Abstract
Transcranial direct current stimulation (tDCS) has emerged as a valuable neuromodulation technique. Many clinical conditions are associated with brain damage, and in severe cases, structural changes such as skull defects are common. These clinical characteristics result in distinct electrical flow patterns during tDCS application compared to cases without brain damage. Recently, notable advancements have been made in both the medical and engineering fields pertaining to the use of in silico modelling and simulation with the aid of magnetic resonance imaging (MRI). As a result, it is now possible to conduct simulations tailored to the unique structural anatomy of an individual’s brain, using their own MRI data, to provide targeted tDCS. We have developed software that performs both segmentation and simulation, and have conducted randomized controlled trials using optimized tDCS for stroke and disorders of consciousness. Additionally, we have carried out simulation-related research on stroke and burr hole surgery. This review examines various articles related to simulation and optimized tDCS, evaluating their clinical implications. We believe that these insights will provide valuable guidance for both current and future applications of tDCS.
Keywords: Transcranial Direct Current Stimulation, Neuromodulation, Optimization, Simulation, Segmentation
Highlights
• Transcranial direct current stimulation (tDCS) has been used for neuromodulation.
• Stroke or other brain damage may change the electric flow with tDCS.
• Simulation would be necessary for tDCS in cases with or without structural damage.
INTRODUCTION
Transcranial direct current stimulation (tDCS) has become a widely utilized method of neuromodulation for a variety of medical conditions [1,2,3]. Attempts to treat neuropsychiatric disorders through electrical brain stimulation have persisted from ancient times to the modern era. In the early 2000s, Nitsche and Paulus published influential studies showing that tDCS could effectively modulate cortical excitability [4]. These findings, along with growing interest in non-invasive brain stimulation techniques, led to a surge of research into the therapeutic potential of tDCS for a range of conditions, including depression [5], addiction [6], chronic pain [7], and cognitive enhancement [8]. The non-invasive nature, relative safety, and low cost of tDCS have made it an attractive option for research into brain function and neuroplasticity [3]. In stroke rehabilitation, the initial study applied tDCS to improve hand motor function in stroke patients [9]. Since then, subsequent studies have reported that tDCS is an effective intervention for enhancing language abilities, upper limb motor function, and activities of daily living in patients with subacute and chronic stroke [7,9,10]. Recent evidence-based guidelines also recommend the application of tDCS for patients who have suffered a stroke [11].
Although tDCS shows significant potential as a neuromodulation method, determining the optimal therapeutic protocol is challenging due to the complexity of stimulation parameters and the substantial differences in conductivity among various tissues, such as the scalp, skull, cerebrospinal fluid (CSF), and gray matter [12]. Conventionally, stimulating electrodes are placed directly over the target brain area, but this does not guarantee optimal stimulation of that region. In addition to electrode placement, other parameters—such as current amplitude, number of electrodes, electrode size, and electrode montage—must also need to be optimized. Computer simulations can assist in designing an optimal tDCS treatment protocol [13].
Recently, tDCS simulation software based on brain imaging data has become increasingly prevalent [14]. These simulations accurately delineate the human brain structure, thereby enabling the calculation of the brain’s electrical current flow and the determination of optimal stimulation locations and intensities [15]. Among various software programs for segmentation and simulations, tES LAB (Neurophet®, Seoul, Korea) has already been developed and commercialized in South Korea. Increasingly, medical procedures have evolved from being performed without expert guidance to being conducted with specialist assistance. Accordingly, we propose that electrical simulations based on in silico modeling should become a necessary component of tDCS. We have recently engaged in research aimed at optimizing this process through software development, in silico modeling simulations, and clinical trials. As a result, we present a review and discussion of optimized tDCS from both technical and clinical perspectives, drawing on our previous research.
SIMULATION WITH PATIENTS WITHOUT STRUCTURAL DAMAGE
There are several methods to localize electrode placement for tDCS. The 10-20 electroencephalogram (EEG) measurement system is commonly used to guide electrode placement [16,17,18]. Alternatively, neuro-navigation software, which may offer greater accuracy than the 10-20 EEG system, can also be used. Studies have shown that electrode positioning using the 10-20 system can differ significantly from magnetic resonance imaging (MRI)-based neuro-navigation, even in healthy brains [18,19]. One common target area for motor recovery following stroke is the primary motor cortex (M1), which is typically located at the C3/C4 region in the 10-20 system. However, Silva et al. [18] demonstrated that C3/C4 was among the least accurate locations, with the longest distance to the M1-hand area. Specifically, the distance from the C3h/C4h point (using the international 10-5 system) to M1 was only 0.98 cm, while the distance from C3/C4 reached 2.6 cm. Similarly, a comparative study on dorsolateral prefrontal cortex (DLPFC) stimulation found that the 10-20 EEG system targets different parts of the superior frontal cortex (a region within the DLPFC) compared to MRI-guided neuro-navigation [19]. The neuro-navigation positioned the left DLPFC anodal electrode more laterally and posteriorly than the 10-20 system, leading to distinct underlying electric field distributions when simulated.
Moreover, several studies have noted that the peak electric field tends to occur approximately midway between the anode and cathode, slightly favoring the anode [20,21]. This suggests that conventional electrode montages, which rely on the 10-20 system for localization, may result in suboptimal electric field strengths in the targeted brain region. To address these limitations, optimal stimulation can be achieved by calculating electrode positions and current strengths that maximize the electric field strength or its focality at the target point using computational modeling [22].
Although tDCS has shown promising results in many studies, its outcomes have been inconsistent among subjects, even when the same protocol is applied [23,24]. This significant variability of results complicates the establishment of standardized treatment protocols. As the stimulating current passes through a complex path—including the scalp, skull, meninges, and CSF—before reaching the brain, the resulting cortical electric field differs based on each individual’s unique anatomical features. Cross-sectional studies investigating tDCS-induced electric fields in groups of healthy adults have shown variability in the electric field strength across different cortical regions [25]. Contributing factors to this variability include gyral and sulcal geometry, differences in white and gray matter architecture, variations in skull thickness, and current shunting through the highly conductive CSF [26,27]. These anatomical differences can lead to different electric fields, even with the same tDCS stimulation, which may account for the variability in tDCS outcomes. Therefore, it is essential to develop an optimized tDCS strategy that takes into consideration the unique anatomical structures of each individual, even in the absence of structural brain damage.
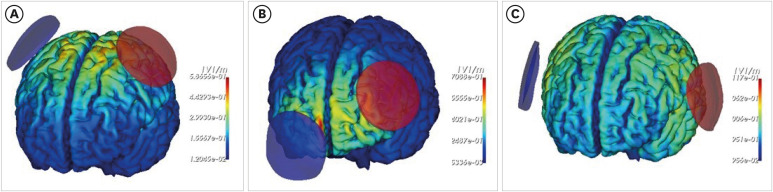
Computational modeling simulates tDCS applications to estimate the magnitude, distribution, and direction of the current delivered to the brain for a given protocol. This is achieved by classifying different tissue types using MRI scans and assigning conductivity values to the segmented compartments. The head, which consists of various tissues including skin, skull, CSF, gray matter, and white matter, has anatomically complex electrical properties [13,15]. Therefore, accurately identifying these tissues is crucial for estimating the brain’s electric field. Based on these electric field estimations, electrode placement and current strength can be adjusted to optimally target specific brain regions. Thus, the author developed a more precise and accurate segmentation methodology with our research team [15]. The deep learning method, which has recently become a breakthrough approach, has been employed to overcome the limitations of conventional segmentation methods. Furthermore, a novel framework for brain tissue segmentation has been proposed, whereby brain structure is utilized as prior information and a convolutional neural network is employed. Fig. 1 shows the results of computational modeling using tES LAB in healthy adults, targeting the hand knob of the primary motor cortex, the left Broca area, and the DLPFC. With this current flow modeling software, optimal electrode placement can be determined based on the targeted brain region, and the resulting electric field distribution can be evaluated.
Fig. 1. The simulation of targeting multiple brain regions on a single subject without structural damage. The regions included are the hand knob within the primary motor cortex (A), the Broca area (B), and the dorsolateral prefrontal cortex (C). The color represents the electric field and intensity. The red electrode indicates the anode and blue indicates the cathode.
SIMULATION WITH PATIENTS WITH MILD TO MODERATE STRUCTURAL DAMAGE
A significant number of patients who could benefit from tDCS treatment, such as those with stroke or traumatic brain injury, often exhibit varying degrees of structural head damage. In clinical practice, it is generally assumed that the severity of brain damage is related to the degree of functional impairment. Therefore, alongside conventional rehabilitation, neuromodulation treatments like tDCS are considered crucial adjuncts for functional recovery in patients with significant brain damage. However, brain lesions can influence the electric field distribution by tDCS, which may hinder adequate stimulation of the intended target area. Stroke or traumatic brain injury typically results in structural changes, initially manifesting as edema, which leads to an increase in size and a decrease in electrical resistance [28]. This is followed by chronic astrocytosis and tissue shrinkage, leading to increased electrical resistance. In addition, structural alterations, like cavities or ventricular hypertrophy filled with CSF, further contribute to the heterogeneity of tDCS outcomes [29].
Computational modeling studies revealed that, an electric field was stably generated at the target point in a healthy brain, but the overall electric field intensity in the cerebral cortex was significantly reduced in stroke patients [29,30]. As a result, conventional tDCS may fail to deliver sufficient stimulation to the target region in patients with structural brain injuries, thus limiting its therapeutic effectiveness. To address this challenge, computational modeling of tDCS is especially crucial for individuals with substantial brain damage. The advent of in silico modeling allows for simulations that reflect the specific damaged brain structure, enabling precise calculation of electric field values and optimization of electrode positioning to maximize stimulation in the target area. This approach also helps in determining the optimal current levels that can be safely administered to achieve the desired therapeutic effect.
We hypothesized that optimized tDCS would entail a distinct electrode positioning for each stroke patient, thereby generating more robust electric fields at target sites than conventional tDCS. In our study, an optimized tDCS montage was identified, designed to maximize the electric field in hand motor areas. To test the efficacy of optimized tDCS, a within-subject design was employed to simulate conventional and optimized tDCSs in 21 stroke patients, with subsequent comparisons made between the 2 [31]. The optimized tDCS configuration yielded higher-intensity electric fields at the targeted regions than the conventional tDCS approach. Moreover, electrode placement for optimized tDCS varied significantly between patients, demonstrating notable differences from the conventional placement [31]. These findings support our initial hypothesis, indicating that tDCS optimized via computational modeling has the potential to enhance stroke motor rehabilitation more effectively than conventional tDCS [31].
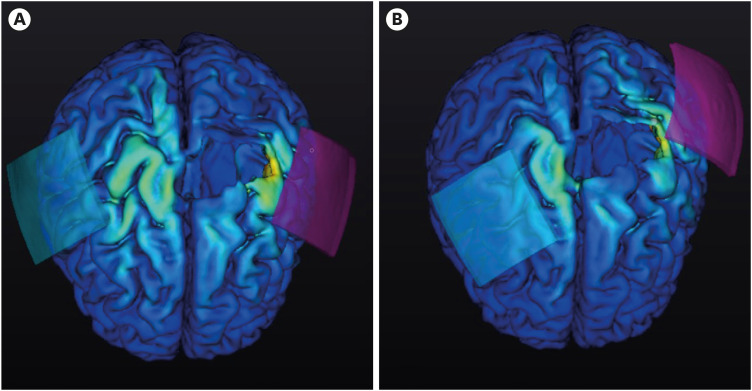
In addition, we conducted a double-blind, randomized controlled trial for enhancing motor recovery of the upper limbs in the patients with subacute to chronic stroke, which proved the efficacy of tDCS based on personalized MRI-based electrical field simulation and optimized stimulation [32]. Fig. 2 illustrates the representative electrode positioning for both conventional tDCS and the optimized simulation. Our earlier study found that patients with cortical lesions or low initial Fugl-Meyer Assessment for the Upper Extremity (FMA-UE) scores require electrode positions for optimized tDCS that are different from conventional tDCS montage [31]. These findings indicate that cortical lesions have a considerable impact on the electric field, thereby increasing the complexity of the computational modeling process when selecting the optimal montage. In patients with low initial FMA-UE scores, optimized electrode placements were located farther from conventional electrode positions. Studies have shown that stroke patients often experience a decrease in cerebral cortex thickness and surface area, which is associated with damage to the corticospinal tract [33,34]. This cortical atrophy, coupled with an increase in local CSF thickness, can significantly attenuate the electric field induced by tDCS [30]. Therefore, when treating stroke patients with cortical lesions or severely compromised initial upper limb function, employing an optimal montage determined through computational modeling can enhance the effectiveness of tDCS.
Fig. 2. The representative positioning of electrodes for transcranial direct current stimulation with conventional placement and with optimization based on magnetic resonance imaging simulation. The conventional placement is shown in (A), while the optimized placement is shown in (B). The color represents the electric field and intensity. The red electrode indicates the anode and blue indicates the cathode.
SIMULATION WITH PATIENTS WITH SEVERE STRUCTURAL DAMAGE OR SKULL DAMAGE
As a physiatrist specializing in neuro-rehabilitation, a significant portion of our clinical caseload comprises patients with severe brain diseases who have undergone a range of structural interventions, including craniectomy or cranioplasty with titanium, ventriculoperitoneal shunt placement, or titanium clip surgery. Traditionally, it has been accepted that non-invasive neuromodulation treatments are contraindicated for patients who have undergone such brain surgeries. Currently, conventional tDCS is also considered contraindicated for patients with craniotomy or metallic implants in their skull [35]. However, a recent case has reported the use of 2 mA cerebellar tDCS in patients with deep brain stimulators in the hypothalamus for the treatment of generalized dystonia [36]. The skull and scalp serve as high- and low-frequency filters, respectively [37]. Most neurosurgical procedures have the potential to alter this filter by forming scars, and as a result, the injected current may not flow as we intended. Therefore, the implementation of tDCS treatment without prior simulation may not be acceptable in clinical settings.
A prospective, randomized, placebo-controlled, crossover, double-blind, multicenter phase 2 feasibility study was conducted to assess the feasibility of using tDCS based on electrical field simulation with MRI for the restoration of consciousness in patients with prolonged disorders of consciousness [38]. During the trial, we identified several patients who were deemed ineligible for tDCS due to contraindications resulting from prior brain surgeries. In response, we conducted an additional simulation study focusing on patients who had undergone burr hole surgery [39]. The objective of this study was to investigate how small skull defects from burr hole surgery affect electric field distribution. To accomplish this, we compared the electric field distributions in patients with burr holes to those without. Additionally, we examined changes in the cortical electric field generated at the burr hole while varying the position of the electrode. Our in-silico modeling revealed that the cortical electric field intensity at the burr hole increased as the anode approached it, but remained constant when the anode was more than 60 mm away. We also found that a high electric field is generated deep within the brain when the anode is positioned directly above the burr hole. Therefore, we propose that patient-specific head modeling and development of an optimized tDCS protocol are essential to effectively and safely apply tDCS to stroke patients who have undergone skull surgery.
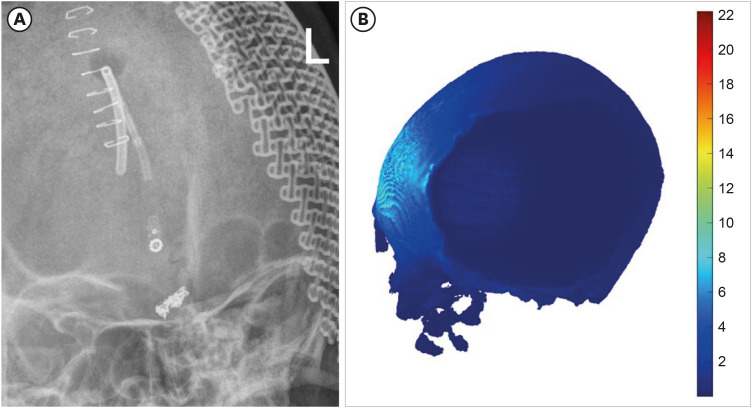
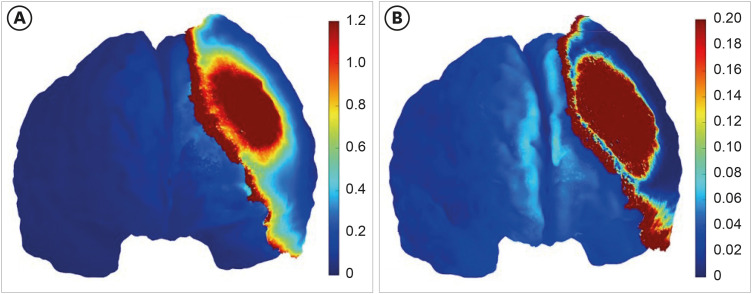
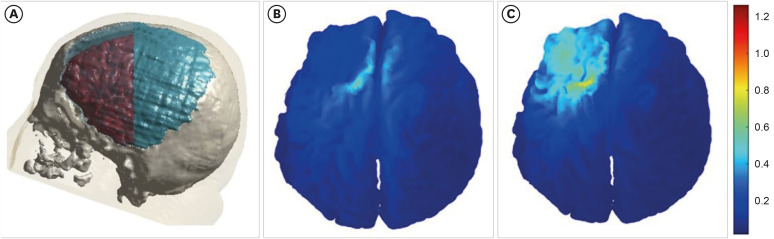
Finally, we reviewed the simulation data from our recent case report involving a patient with prolonged disorder of consciousness who underwent cranioplasty with a titanium plate after craniectomy and was treated with tDCS based on MRI-based simulation [40]. When a titanium plate is used, the current is redirected to flow beneath the surgical site (Fig. 3). Given that the conductivity of skin is low (0.465 s/m) and that of titanium is high (5,000 s/m), it would be beneficial to simulate by changing the conductivity of skin and titanium. The skin does not exhibit a shunting effect, resulting in an electric field strength of 0.4–0.6 V/m in the DLPFC (Fig. 4). In contrast, the current flowing to the edge of the titanium plate indicates a shunting effect, leading to a significantly lower electric field strength of less than 0.2 V/m (Fig. 4). In conclusion, the procedure entailed layer-specific targeting of the right DLPFC, along with the simulation of conventional and optimization techniques, and treatment based on the recommendations outlined in Fig. 5.
Fig. 3. The shunting effect with simulation. Titanium plate is shown in (A), the shunting effect is shown in (B). The color represents the electric field and intensity.
Fig. 4. The electrical simulation with skin and metal as variables. Given the low conductivity of skin (0.465 s/m) and the high conductivity of titanium (5,000 s/m), it would be advantageous to simulate the process by modifying the variables associated with the skin and titanium. The simulation with skin is shown in (A), the simulation with titanium is shown in (B). The color represents the electric field and intensity.
Fig. 5. The electrical simulation by conventional and optimization for targeting the right dorsolateral prefrontal cortex. (A) The simulation montages used to simulate the effects on cortical current flow from transcranial direct current stimulation. (B) The simulation via conventional stimulation with 10-20 system. (C) The simulation via optimization based on the patient’s magnetic resonance imaging. The color represents the electric field and intensity [39].
Following neurosurgical procedures involving the skull, the electrical conductivity of large skull defects/plates may be increased, resulting in the diversion of current away from the directly underlying cortex and the accumulation of electrical activity in the cortex beneath the defect’s perimeter [41]. As a result, neuromodulation with tDCS in such cases may result in uneven current flow, leading to unpredictable and potentially adverse stimulation effects. Therefore, in silico modeling and simulation would be essential for patients with severe structural or skull damage [39]. By incorporating the structural characteristics of each patient, these simulations can calculate the electric field magnitude and determine appropriate stimulation parameters, thus effectively addressing these challenges. With the increasing adoption of guided treatments in medical procedures, optimization based on patient brain imaging will become the new standard practice for tDCS in the near future.
CONCLUSION
A novel future treatment framework for tDCS was proposed based on the patients' brain structure and computation simulation. This contribution is 3-fold: (1) the exact stimulation site was defined based on structure rather than the conventional 10-20 system; (2) a simulated electric field and Enorm values for the intensity of tDCS were proposed; and (3) the proposed method was subjected to quantitative and qualitative analyses through various experiments.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea funded by the Korean government (No. NRF 2021R1A2C1012113), and the Basic Medical Science Facilitation Program through the Catholic Medical Center of The Catholic University of Korea funded by the Catholic Education Foundation.
Conflict of Interest: The corresponding author of this manuscript is an Editor-in-Chief of Brain & NeuroRehabilitation. The other author has no potential conflicts of interest to disclose.
References
- 1.Aloi D, Della Rocchetta AI, Ditchfield A, Coulborn S, Fernández-Espejo D. Therapeutic use of transcranial direct current stimulation in the rehabilitation of prolonged disorders of consciousness. Front Neurol. 2021;12:632572. doi: 10.3389/fneur.2021.632572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camacho-Conde JA, Gonzalez-Bermudez MD, Carretero-Rey M, Khan ZU. Brain stimulation: a therapeutic approach for the treatment of neurological disorders. CNS Neurosci Ther. 2022;28:5–18. doi: 10.1111/cns.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang J, Lee H, Yu S, Lee M, Kim HJ, Kwon R, Kim S, Fond G, Boyer L, Rahmati M, Koyanagi A, Smith L, Nehs CJ, Kim MS, Sánchez GF, Dragioti E, Kim T, Yon DK. Effects and safety of transcranial direct current stimulation on multiple health outcomes: an umbrella review of randomized clinical trials. Mol Psychiatry. 2024 doi: 10.1038/s41380-024-02624-3. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 4.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunoni AR, Boggio PS, De Raedt R, Benseñor IM, Lotufo PA, Namur V, Valiengo LC, Vanderhasselt MA. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J Affect Disord. 2014;162:43–49. doi: 10.1016/j.jad.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Ekhtiari H, Tavakoli H, Addolorato G, Baeken C, Bonci A, Campanella S, Castelo-Branco L, Challet-Bouju G, Clark VP, Claus E, Dannon PN, Del Felice A, den Uyl T, Diana M, di Giannantonio M, Fedota JR, Fitzgerald P, Gallimberti L, Grall-Bronnec M, Herremans SC, Herrmann MJ, Jamil A, Khedr E, Kouimtsidis C, Kozak K, Krupitsky E, Lamm C, Lechner WV, Madeo G, Malmir N, Martinotti G, McDonald WM, Montemitro C, Nakamura-Palacios EM, Nasehi M, Noël X, Nosratabadi M, Paulus M, Pettorruso M, Pradhan B, Praharaj SK, Rafferty H, Sahlem G, Salmeron BJ, Sauvaget A, Schluter RS, Sergiou C, Shahbabaie A, Sheffer C, Spagnolo PA, Steele VR, Yuan TF, van Dongen JD, Van Waes V, Venkatasubramanian G, Verdejo-García A, Verveer I, Welsh JW, Wesley MJ, Witkiewitz K, Yavari F, Zarrindast MR, Zawertailo L, Zhang X, Cha YH, George TP, Frohlich F, Goudriaan AE, Fecteau S, Daughters SB, Stein EA, Fregni F, Nitsche MA, Zangen A, Bikson M, Hanlon CA. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev. 2019;104:118–140. doi: 10.1016/j.neubiorev.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolognini N, Spandri V, Ferraro F, Salmaggi A, Molinari AC, Fregni F, Maravita A. Immediate and sustained effects of 5-day transcranial direct current stimulation of the motor cortex in phantom limb pain. J Pain. 2015;16:657–665. doi: 10.1016/j.jpain.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Buss SS, Fried PJ, Pascual-Leone A. Therapeutic noninvasive brain stimulation in Alzheimer’s disease and related dementias. Curr Opin Neurol. 2019;32:292–304. doi: 10.1097/WCO.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 10.Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial direct current stimulation vs sham stimulation to treat aphasia after stroke: a randomized clinical trial. JAMA Neurol. 2018;75:1470–1476. doi: 10.1001/jamaneurol.2018.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DY, Ryu B, Oh BM, Kim DY, Kim DS, Kim DY, Kim DK, Kim EJ, Lee HY, Choi H, Kim HS, Lee HH, Kim HJ, Oh HM, Seok H, Park J, Park J, Park JG, Kim JM, Lee J, Shin JH, Lee JK, Oh JS, Park KD, Kim KT, Chang MC, Chun MH, Kim MW, Kang MG, Song MK, Choi M, Ko MH, Kim NY, Paik NJ, Jung SH, Yoon SY, Lim SH, Lee SJ, Yoo SD, Lee SH, Yang SN, Park SW, Lee SY, Han SJ, Lee SJ, Bok SK, Ohn SH, Im S, Pyun SB, Hyun SE, Kim SH, Ko SH, Jee S, Kwon S, Kim TW, Chang WH, Chang WK, Yoo WK, Kim YH, Yoo YJ, Kim YW, Shin YI, Park YG, Choi YH, Kim Y KSNR Stroke CPG Writing Group. Clinical practice guideline for stroke rehabilitation in Korea-Part 1: Rehabilitation for Motor Function (2022) Brain Neurorehabil. 2023;16:e18. doi: 10.12786/bn.2023.16.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales-Quezada L, El-Hagrassy MM, Costa B, McKinley RA, Lv P, Fregni F. Transcranial direct current stimulation optimization - from physics-based computer simulations to high-fidelity head phantom fabrication and measurements. Front Hum Neurosci. 2019;13:388. doi: 10.3389/fnhum.2019.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bikson M, Rahman A, Datta A. Computational models of transcranial direct current stimulation. Clin EEG Neurosci. 2012;43:176–183. doi: 10.1177/1550059412445138. [DOI] [PubMed] [Google Scholar]
- 14.Hunold A, Haueisen J, Nees F, Moliadze V. Review of individualized current flow modeling studies for transcranial electrical stimulation. J Neurosci Res. 2023;101:405–423. doi: 10.1002/jnr.25154. [DOI] [PubMed] [Google Scholar]
- 15.Lee J, Lee M, Lee J, Kim RE, Lim SH, Kim D. Fine-grained brain tissue segmentation for brain modeling of stroke patient. Comput Biol Med. 2023;153:106472. doi: 10.1016/j.compbiomed.2022.106472. [DOI] [PubMed] [Google Scholar]
- 16.Rich TL, Gillick BT. Electrode placement in transcranial direct current stimulation-how reliable is the determination of C3/C4? Brain Sci. 2019;9:69. doi: 10.3390/brainsci9030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich TL, Menk JS, Rudser KD, Chen M, Meekins GD, Peña E, Feyma T, Bawroski K, Bush C, Gillick BT. Determining electrode placement for transcranial direct current stimulation: a comparison of EEG- versus TMS-guided methods. Clin EEG Neurosci. 2017;48:367–375. doi: 10.1177/1550059417709177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva LM, Silva KM, Lira-Bandeira WG, Costa-Ribeiro AC, Araújo-Neto SA. Localizing the primary motor cortex of the hand by the 10-5 and 10-20 systems for neurostimulation: an MRI study. Clin EEG Neurosci. 2021;52:427–435. doi: 10.1177/1550059420934590. [DOI] [PubMed] [Google Scholar]
- 19.De Witte S, Klooster D, Dedoncker J, Duprat R, Remue J, Baeken C. Left prefrontal neuronavigated electrode localization in tDCS: 10-20 EEG system versus MRI-guided neuronavigation. Psychiatry Res Neuroimaging. 2018;274:1–6. doi: 10.1016/j.pscychresns.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8:046011. doi: 10.1088/1741-2560/8/4/046011. [DOI] [PubMed] [Google Scholar]
- 21.Rampersad SM, Janssen AM, Lucka F, Aydin Ü, Lanfer B, Lew S, Wolters CH, Stegeman DF, Oostendorp TF. Simulating transcranial direct current stimulation with a detailed anisotropic human head model. IEEE Trans Neural Syst Rehabil Eng. 2014;22:441–452. doi: 10.1109/TNSRE.2014.2308997. [DOI] [PubMed] [Google Scholar]
- 22.Dmochowski JP, Datta A, Huang Y, Richardson JD, Bikson M, Fridriksson J, Parra LC. Targeted transcranial direct current stimulation for rehabilitation after stroke. Neuroimage. 2013;75:12–19. doi: 10.1016/j.neuroimage.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strube W, Bunse T, Malchow B, Hasan A. Efficacy and interindividual variability in motor-cortex plasticity following anodal tDCS and paired-associative stimulation. Neural Plast. 2015;2015:530423. doi: 10.1155/2015/530423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimulat. 2014;7:372–380. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Laakso I, Tanaka S, Koyama S, De Santis V, Hirata A. Inter-subject variability in electric fields of motor cortical tDCS. Brain Stimulat. 2015;8:906–913. doi: 10.1016/j.brs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Opitz A, Paulus W, Will S, Antunes A, Thielscher A. Determinants of the electric field during transcranial direct current stimulation. Neuroimage. 2015;109:140–150. doi: 10.1016/j.neuroimage.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Datta A, Bansal V, Diaz J, Patel J, Reato D, Bikson M. Gyri-precise head model of transcranial direct current stimulation: improved spatial focality using a ring electrode versus conventional rectangular pad. Brain Stimul. 2009;2:201–207. 207.e1. doi: 10.1016/j.brs.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song J, Chen R, Yang L, Zhang G, Li W, Zhao Z, Xu C, Dong X, Fu F. Electrical impedance changes at different phases of cerebral edema in rats with ischemic brain injury. BioMed Res Int. 2018;2018:9765174. doi: 10.1155/2018/9765174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minjoli S, Saturnino GB, Blicher JU, Stagg CJ, Siebner HR, Antunes A, Thielscher A. The impact of large structural brain changes in chronic stroke patients on the electric field caused by transcranial brain stimulation. Neuroimage Clin. 2017;15:106–117. doi: 10.1016/j.nicl.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Cruijsen J, Dooren RF, Schouten AC, Oostendorp TF, Frens MA, Ribbers GM, van der Helm FC, Kwakkel G, Selles RW 4D EEG Consortium. Addressing the inconsistent electric fields of tDCS by using patient-tailored configurations in chronic stroke: Implications for treatment. Neuroimage Clin. 2022;36:103178. doi: 10.1016/j.nicl.2022.103178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon MJ, Park HJ, Yoo YJ, Oh HM, Im S, Kim TW, Lim SH. Electric field simulation and appropriate electrode positioning for optimized transcranial direct current stimulation of stroke patients: an in silico model. Sci Rep. 2024;14:2850. doi: 10.1038/s41598-024-52874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo YJ, Park HJ, Kim TY, Yoon MJ, Oh HM, Lee YJ, Hong BY, Kim D, Kim TW, Lim SH. MRI-based personalized transcranial direct current stimulation to enhance the upper limb function in patients with stroke: study protocol for a double-blind randomized controlled trial. Brain Sci. 2022;12:1673. doi: 10.3390/brainsci12121673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Wang C, Qin W, Ding H, Peng Y, Guo J, Han T, Cheng J, Yu C. Cortical structural changes after subcortical stroke: patterns and correlates. Hum Brain Mapp. 2023;44:727–743. doi: 10.1002/hbm.26095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Meng L, Qin W, Liu N, Shi FD, Yu C. Structural damage and functional reorganization in ipsilesional M1 in well-recovered patients with subcortical stroke. Stroke. 2014;45:788–793. doi: 10.1161/STROKEAHA.113.003425. [DOI] [PubMed] [Google Scholar]
- 35.Ko MH. Safety of transcranial direct current stimulation in neurorehabilitation. Brain Neurorehabil. 2021;14:e9. doi: 10.12786/bn.2021.14.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iannone A, Allam N, Brasil-Neto JP. Safety of transcranial direct current stimulation in a patient with deep brain stimulation electrodes. Arq Neuropsiquiatr. 2019;77:174–178. doi: 10.1590/0004-282X20190019. [DOI] [PubMed] [Google Scholar]
- 37.Liu A, Vöröslakos M, Kronberg G, Henin S, Krause MR, Huang Y, Opitz A, Mehta A, Pack CC, Krekelberg B, Berényi A, Parra LC, Melloni L, Devinsky O, Buzsáki G. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. 2018;9:5092. doi: 10.1038/s41467-018-07233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon MJ, Oh HM, Kim T, Choi SJ, Choi WH, Jung HS, Lim SC, Yoo YJ, Park HJ, Hong BY, Park GY, Kim D, Kim TW, Im S, Lim SH. Safety and therapeutic effects of personalized transcranial direct current stimulation based on electrical field simulation for prolonged disorders of consciousness: study protocol for a multi-center, double-blind, randomized controlled trial. Front Neurol. 2023;14:1184998. doi: 10.3389/fneur.2023.1184998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon MJ, Kim H, Yoo YJ, Im S, Kim TW, Dhaher YY, Kim D, Lim SH. In silico modeling of electric field modulation by transcranial direct current stimulation in stroke patients with skull burr holes: implications for safe clinical application. Comput Biol Med. 2024;184:109366. doi: 10.1016/j.compbiomed.2024.109366. [DOI] [PubMed] [Google Scholar]
- 40.Im S, Park GY, Kim TW, Lim SH. Optimized trans-cranial direct current stimulation for prolonged consciousness disorder in a patient with titanium mesh cranioplasty. Neurol Sci. 2024;45:3513–3516. doi: 10.1007/s10072-024-07516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datta A, Bikson M, Fregni F. Transcranial direct current stimulation in patients with skull defects and skull plates: high-resolution computational FEM study of factors altering cortical current flow. Neuroimage. 2010;52:1268–1278. doi: 10.1016/j.neuroimage.2010.04.252. [DOI] [PMC free article] [PubMed] [Google Scholar]