as the investigated factors based on their significant results in the Mann-Whitney U test. Of these, only disease duration emerged as a significant predictor. Specifically, the results of the present study indicated that a longer disease duration was associated with an increased risk of poor response to omalizumab treatment (odds ratio, 0.975; 95% confidence interval [CI], 0.955–0.995) (Table 3). When predicting omalizumab response using only serum total IgE and MAST food excluding duration, the area under the curve (AUC) was 0.473 (95% CI, 0.362–0.692) with a cut-off value of 0.869. This AUC value rose to 0.749 (95% CI, 0.522–0.977) with a cut-off of 0.855 when duration was incorporated. Analyzing each variable individually, duration had the highest AUC at 0.705, compared to MAST food’s 0.687 and serum total IgE’s 0.652, underlining duration's superior standalone predictive capability for omalizumab treatment. These findings emphasize duration’s central role in forecasting the omalizumab treatment outcome, suggesting that patients who have suffered from CSU for a longer time may benefit from personalized therapeutic interventions. An interesting observation was that the combined AUC for duration and serum total IgE was 0.795, marginally surpassing the AUC from duration alone, hinting at a possible synergy between the 2 that merits further exploration (Fig. 3).
Table 3. Logistic regression analysis.
| Variables | Logistic regression | ||
|---|---|---|---|
| OR (95% CI) | p-value | c-statistics* | |
| Disease duration | 0.975 (0.955–0.995) | 0.016* | 0.749 |
| Serum total IgE | 1.001 (0.995–1.007) | 0.769 | |
| MAST food | 0.965 (0.798–1.168) | 0.717 | |
Bold denotes statistical significance (p<0.05).
Nagelkerke R2: 0.213; Hosmer & Lemeshow test: χ2=5.444 (p=0.709).
OR: odds ratio, CI: confidence interval, IgE: immunoglobulin E, MAST: multiple antigen simultaneous test.
*c-statistics: area under the curve value of the logistic regression model.
Fig. 3. ROC curve for logistic regression model.
ROC: receiver operating characteristic, IgE: immunoglobulin E, MAST: multiple antigen simultaneous test, AUC: area under the curve, CI: confidence interval.
There have been several reports published which suggest serum total IgE level could be used as a predictive biomarker for the response to omalizumab treatmeant8,17. Nettis et al.1 studied the omalizumab response by dividing serum total IgE into 3 groups, and found that higher pretreatment serum total IgE levels were associated with better treatment responses. Straesser et al.18 also found that lower serum total IgE (≤15.2 IU/mL) correlated with significantly lower responses to omalizumab treatment. Based on these studies, our data on Korean patients was consistent with the previous findings involving the Caucasian population. In previous studies, Marzano et al.16, Weller et al.17, and Cugno et al.19 reported that the average total IgE level was significantly higher in patients who responded to omalizumab treatment than those who did not. Of note, the results of the present study showed relatively elevated total serum IgE levels in both the responder and non-responder groups (185.0 vs. 90.5 IU/mL), which is consistent with previous studies by Litonjua et al.20 and Park et al.21. According to the results of the aforementioned studies, the serum total IgE levels of White, Black, and Hispanic individuals were much lower than those of Koreans, when evaluating healthy patients. This provides a possible explanation for the potential use of serum total IgE level as a biomarker to predict omalizumab response in Korean patients with CSU. In our correlation analysis, we found a significant positive correlation between total serum IgE levels and both MAST food and inhalant sensitivities.
In the present study, we examined the association between sensitization rates to MAST panels and omalizumab treatment outcomes in patients with CSU. Our results revealed a significant correlation between higher sensitization rates to certain MAST food test items and poorer responses to omalizumab treatments. Although CSU is typically not associated with food allergens, there are rare cases in which patients may experience sensitivity to certain foods. In such cases, avoiding specific foods may be beneficial to symptom management. Therefore, in the non-responder group, the addition of food allergy evaluations, in conjunction with omalizumab treatment, may be considered for patients with high sensitization rates to improve treatment outcomes. However, food allergies and their associated immune responses are complex and multifaceted, and further research is needed to fully understand their underlying mechanisms. Overall, the present study highlights the importance of personalized approaches to the management of CSU, taking into account the characteristics of individual patients, such as sensitization rates to specific food items. This approach could lead to better outcomes and more effective treatment decisions in patients with CSU. Nonetheless, the retrospective design of our study introduces certain limitations. Specifically, it was not possible to directly verify with patients if the foods detected through MAST testing were directly linked to exacerbations of CSU symptoms. Consequently, we lack definitive evidence that these foods directly contributed to the worsening of symptoms. Furthermore, while positive results on the MAST panel indicate sensitization, they do not unequivocally establish a direct exacerbation of CSU symptoms, potentially reflecting non-specific increases in total serum IgE levels rather than specific allergic reactions.
CSU encompasses 2 types of autoimmunity, type I and type IIb. Type I autoimmunity, also known as autoallergy, occurs when the body’s immune system produces IgE autoantibodies that recognize and bind to self-antigens, such as thyroid peroxidase (TPO), thyroglobulin (TG), tissue factor, IL-24, or double stranded deoxyribonucleic acid (dsDNA). When these autoantibodies cross-link with their target antigens, they trigger the release of histamine and other inflammatory molecules from mast cells, leading to the development of hives. Type IIb autoimmunity, on the other hand, is caused by the presence of IgG autoantibodies that activate mast cells by binding to their IgE receptors (FcεRI). The activation of mast cells leads to the release of histamine and other inflammatory molecules, leading to the development of hives. Both types of autoimmunity can contribute to the development of CSU; therefore, the treatment of CSU typically involves the use of antihistamines and other medications to alleviate symptoms and modulate the immune response19,20,21. The target of omalizumab might be type I autoimmunity22, although the exact reason why serum total IgE is correlated with omalizumab response is not well understood. It is possible that patients who have elevated type II autoimmunity may respond less to omalizumab, and there could be a link between type II autoimmunity and low serum total IgE levels22,23,24,25. Therefore, further studies are needed to evaluate the linkage between low serum total IgE levels and type II autoimmunity.
The function and number of basophils or eosinophils may also be correlated with the response to omalizumab treatment, as it has been reported that impaired basophil function may contribute to a decreased omalizumab response. Regarding the functional aspects of basophils, increased basophil CD203c upregulation through FcεRI stimulation showed good response to omalizumab treatment in patients with CSU26. Deza et al.9 discovered that lower baseline levels of the basophil FcεRI receptor are correlated with a poor therapeutic response. Additionally, eosinopenia is also associated with type IIb autoimmunity, along with increased disease activity and a limited response to treatment27. There are few reports, however, on the relationship of the number of eosinophils and basophils with the response to omalizumab treatment. Interestingly, discordant findings were observed in the present study compared to those of previous studies, and in the present study, the eosinophil and basophil counts were lower, although statistical significance was not found, in the responder group than the non-responder group.
According to recent research on omalizumab response in a small Korean population (n=26), the lower the body mass index or higher the serum total IgE level, the better the improvement in the UAS7 score after the 12th week of treatment, showing a good treatment response that is statistically significant12.
The present study did have several limitations. Firstly, this was a retrospective, single-center, cross-sectional study, with a smaller number of patients (n=90). As such, there was a possibility of selection bias. Secondly, another cohort study should be evaluated to re-confirm the reliability of biomarkers, particularly those for serum total IgE levels, and MAST results. Lastly, our analysis was limited to the utilization of UAS7 as the primary metric for assessing disease activity, without the inclusion of other significant indicators such as the Dermatology Life Quality Index and the Weekly Itch Severity Score. This exclusion may limit the comprehensiveness of our findings in reflecting the full impact of Chronic Spontaneous Urticaria on patients’ quality of life.
In conclusion, the present study involved nearly 100 individuals, complementing previous studies that identified predictive biomarkers of omalizumab treatment response in Korean patients with CSU. The present study has a significant advantage, in that it can predict the treatment response in Korean patients with CSU prior to starting omalizumab treatment. Longer disease duration, lower initial total IgE levels, and greater MAST food sensitivity may serve as potential biomarkers indicating a poor response to omalizumab treatment in patients with CSU, with disease duration being a stronger predictive factor than serum total IgE levels and MAST food sensitivity. These biomarkers may, therefore, lead to an individualized approach to treat patients with CSU, considering the clinical and laboratory characteristics of omalizumab treatment.
Footnotes
FUNDING SOURCE: This study was supported by the grant of the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (grant No. HI14C1324) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT; grant No. NRF-2021R1A4A5032185).
CONFLICTS OF INTEREST: The authors have nothing to disclose.
DATE SHARING STATEMENT: The data supporting the findings of the present study are available from the corresponding author upon reasonable request.
References
- 1.Nettis E, Cegolon L, Di Leo E, Lodi Rizzini F, Detoraki A, Canonica GW, et al. Omalizumab in chronic spontaneous urticaria: efficacy, safety, predictors of treatment outcome, and time to response. Ann Allergy Asthma Immunol. 2018;121:474–478. doi: 10.1016/j.anai.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Johal KJ, Saini SS. Current and emerging treatments for chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2020;125:380–387. doi: 10.1016/j.anai.2019.08.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz M, Vadasz Z, Kocatürk E, Giménez-Arnau AM. Omalizumab updosing in chronic spontaneous urticaria: an overview of real-world evidence. Clin Rev Allergy Immunol. 2020;59:38–45. doi: 10.1007/s12016-020-08794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolkhir P, Altrichter S, Munoz M, Hawro T, Maurer M. New treatments for chronic urticaria. Ann Allergy Asthma Immunol. 2020;124:2–12. doi: 10.1016/j.anai.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Licari A, Marseglia A, Caimmi S, Castagnoli R, Foiadelli T, Barberi S, et al. Omalizumab in children. Paediatr Drugs. 2014;16:491–502. doi: 10.1007/s40272-014-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham J, McBride D, Stull D, Halliday A, Alexopoulos ST, Balp MM, et al. Cost utility of omalizumab compared with standard of care for the treatment of chronic spontaneous urticaria. Pharmacoeconomics. 2016;34:815–827. doi: 10.1007/s40273-016-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altrichter S, Hawro T, Hänel K, Czaja K, Lüscher B, Maurer M, et al. Successful omalizumab treatment in chronic spontaneous urticaria is associated with lowering of serum IL-31 levels. J Eur Acad Dermatol Venereol. 2016;30:454–455. doi: 10.1111/jdv.12831. [DOI] [PubMed] [Google Scholar]
- 8.Ertas R, Ozyurt K, Atasoy M, Hawro T, Maurer M. The clinical response to omalizumab in chronic spontaneous urticaria patients is linked to and predicted by IgE levels and their change. Allergy. 2018;73:705–712. doi: 10.1111/all.13345. [DOI] [PubMed] [Google Scholar]
- 9.Deza G, Bertolín-Colilla M, Pujol RM, Curto-Barredo L, Soto D, García M, et al. Basophil FcεRI expression in chronic spontaneous urticaria: a potential immunological predictor of response to omalizumab therapy. Acta Derm Venereol. 2017;97:698–704. doi: 10.2340/00015555-2654. [DOI] [PubMed] [Google Scholar]
- 10.Palacios T, Stillman L, Borish L, Lawrence M. Lack of basophil CD203c-upregulating activity as an immunological marker to predict response to treatment with omalizumab in patients with symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2016;4:529–530. doi: 10.1016/j.jaip.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung BY, Um JY, Kang SY, Kim HO, Park CW. Natural history of chronic urticaria in Korea. Ann Dermatol. 2020;32:38–46. doi: 10.5021/ad.2020.32.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang MJ, Kim HS, Kim HO, Park YM. The impact of chronic idiopathic urticaria on quality of life in Korean patients. Ann Dermatol. 2009;21:226–229. doi: 10.5021/ad.2009.21.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HY, Koo DW, Lee JS. Analysis of predictive factors of response to omalizumab treatment in chronic spontaneous urticaria patients. Korean J Dermatol. 2021;59:685–692. [Google Scholar]
- 14.Stull D, McBride D, Tian H, Gimenez Arnau A, Maurer M, Marsland A, et al. Analysis of disease activity categories in chronic spontaneous/idiopathic urticaria. Br J Dermatol. 2017;177:1093–1101. doi: 10.1111/bjd.15454. [DOI] [PubMed] [Google Scholar]
- 15.Ye YM, Koh YI, Choi JH, Kim MA, Park JW, Kim TB, et al. The burden of symptomatic patients with chronic spontaneous urticaria: a real-world study in Korea. Korean J Intern Med. 2022;37:1050–1060. doi: 10.3904/kjim.2022.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzano AV, Genovese G, Casazza G, Fierro MT, Dapavo P, Crimi N, et al. Predictors of response to omalizumab and relapse in chronic spontaneous urticaria: a study of 470 patients. J Eur Acad Dermatol Venereol. 2019;33:918–924. doi: 10.1111/jdv.15350. [DOI] [PubMed] [Google Scholar]
- 17.Weller K, Ohanyan T, Hawro T, Ellrich A, Sussman G, Koplowitz J, et al. Total IgE levels are linked to the response of chronic spontaneous urticaria patients to omalizumab. Allergy. 2018;73:2406–2408. doi: 10.1111/all.13586. [DOI] [PubMed] [Google Scholar]
- 18.Straesser MD, Oliver E, Palacios T, Kyin T, Patrie J, Borish L, et al. Serum IgE as an immunological marker to predict response to omalizumab treatment in symptomatic chronic urticaria. J Allergy Clin Immunol Pract. 2018;6:1386–1388.e1. doi: 10.1016/j.jaip.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cugno M, Genovese G, Ferrucci S, Casazza G, Asero R, Marzano AV. IgE and D-dimer baseline levels are higher in responders than nonresponders to omalizumab in chronic spontaneous urticaria. Br J Dermatol. 2018;179:776–777. doi: 10.1111/bjd.16593. [DOI] [PubMed] [Google Scholar]
- 20.Litonjua AA, Celedón JC, Hausmann J, Nikolov M, Sredl D, Ryan L, et al. Variation in total and specific IgE: effects of ethnicity and socioeconomic status. J Allergy Clin Immunol. 2005;115:751–757. doi: 10.1016/j.jaci.2004.12.1138. [DOI] [PubMed] [Google Scholar]
- 21.Park GS, Lee GS, Kim CW. Study on serum IgE levels in healthy Korean. Korean J Clin Pathol. 1982;2:65–72. [Google Scholar]
- 22.Kolkhir P, Church MK, Weller K, Metz M, Schmetzer O, Maurer M. Autoimmune chronic spontaneous urticaria: what we know and what we do not know. J Allergy Clin Immunol. 2017;139:1772–1781.e1. doi: 10.1016/j.jaci.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 23.Bracken SJ, Abraham S, MacLeod AS. Autoimmune theories of chronic spontaneous urticaria. Front Immunol. 2019;10:627. doi: 10.3389/fimmu.2019.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer M, Eyerich K, Eyerich S, Ferrer M, Gutermuth J, Hartmann K, et al. Urticaria: Collegium Internationale Allergologicum (CIA) update 2020. Int Arch Allergy Immunol. 2020;181:321–333. doi: 10.1159/000507218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolkhir P, Muñoz M, Asero R, Ferrer M, Kocatürk E, Metz M, et al. Autoimmune chronic spontaneous urticaria. J Allergy Clin Immunol. 2022;149:1819–1831. doi: 10.1016/j.jaci.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Oda Y, Fukunaga A, Washio K, Imamura S, Mizuno M, Hatakeyama M, et al. Improved FcεRI-mediated CD203c basophil responsiveness reflects rapid responses to omalizumab in chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2021;9:1166–1176.e8. doi: 10.1016/j.jaip.2020.08.048. [DOI] [PubMed] [Google Scholar]
- 27.Kolkhir P, Church MK, Altrichter S, Skov PS, Hawro T, Frischbutter S, et al. Eosinopenia, in chronic spontaneous urticaria, is associated with high disease activity, autoimmunity, and poor response to treatment. J Allergy Clin Immunol Pract. 2020;8:318–325.e5. doi: 10.1016/j.jaip.2019.08.025. [DOI] [PubMed] [Google Scholar]