Abstract
Dung serves as a critical resource for diverse organisms, including dung-inhabiting fungi, which play a key role in nutrient cycling. In this study, we examined the decomposition rates and half-lives of dung from ruminant and monogastric herbivores in a microcosm experiment, assessing the impact of autoclaving (fungal exclusion) on decomposition dynamics. Over six months, autoclaved dung decomposed more slowly, retaining greater biomass and highlighting the fungi’s role in matter cycling. Decomposition followed a Gaussian linear model, with constants k ranging from 0.02 to 0.03 and half-lives of 19–23 days. Nutrient mineralization varied significantly between the start and end of the experiment, underscoring the contribution of the fungi to nutrient release. Our findings emphasize the ecological importance of dung-inhabiting fungi and suggest areas for future research on factors influencing dung decomposition in terrestrial ecosystems.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82059-6.
Keywords: Brazilian Cerrado, Coprophilous fungi, Dung decomposition, Rate of decay, substrates
Subject terms: Fungal ecology, Biodiversity
Introduction
Dung is an essential resource for a wide range of organisms that utilize it in various ways. Among these, dung beetles are the most extensively studied, having evolved diverse strategies to exploit this substrate for their survival1. These beetles belong to the Scarabaeinae subfamily, which has almost 7,000 known species, with cosmopolitan distribution and diversity hotspots in tropical areas, and are important components of terrestrial ecosystems, performing ecosystem functions such as nutrient cycling, bioturbation, and seed dispersal, among others2,3.
In addition to these dung beetles, several other organisms use the dung of a wide variety of animals, especially herbivores, as a resource at some point in their life history [e.g.,4,5]. As it is a material rich in nutrients and water not absorbed during the digestion process, being, therefore, a source of stored energy, dung is used mainly as a food resource in practically all forms of reuse found in nature6–9. It is estimated that in the fresh dung of herbivores there is about 70 to 85% water. Undigested fibers, basically composed of lignocellulose, represent about 70% of the defecated organic material, which can contain up to 3% of N, most of this (75%) as a fecal metabolic6.
Due to this high content of nutrients and high humidity, dung also favors the development of a wide diversity of microorganisms that use it as a substrate for growth and development, among which bacteria, protists, and fungi stand out10–12. While macrorganisms are responsible for bioturbation and direct consumption of dung, microorganisms such as fungi and bacteria carry out the decomposition of fecal biomass, playing key roles in the process of energy cycling in terrestrial ecosystems3,10. The dung-inhabiting fungi, known as coprophilous fungi (Greek: kópros, κόπρος: dung; -phílos, φιλεω: “to love, to have an affinity with”), depend on the ingestion of spores by animals to break dormancy and/or stimulation of their germination and subsequent mycelial development in the dung after being defecated11,13. For these fungi, this is a crucial stage in their development and many groups present evolutionary convergences in their life histories to adapt to coprophilous life, such as minute sporulating structures, small and resistant spores, and dispersal strategies, among others11,14,15.
As highlighted, dung represents an important resource for the maintenance of terrestrial ecosystems. Through its physicochemical characteristics, it is believed that dung modulates both the microbial communities of fungi and bacteria associated with this substrate and the balance of nutrients available in the soil for the development of plant communities12,16–18. For the community of fungi that inhabit the dung, not only the stoichiometry is relevant, but also the origin of the dung, that is, if it was produced by an omnivore, carnivore, or herbivore and, in the latter case if a ruminant or monogastric animal. These factors are decisive for the evaluation of which variations will be observed in the fungal communities that will be expressed in these substrates19–21.
In addition to these factors, there are still a few known parameters for the ecology of these fungal communities, which is the rate of decomposition of dung in these environments, as an available substrate for growth and reproduction. Dung piles deposited in a landscape represent potential islands for colonization in a matrix and are characterized as ephemeral habitats, as well as animal carcasses and plant litter, which are vital for communities dependent on them22,23. Once durability of these habitats will directly influence the expression of communities, understanding this parameter will help us to better understand how the intrinsic characteristics of the substrates modulate the occurrence of these organisms, especially the fungi that, as presented, are the main cyclers of the matter and energy egested in these substrates.
Dung-inhabiting fungi can be considered coprophilous, when they are totally dependent on the animal to complete their life cycle (e.g., genus Pilobolus spp.), or fimicolous, when they can use other substrates for their development, such as decaying plant debris, in addition to dung, being many opportunistic soil fungi24. When in the coprophilous cycle, swallowing of the spores, which would otherwise not germinate, favors breaking their dormancy by scarifying them chemically and mechanically as they pass through the digestive system12,13,25,26. Although the type of digestive system, ruminant or monogastric, has little influence on the structure of the community of dung-inhabiting fungi27, there is no information on whether or not these types of dung could favor the rate of decomposition and mineralization of fecal matter due to their physical-chemical composition, which could be a driving factor for fungal communities12.
Recent studies have shown the influence of dung composition on plant community diversity in temperate and tropical regions18,28 and how stoichiometry and macrofauna using dung as a resource affect decomposition rates in African savannas16,17. However, no research has directly assessed the decomposition rate (half-life and total decomposition time) of dung and its role in shaping fungal communities in tropical areas, where heat and humidity accelerate this process. This study aimed to investigate the half-life and decomposition rate of dung from ruminant and monogastric herbivores in a six-month microcosm experiment. Additionally, we evaluate the nutritional content of dung and the impact of dung sterilization via autoclaving, simulating microbiota exclusion, on decomposition dynamics.
Results
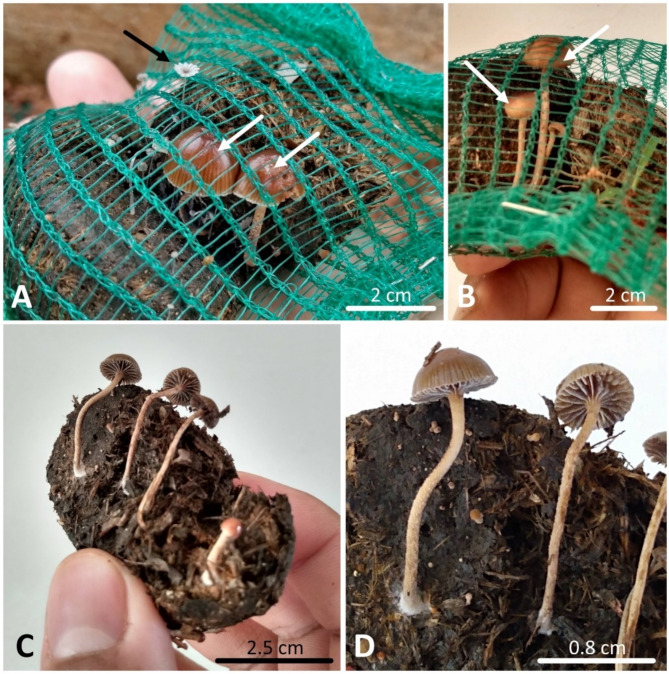
The decomposition of both dung types was slow, with approximately 75% of the biomass remaining after half the experiment. By the end of the 200-day evaluation, the remaining biomass had reduced to 30–15% for non-autoclaved horse and cow dung. These were the only treatments to show mycelial growth, triggered by the onset of the rainy season, which allowed spore germination and development of the dung-inhabiting fungi Parasola misera (P. Karst.) Redhead, Vilgalys & Hopple (Basidiomycota: Psathyrellaceae) and Deconica coprophila (Bull.) P. Kumm. (Basidiomycota: Strophariaceae). This study represents the third documented occurrence of D. coprophila in the country (Fig. 1).
Fig. 1.
Presence of coprophilous basidiomycete in non-autoclaved dung after the onset of the rainy season.A, B Delicate sporomes of Parasola misera (black arrow) and Deconica coprophila (white arrows), with a glabrous, subviscous, translucent-striated surface cap (when young). C, D Development of several sporomes of D. coprophila on horse dung, where it is possible to observe countless other basidiomes emerging from the dung.
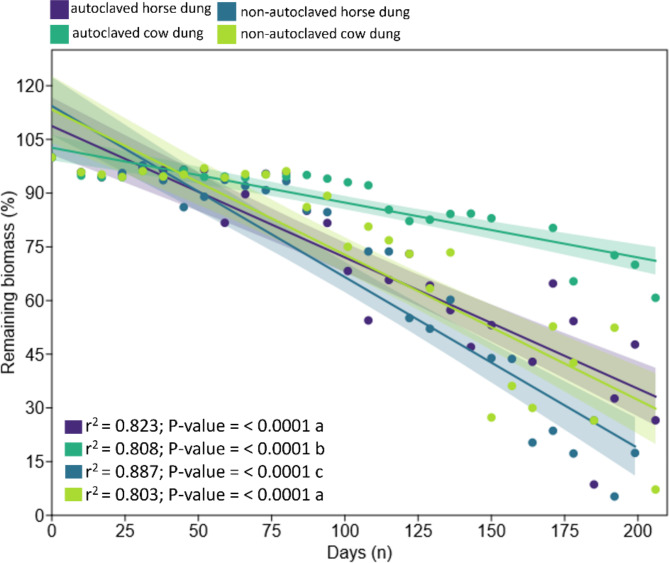
Autoclaved dung proved to be more durable in the microcosm, losing little mass over time. The autoclaved horse dung remained with just over 30% biomass remaining at the end of the experiment. On the other hand, autoclaved cow dung, among all, was the most inert, with just over 75% of remaining biomass remaining on average at the end of the evaluation. These differences were observed and verified by the physical and visual evaluation of the remaining material, after weighing and statistically, through regression models, where we obtained significant differences between all treatments (P-value = < 0.0001; F = 18.559, P-valuesame slope = < 0.0001) (Fig. 2). The model with the best fit to estimate the mass loss in all treatments was the Gaussian [equation: 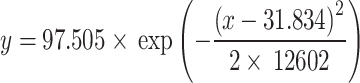 ; Akaike IC = 29.471, r2=0.67]. Analysis of variance (ANOVA) comparisons performed to the ordinary least squares regressions to the all treatments of the bivariate generalized linear model to remaining dung biomass (%) throughout the decomposition process are provided in Table S1 (Supplementary Material).
; Akaike IC = 29.471, r2=0.67]. Analysis of variance (ANOVA) comparisons performed to the ordinary least squares regressions to the all treatments of the bivariate generalized linear model to remaining dung biomass (%) throughout the decomposition process are provided in Table S1 (Supplementary Material).
Fig. 2.
Bivariate generalized linear model of remaining dung biomass (%) throughout the decomposition process and between different treatments (autoclaved and non-autoclaved) during 206 days of dungbag exposure in the field. Different letters after statistical values indicate significant differences.
The decomposition constant k calculated from the equation 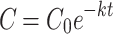 for each type of dung and treatment, as well as the half-life values, obtained by the equation
for each type of dung and treatment, as well as the half-life values, obtained by the equation 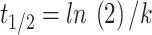 , are shown in Table 1. The k values remained similar for all treatments, with non-autoclaved horse dung slightly higher (0.0350) compared to autoclaved cow dung (0.0298). Conversely, the half-life was longer for autoclaved cow dung (23.2 days), while non-autoclaved horse dung had the shortest half-life (19.7 days).
, are shown in Table 1. The k values remained similar for all treatments, with non-autoclaved horse dung slightly higher (0.0350) compared to autoclaved cow dung (0.0298). Conversely, the half-life was longer for autoclaved cow dung (23.2 days), while non-autoclaved horse dung had the shortest half-life (19.7 days).
Table 1.
Estimation of remaining biomass (%), exponential decomposing equation (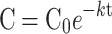 ) fitted to these values of the samples after 206 days on field exposure and respective decomposition constants (k) and time necessary to decompose half of the biomass (t).
) fitted to these values of the samples after 206 days on field exposure and respective decomposition constants (k) and time necessary to decompose half of the biomass (t).
| Sample1 | Biomass remaining (%) | Equation | k (days− 1) | t0.5 (days) |
|---|---|---|---|---|
| HORa | 26.55 |
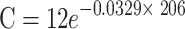
|
0.0329 | 21.05 |
| HORn | 0 |
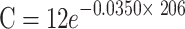
|
0.0350 | 19.75 |
| COWa | 60.76 |
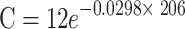
|
0.0298 | 23.20 |
| COWn | 7.20 |
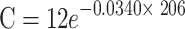
|
0.0340 | 20.35 |
1HORa: autoclaved horse dung, HORn: non-autoclaved horse dung, COWa: autoclaved cow dung, COWn: non-autoclaved cow dung.
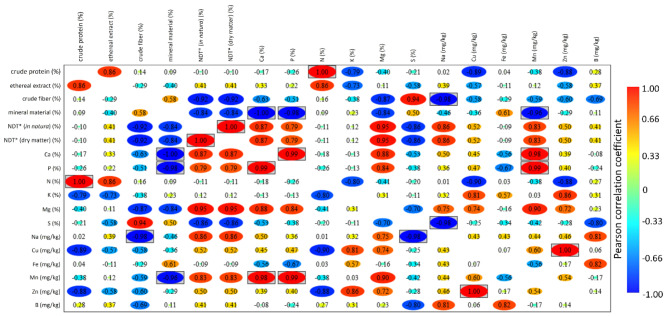
The availability of nutrients at the end of the experimental trial showed a large percentage variation in nutrient contents, especially those considered secondary nutrients and micronutrients (Ca, Mg, S, Na, Cu, Fe, Mn, Zn, and B), whose mineralization is responsible by a high percentage change over time (Table 2). The micronutrients with the greatest variations were B (range between 9,000 and 15,900%), Fe (range between 345 and 733%), Cu (range between 61 and 558%), S (range between 58 and 112,400%), K (range between 85 and 677%) and P (variation between 51 and 255%) (Table 2). Figure 3 lists Pearson’s correlation coefficients between the different nutritional parameters evaluated in both substrates, relating which ones presented greater or lesser correlation. The parameters that showed the highest positive and statistically significant correlation were N–crude protein (r = 1.0; P-value = < 0.0001), Mn–Ca (r = 0.98; P-value = 0.02), Mn–P (r = 0.99; P-value = 0.01), Zn–Cu (r = 1.0; P-value = 0.004), P–Ca (r = 0.99; P-value = 0.01), NDT dry matter (total digestive nutrientes)–NDT in natura (r = 1.0; P-value = < 0.0001). Significant and negative correlations were observed between Ca–mineral matter (r = -1.0; P-value = 0.004), Na–S (r = -0.98; P-value = 0.02), P– mineral matter (r = -0.98; P-value = 0.01), Na–crude fiber (r = -0.98; P-value = 0.02), and Mn–mineral material (r = -0.96; P-value = 0.04). P-values for all comparisons are provided in Table S2 (Supplementary Material).
Table 2.
Parameters and the percentage variation of the nutritional contents of samples of cattle (COW) and horse dung (HOR), autoclaved and non-autoclaved, used to evaluate the decomposition rate.
| Parameter | t = 0 days1 | t = 206 days | Variation (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cow | hor | cowa | hora | cown | horn | ∆cowa | ∆hora | ∆cown | ∆horn | |
| Crude protein (%) | 12.5 | 10.4 | 11.3 | 10 | 8.2 | 13.2 | 10.6 | 4 | 52.4 | 27 |
| Ethereal extract (%) | 1.8 | 2 | 1.5 | 1.1 | 1.1 | 1.5 | 20 | 81.8 | 63.6 | 33.3 |
| Crude fiber (%) | 13 | 21 | 14.5 | 19.9 | 13.2 | 15.7 | 11.5 | 5.5 | 1.5 | 33.7 |
| Mineral material (%) | 15 | 15 | 22.1 | 26 | 24.5 | 25.9 | 47.3 | 73.3 | 63.3 | 72.6 |
| NDT* (in natura) (%) | 59.5 | 53.7 | 58.9 | 50.4 | 57.5 | 54.3 | 1 | 6.1 | 3.4 | 1.1 |
| NDT* (dry matter) (%) | 66.9 | 60.3 | 58.9 | 50.4 | 57.5 | 54.3 | 13.5 | 16.4 | 16.3 | 11 |
| Ca (%) | 1.6 | 1 | 1.1 | 0.49 | 0.78 | 0.50 | 45.5 | 104 | 105 | 100 |
| P (%) | 0.96 | 0.52 | 0.3 | 0.25 | 0.27 | 0.24 | 176 | 51.9 | 255.5 | 116.6 |
| N (%) | 2 | 1.66 | 1.8 | 1.60 | 1.3 | 2.1 | 11.1 | 3.6 | 53.8 | 26.5 |
| K (%) | 0.48 | 0.7 | 0.07 | 0.10 | 0.17 | 0.09 | 585 | 85.7 | 182.3 | 677.7 |
| Mg (%) | 0.57 | 0.3 | 0.25 | 0.20 | 0.25 | 0.21 | 128 | 33.3 | 128 | 42.8 |
| S (%) | 0.15 | 0.12 | 0.25 | 135 | 0.30 | 0.19 | 66.6 | 112,400 | 100 | 58.3 |
| Na (mg/kg) | 130 | 120 | 120 | 0 | 151 | 122 | 8.3 | 100 | 16.2 | 1.6 |
| Cu (mg/kg) | 12 | 18 | 50 | 42 | 79 | 29 | 316 | 133.3 | 558.3 | 61.1 |
| Fe (mg/kg) | 2,400 | 1,800 | 10,700 | 12,000 | 14,500 | 15,000 | 345 | 566.7 | 504.2 | 733.3 |
| Mn (mg/kg) | 380 | 320 | 395 | 240 | 330 | 210 | 3.9 | 33.3 | 15.2 | 52.3 |
| Zn (mg/kg) | 150 | 80 | 100 | 96 | 120 | 90 | 50 | 20 | 25 | 12.5 |
| B (mg/kg) | 0.6 | 0.4 | 55 | 50 | 61 | 64 | 9,066 | 12,400 | 10,066 | 15,900 |
COWa: autoclaved bovine dung; HORa: autoclaved equine dung; COWn: non-autoclaved bovine dung; HORn: non-autoclaved equine dung.
*Total digestible nutrients (NDT) = digestive protein + crude fiber + digestive ethereal extract + non-nitrogenous digestive extract. Units conversion: (%) = g/Kg / 10; Dry matter (%) = 100 – humidity; crude protein = Ntotal × 6.25. ∆: percentage variation of nutritional contents at t = 206.
Fig. 3.
Heatmap showing Pearson’s correlation coefficients for the nutritional parameters of the evaluated substrates. Nutritional parameters with a highly statistically significant and positive correlation are highlighted in boxes, represented by those values with the most intense red coloration. Statistically significant low correlations are highlighted in boxes, being those with values with the most intense staining for blue.
Discussion
The contribution of organic material of plant origin is one of the largest sources of carbon and nutrients in ecosystems. In addition to these residues, there are also other residues of organic origin, such as animal remains and microorganisms29–32. Another important source of nutrients, still little studied in terms of its contribution as a resource to terrestrial ecosystems, is the dung of different animal species, which are produced in tons daily. It is estimated that just one individual of beef or dairy cattle produces, on average, between 16.5 and 43.2 kg of dung daily, an amount that can reach an average of 64 kg/day33–35.
As it constitutes a rich source of minerals allocated above ground by plants via processes of gross primary production, the contribution and decomposition of plant litter, as well as the consumption of plants by herbivorous animals and, indirectly, omnivores and carnivores and the consequent defecation, contribute to the return of these nutrients to the soil36,37. Thus, the cycling of these nutrients stands out as an essential function for the maintenance of ecosystems, whether terrestrial or aquatic. This process is mediated by several factors that together modulate the nutrient cycling process at large ecological scales38,39. Saprophytic fungi are essential for plant litter decomposition, while dung-inhabiting fungi, also known as coprophilous or fimicolous fungi, depending on their life story10,12,25,26, are primarily responsible for the decomposition of these substrates. Although there are studies relating the influence of the physicochemical characteristics of dung (defined here as the feces of herbivorous animals) with the dynamics of dung beetle populations e.g1,6–8, very few studies have highlighted how the physicochemical characteristics of dung and its durability in the environment influence the populations of fungi that decompose it27. Our results highlighted a slow decomposition rate (Fig. 4), with 75% of the remaining biomass of the different dungs still present in half of the experimental time, highlighting the potential influence of the environmental factor in this process.
The beginning of the experiment took place during the dry season of the Brazilian Cerrado biome, between the months of April/May to September/October40. The high temperatures and high rate of insolation in the Cerrado41, directly affect the rate of decomposition, either positively, by accelerating the biochemical processes involved in decomposition, or negatively, by causing desiccation of substrates42 or by the low availability of moisture, necessary for the development of fungi. The potential entry of moisture, with the advent of the rainy season, which began in mid-October 2021, favored decomposition, so that it was only six months after the start of the experiment that we observed a marked reduction in the remaining biomass.
In the decomposition equations that best represent the expected decomposition rate for each type of dung and treatment (Table 1), we observed that the most adjusted equation for the decomposition of non-autoclaved horse dung was the one with 0% remaining biomass, with a decomposition constant k of 0.03 and a half-life of 19 days. In contrast, in autoclaved horse dung, the best fit observed, with similar constant k and half-life, a biomass remnant of 26% is still observed. This pattern is also observed for cow dung, with similar k constants and half-life, but with different percentages of remaining biomass, with non-autoclaved dung decomposing the best.
The role of the microbiota associated with dung at this stage is highlighted. The role that dung-inhabiting fungi play in the decomposition of this substrate is widely known, given the large number of studies that attest to their occurrence in these substrates during all periods of the year11,12,21,43–46. We observed that the autoclaved dung, i.e., excluding the microbiota associated with them, decomposed more slowly, with a remainder of just over 30% and 75% for autoclaved horse and cow dung, respectively (Fig. 2, Table S2). The influence of the mycobiota can still be confirmed by the fact that, after the beginning of the rainy season, we observed the development of mycelium and, later, sporomes of specimens of Parasola misera and Deconica coprophila, which represents, according to the most recent surveys, the third citation of the occurrence of this coprophilous basidiomycete in Brazil44,45. The non-autoclaved dung presented, for both types, the best decomposition rate, remaining between 30 and 15% of the total biomass at the end of the experiment (Fig. 2). Given the role that basidiomycetes play in the decomposition of the recalcitrant material that composes the dung (e.g. cellulose and hemicellulose), it is possible to affirm that the development of these species in the dung has favored the decomposition and consequent release of nutrients observed over time (Table 2).
Despite the slow mass loss over time, the best-fit linear model (Gaussian) allows us to infer that, extending the experimental time, practically all the biomass would be cycled within a year, as evidenced by the calculated half-life times for each type of dung (Table 2). Slow decomposition rates for dung have been observed in other studies, such as in decomposing bovine dung in forestry systems in Japan35,47. Although we have not evaluated the effect of excluding mesofauna from the soil, since our objective was to quantify the durability of dung as a viable substrate for the development of fungi, studies show that the presence of arthropods is very relevant because they act either as consumers of this dung or as detritivores, accelerating the performance of fungi6,8,48. The presence of termites in the process of cycling dung, especially herbivores, is relevant. About 126 species of termites are known to eat the dung of different herbivores species, the action of these insects is very fast, especially in savannas during the dry seasons. About one third of the dung deposited in these environments is estimated to be consumed by termites49.
Regarding the mineralization of nutrients in the dung (Table 2; Fig. 3), we observed a slow release of N over time, with a low percentage variation over time, as described in other studies, which emphasize the low mobility of N in the decomposition of dung35,48,50. In contrast, other micronutrients were released into the dung and became liable to be released into the soil as decomposition proceeded. Large percentage amounts of Cu, Fe, Mn, Zn and B, similar to what was observed in other studies that evaluated the release of micronutrients from dung to the soil18,51. Mineralization of limiting nutrients in the soil is therefore of utmost importance, considering the role that dung decomposition plays in modulating the development of plant communities18,28,50.
Dung inhabiting-fungi, in general, can be framed in an ecological succession scheme, following a nutritional hypothesis, where, initially, the dung is colonized by species that exploit, as a resource, simpler components of the dung, such as less complex sugars, as members of the phylum Mucoromycota (e.g. Pilobolus spp.). Then, representatives of the phylum Ascomycota appear, with the emergence of apothecia-forming species (e.g., Ascobolus spp.) followed by peritecioid species (e.g., Podospora spp.), more specialized in degrading and exploiting more complex resources as a carbon source. Finally, there are species with high enzymatic capacity to degrade more complex and recalcitrant substances, such as lignin and hemicellulose, represented by fungi of the phylum Basidiomycota (e.g., Coprinopsis spp., Psilocybe spp.) and, in some cases, species of ascomycetes of the family Xylariaceae (e.g., Hypocopra spp., Poronia spp.)10,11,13,21,52.
Our data demonstrate the viability of ruminant and non-ruminant dung as a substrate for fungal development, based on a six-month microcosm experiment in a tropical area. Mycelium growth and significant fecal biomass retention suggest that, under natural conditions, dung decomposes more slowly due to environmental constraints on microbial communities. As fungi require moisture for spore germination, decomposition is slower in dry seasons, with water availability acting as a trigger for mycelial growth and nutrient cycling. Considering the half-life observed for each dung type, r-strategist fungi likely prioritize spore production to ensure species persistence in ecosystems. However, our results indicate that in wetter environments, accelerated decomposition and limited residual biomass may restrict the development of all potential fungal species51.
Dung decay time and the presence of dung-inhabiting fungi are critical factors when using dung as a nutrient source for soil fertilization. These fungi not only enhance decomposition but also accelerate the release of essential nutrients such as P and K. Further studies are needed to evaluate: (a) nutrient mobilization during decomposition, (b) the impact of mesofaunal exclusion (e.g., termites) on decomposition rates, (c) the behavior of mineralized nutrients in soil and their effects on above- and belowground communities, and (d) the biogeochemical dynamics of nutrient cycling across ecosystems under varying climates, animals, and interactions12. This broader approach would deepen our understanding of dung composition, its influence on coprophilous fungi, and its role in matter and egested energy cycling within terrestrial ecosystems.
Materials and methods
Study area and samples collection
The experiment was carried out in a microcosm developed in an urban area of the municipality of Anápolis, Goiás, Brazil (16°18’10.4"S and 48°53’47.5"W), in the Cerrado biome, between July 2021 and January 2022. The predominant climate in the area is rainy tropical [Aw (tropical savannah) and Cwa (temperate rainy dry winter)], according to the Köppen classification53. There is a predominance of a dry and a rainy season, well delimited by a characteristic seasonal variation; the dry season occurs from May to September, and the rainy season from October to April54. The average rainfall in the region varies between 1200 and 1400 mm, with an average annual temperature between 22 and 23ºC, which is due to the high altitudes observed in the central-west and south regions of the state of Goiás55.
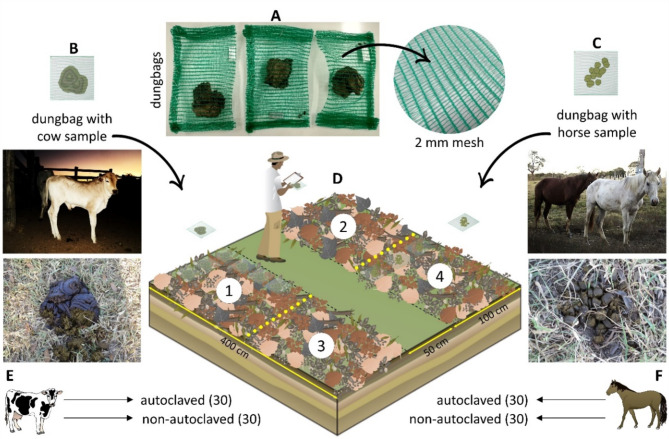
In this area, two parallel plots were constructed with measurements of 400 × 100 cm, 50 cm apart from each other. The soil of the sites (Oxisol) was turned and a layer of about 4 cm of plant litter collected in an area of Cerrado dry forest was deposited uniformly in the place, in order to cover the soil (Fig. 4). The area was irrigated for a week to then receive the experiment. Cattle (Bos taurus L.) and horses (Equus caballus L.) were considered as models of ruminant and non-ruminant herbivores, respectively, since these animals are easily accessible in domesticated herds, and their dung is widely used in several studies on the ecology and biology of dung-inhabiting fungi10,11,21,56. Dung samples were collected in pastures in a rural area of the municipality of Goiás, in the western region of the state of Goiás (15°55’45.8"S, 50°09’15.1"W) in September 2020. About three kilograms of each type of dung were collected and packed in plastic bags and transported to the laboratory. The appearance of the material was always observed before collection, to avoid very old samples, prioritizing the most recent dung possible. To ensure sampling heterogeneity, we sought to collect different animals’ defecations, in the pasture, which were mixed in order to form a composite sample to each dung type.
Fig. 4.
Experimental structure of the microcosm used to evaluate dung decomposition: A model and mesh of the dungbags prepared for the experiment, (B, C, arrangement of pellets and dung-pads inside the dungbags, D experimental microcosm, subdivided into the four-part distribution of dungbags: 1–2: plots for autoclaved and non-autoclaved cow dung, 3–4: plots for autoclaved and non-autoclaved horse dung, E, F animal models of ruminant and non-ruminant herbivores and dung type.
Source: the authors.
In the laboratory, the samples were placed in paper bags, which were closed and then dried in an oven with forced air circulation at 35–40º C for a week to prevent decomposition before the experiment. A composed dung sample, totaling 500 g, was separated for the analysis of nutritional content. The rest of the material was packed in plastic bags, sealed, identified, and stored in cardboard boxes for use in the other stages of the experiment.
Analysis of the dung nutritional content
For the dung nutritional analysis before the beginning of decomposition (t = 0 days) and in the final stage (t = 206 days), 500 g of dung from the composite sample was used. We determined the composition of macro and micronutrients [nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), sulfur (S), manganese (Mn), sodium (Na), iron (Fe), copper (Cu), zinc (Zn) and boron (B)], following the protocols established by Tedesco et al.57 and Miyazawa et al.58. The parameters moisture and dry matter, used to determine the biomass, total digestible nutrients (TDN), protein and crude fibers, ether extract and mineral matter followed the protocols of Silva and Queiroz56 and Souza et al.59.
Microcosm decomposition assay
In order to evaluate the half-life and decomposition rate of the two dung types, 120 litterbags were created, henceforth called dungbags60, made with 2 mm nylon mesh and stapled in double folds on the sides. We opted for metal clips as they make the closure more secure, reducing the risk of breakage and loss of contents from the bags. The samples of each dung type were then divided into two parts, one of which was autoclaved for 15 min at 120 °C by using a Fanem 415 vertical autoclave, with distilled water, for total destruction of any organisms that inhabit dung. For each dung type, 30 bags with autoclaved dung and 30 bags with non-autoclaved dung were prepared, each containing about 10 to 16 g of dung. The weighing was performed on a precision scale (minimum reading of 0.001 g), with the weight of each bag being previously disregarded (tare).
Each dungbag received a metal label that identifies the dung type (equine or bovine) and whether or not it is autoclaved (treatment). The bags were taken to the microcosm, where they were evenly distributed over the previously prepared soil, being removed only for weekly collections (Fig. 4). Since the Brazilian Cerrado is markedly seasonal in terms of precipitation55,40, we started the microcosm experiment in the dry season, transitioning to the rainy season, to avoid that excessive rainfall would affect the objective of the experiment, dissolving the substrate in the dungbags at the beginning of the experiment.
Weekly, for six months (total of 206 days), four bags of the microcosm were randomly collected, one from each treatment. These bags were packed in paper bags and taken carefully to the Laboratory and, after removing litter and soil residues, taking care not to lose the contents of the bags, the material was dried in an oven and weighed using the same precision scale and the values were tabulated. The evaluation of fungi developing on non-autoclaved manure was conducted following protocols described by Bell10 and Doveri11, with some modifications. Dungbags were inspected under a Leica EZ4 stereoscopic microscope with a 4.4:1 zoom. When sporomes were observed, they were carefully removed using tweezers or dissecting needles. For taxonomic identification, microscopic structures were examined from freehand sections of fresh material cut with a razor blade. The sections were hydrated in 3% KOH and stained with fuchsin or Melzer’s reagent. Microscopic structures were photographed at ×1000 magnification using an Olympus CX31 optical microscope equipped with a digital camera. Measurements were obtained using the software Piximètre (v. 5.10 R 1541) [http://www.piximetre.fr/]. Species identification was based on morphological features, referencing specialized literature10,11,46.
Statistical analyses
The mass values obtained for each sample at the end of each collection were corrected and adjusted to percentages considering a standard mass of 12 g, in order to guarantee a standardization in the mass values, given in percentage, as a function of the actual masses lost. The mass values were submitted to a linear regression model and subsequently compared using analysis of variance to verify the effect of treatments (dung type and autoclaved and non-autoclaved dung) on mass loss over time. The variation or percentage reduction between the values of nutrient contents quantified at t = 0 and t = 206 was calculated for each parameter, using the equation 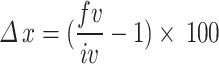 , where
, where 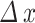 represents the variation in the content of parameter x, fv represents the final value (at t = 206) quantified for parameter x and iv is the initial value (t = 0) for parameter x. Finally, we performed a Pearson correlation (P-value < 0.05) between the different nutritional parameters evaluated at t = 206 in order to verify which of these parameters were positively or negatively correlated. These analyzes were performed in the statistical environment Past 4 (v. 4.17)61, https://www.nhm.uio.no/english/research/resources/past/].
represents the variation in the content of parameter x, fv represents the final value (at t = 206) quantified for parameter x and iv is the initial value (t = 0) for parameter x. Finally, we performed a Pearson correlation (P-value < 0.05) between the different nutritional parameters evaluated at t = 206 in order to verify which of these parameters were positively or negatively correlated. These analyzes were performed in the statistical environment Past 4 (v. 4.17)61, https://www.nhm.uio.no/english/research/resources/past/].
The rate of decomposition and, consequently, nutrient release, follows a simple exponential model. We calculated this rate using the equations proposed by Olson62, where the decomposition rate (k) was obtained by deriving the equation: 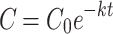 where C represents the final mass of the sample in the dungbag, C0 is the initial mass (10 g), e represents a constant (base of natural logarithms), t represents the elapsed time of the experiment, in days, and k is the decomposition constant. We obtained the decomposition constant k by rearranging the exponential model equation:
where C represents the final mass of the sample in the dungbag, C0 is the initial mass (10 g), e represents a constant (base of natural logarithms), t represents the elapsed time of the experiment, in days, and k is the decomposition constant. We obtained the decomposition constant k by rearranging the exponential model equation: 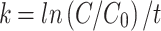 62,63. The half-life, which represents the time required for the dung to lose half its mass, decomposing and releasing nutrients, was calculated using the equation:
62,63. The half-life, which represents the time required for the dung to lose half its mass, decomposing and releasing nutrients, was calculated using the equation: 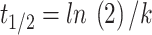 , where t1/2 represents the half-life, ln(2) is a constant and k is the decomposition constant. The equations that best demonstrate the decomposition and loss of matter were calculated and obtained in Microsoft Excel software (v. 2410) [https://products.office.com/excel/].
, where t1/2 represents the half-life, ln(2) is a constant and k is the decomposition constant. The equations that best demonstrate the decomposition and loss of matter were calculated and obtained in Microsoft Excel software (v. 2410) [https://products.office.com/excel/].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks to Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG) for doctor scholarships granted to FJSC (proc. n. 201810267000595). To Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for productivity scholarship provided to CMSN and SXS.
Author contributions
FJSC: conceptualization, methodology, visualization, validation, data curation, formal analysis, writing-original draf. FJSC and SXS: conceived the idea of the study, and, with JCA, conducted fieldwork. FJSC, JCA, and CMSN carried out labwork. FSJC and JCA: original draft, writing-review and first editing. SXS and CMSN: funding acquisition. SXS: supervision, resources, validation, writing-review and editing. CMSN: writing-review and editing. All authors reviewed the manuscript.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Goiás under Grant number 201810267000595.
Data availability
The datasets generated during and/or analyzed during the conduction of the study are available from the corresponding author upon reasonable request. Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Philips, T. K. Chichester. The evolutionary history and diversification of dung beetles. In Ecology and Evolution of Dung Beetles (eds Simmons, L. W. & Ridsdill-Smith, J.) (Wiley, New Jersey, 2011).
- 2.Hanski, I., Cambefort, Y. Dung Beetle Ecology (Princeton University Press, Princeton, 1991).
- 3.Nichols, E. et al. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv.141, 1461–1474. 10.1016/j.biocon.2008.04.011 (2008). [Google Scholar]
- 4.Soares, D. et al. Evolution of coprophagy and nutrient absorption in a cave salamander. Subterr. Biol. 24, 1–9. 10.3897/subtbiol.24.15013 (2017). [Google Scholar]
- 5.Lall, K. R., Jones, K. R. & Garcia, G. W. Nutrition of six selected neotropical mammals in Trinidad and Tobago with the potential for domestication. Veterinary Sci.5, 1–18. 10.3390/vetsci5020052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holter, P. Herbivore dung as food for dung beetles: elementary coprology for entomologists. Ecol. Entomol.41, 367–377. 10.1111/een.12316 (2016). [Google Scholar]
- 7.Jones, R. Call of Nature: The Secret Life of Dung (Pelagic Publishing, 2017).
- 8.Frank, K., Brückner, A., Hilpert, A., Heethof, M. & Blüthgen, N. Nutrient quality of vertebrate dung as a diet for dung beetles. Sci. Rep.7, 12141. 10.1038/s41598-017-12265-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricklefs, R. & Relyea, R. Movimento de energia nos ecossistemas. In A Economia da Natureza (eds Ricklefs, R. & Relyea, R.) (Guanabara Koogan, Rio de Janeiro, 2018).
- 10.Bell, A. Dung Fungi: An Illustrated Guide to Coprophilous Fungi in New Zealand (Victoria University, 1983).
- 11.Doveri, F. Fungi Fimicoli Italici: A Guide to the Recognition of Basidiomycetes and Ascomycetes Living on Faecal Material (AMB Fondazione Centro Studi Micologici, 2004).
- 12.Calaça, F. J. S., Araújo, J. C. & Xavier-Santos, S. O status ecológico das comunidades de fungos coprófilos. Pesq Ens C Exat Nat.1, 136–143. 10.29215/pecen.v1i2.452 (2017). [Google Scholar]
- 13.Harper, J. E. & Webster, J. An experimental analysis of the coprophilous fungus succession. Trans. Br. Mycol. Soc.47, 511–530 (1964). [Google Scholar]
- 14.Sakes, A. et al. Shooting mechanisms in nature: a systematic review. PLoS ONE. 11. 10.1371/journal.pone.0158277 (2016). [DOI] [PMC free article] [PubMed]
- 15.Halbwachs, H. & Bässler, C. No bull: dung-dwelling mushrooms show reproductive trait syndromes different from their non-coprophilous allies. Mycol. Progress. 19, 817–824. 10.1007/s11557-020-01604-5 (2020). [Google Scholar]
- 16.Sitters, J., Maechler, M. J., Edwards, P. J., Suter, W. & Venterink, H. O. Interactions between C:N:P stoichiometry and soil macrofauna control dung decomposition of savanna herbivores. Funct. Ecol.28, 776–786. 10.1111/1365-2435.12213 (2014). [Google Scholar]
- 17.Sitters, J. & Venterink, H. O. A stoichiometric perspective of the effect of herbivore dung on ecosystem functioning. Ecol. Evol.8, 1043–1046. 10.1002/ece3.3666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdés-Correcher, E., Sitters, J., Wassen, M., Brion, N. & Venterink, H. O. Herbivore dung quality affects plant community diversity. Sci. Rep.9, 5675. 10.1038/s41598-019-42249-z (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrower, K. M. & Nagy, L. A. Effects of nutrients and water stress on growth and sporulation of Coprophilous Fungi. Trans. Br. Mycol. Soc.72, 459–462. 10.1016/S0007-1536(79)80154-0 (1979). [Google Scholar]
- 20.Wicklow, D. T., Angel, K. & Lussenhop, J. Fungal community expression in lagomorph versus ruminant feces. Mycologia72, 1015–1021. 10.1080/00275514.1980.12021273 (1980). [Google Scholar]
- 21.Richardson, M. J. Diversity and occurrence of coprophilous fungi. Mycol. Res.105, 387–402. 10.1017/S0953756201003884 (2001). [Google Scholar]
- 22.Elton, C. S. & Miller, R. S. The ecological survey of animal communities: with a practical system of classifying habitats by structural characters. J. Ecol.42, 460–496 (1954). https://www.jstor.org/stable/2256872 [Google Scholar]
- 23.O’Neill, B. J. Community disassembly in ephemeral ecosystems. Ecology97, 3285–3292. 10.1002/ecy.1604 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Juniper, A. J. Dung as a source of predacious fungi. Trans. Br. Mycol. Soc.40, 346–348. 10.1016/s0007-1536(57)80030-8 (1957). [Google Scholar]
- 25.Calaça, F. J. S. & Xavier-Santos, S. Fungos Coprófilos: A Biodiversidade oculta nos Excrementos (Editora Oikos, Editora UEG) (São Leopoldo, Anápolis).
- 26.Calaça, F. J. S., Xavier-Santos, S. & Abdel-Azeem, A. M. Recent advances on occurrence of genus Chaetomium on dung. In Recent Developments on Genus Chaetomium (Fungal Biology) (ed. Abdel-Azeem A. ed.) (Springer, Cham, 2020). 10.1007/978-3-030-31612-9_4
- 27.Calaça, F. J. S., Silva-Neto, C. M., Ferreira, A. A. & Xavier-Santos, S. A pooping case: does the structure of dung-inhabiting fungi respond to the type of diet or type of animal’s digestive system? Nova Hedwigia. 116, 325–348. 10.1127/nova_hedwigia/2023/0810 (2023). [Google Scholar]
- 28.Chaudhary, E., Jouquet, P., Rumpel, C. & Sukumar, R. Chemical parameters of decomposing dung in tropical forest as indicators of feeding behaviour of large herbivores: a step beyond classical stoichiometry. Ecol. Ind.115, 106407. 10.1016/j.ecolind.2020.106407 (2020). [Google Scholar]
- 29.Carvalho, E. M. & Uieda, V. S. Input of litter in deforested and forested areas of a tropical headstream. Braz. J. Biol.70, 283–288 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Almeida, E. J., Luizão, F. & Rodrigues, D. J. Produção De Serrapilheira em florestas intactas e exploradas seletivamente no Sul Da Amazônia em função da área basal Da vegetação E Da Densidade De Plantas. Acta Amazonica. 45, 157–166 (2015). [Google Scholar]
- 31.Camargo, M., Giarrizzo, T. & Jesus, A. J. S. Effect of seasonal flooding cycle on litterfall production in alluvial rainforest on the middle Xingu River (Amazon basin, Brazil). Braz. J. Biol.75(S250-S256 (supl.)). 10.1590/1519-6984.00514BM (2015). [DOI] [PubMed]
- 32.Siqueira, T. M., Pinheiro, M. H. O., Silva, D. G. & Franco, T. M. Influências climáticas na produção de serapilheira em um cerradão em Prata. MG Biotemas. 29, 7–15. 10.5007/2175-7925.2016v29n2p7 (2016). [Google Scholar]
- 33.Mathews, B. W., Sollenberger, L. E. & Tritschler, J. P. Grazing systems and spatial distribution of nutrients in pastures: soil considerations. In Nutrient Cycling in Forage Systems (eds Joost, R. E. & Roberts, C. A.) (Proc Potash and Phosphate Inst and the Foundation for Agron Res, Columbia, 1996).
- 34.Statistics Canada. A Geographical profile of manure production in Canada. Catalogue no. 21-601-M (2006). https://www150.statcan.gc.ca/n1/pub/16-002-x/2008004/article/10751-eng.htm
- 35.Hirata, M. et al. Deposition and decomposition of cattle dung in forest grazing in southern Kyushu, Japan. Ecol. Res.24, 119–125. 10.1007/s11284-008-0488-y (2009). [Google Scholar]
- 36.Yoshitake, S., Soutome, H. & Koizumi, H. Deposition and decomposition of cattle dung and its impact on soil properties and plant growth in a cool-temperate pasture. Ecol. Res.29, 673–684. 10.1007/s11284-014-1153-2 (2014). [Google Scholar]
- 37.Feng, C., Wang, Z., Ma, Y., Fu, S. & Chen, H. Y. H. Increased litterfall contributes to carbon and nitrogen accumulation following cessation of anthropogenic disturbances in degraded forests. Ecol. Manag. 432, 832–839. 10.1016/j.foreco.2018.10.025 (2019). [Google Scholar]
- 38.García-Palacios, P., McKie, B., Handa, I. T., Frainer, A. & Hättenschwiler, S. The importance of litter traits and decomposers for litter decomposition: a comparison of aquatic and terrestrial ecosystems within and across biomes. Funct. Ecol.30, 819–829. 10.1111/1365-2435.12589 (2016). [Google Scholar]
- 39.García-Palacios, P., Shaw, E. A., Wall, D. H. & Hättenschwiler, S. Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecol. Lett.19, 554–563. 10.1111/ele.12590 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro, J. F. & Walter, B. M. T. Fitofisionomias do bioma Cerrado. In Cerrado: Ambiente e Flora (eds Sano, S. M. & Almeida, S. P.) (Embrapa-CPAC, Planaltina, 1998).
- 41.Penereiro, J. C., Ferreira, D. H. L. & Badinger, A. Diversidade Funcional De Besouros coprófilos em um fragmento de Cerrado, Uberlândia, MG. Braz. J. Anim. Environ. Res.4, 3561–3568. 10.34188/bjaerv4n4-208 (2021). [Google Scholar]
- 42.Witkamp, M. Decomposition of leaf litter in relation to environment microflora and microbial respiration. Ecology47, 194–201. 10.2307/1933765 (1966). [Google Scholar]
- 43.Richardson, M. J. Records of coprophilous fungi – a data set. Mycotaxon130, 925 (2015). https://www.mycotaxon.com/resources/checklists/Richardson-v130-3-checklist.pdf [Google Scholar]
- 44.Calaça, F. J. S., Silva, N. C. & Xavier-Santos, S. A checklist of coprophilous fungi and other fungi recorded on dung from Brazil. Mycotaxon128, 205 (2014). http://www.mycotaxon.com/resources/checklists/calaca_v128_checklist.pdf
- 45.Calaça, F. J. S., Tereza, V. B. & Xavier-Santos, S. Additions to a checklist of coprophilous fungi and other fungi recorded on dung from Brazil: an overview of a century of research. Mycotaxon135, 901. 10.5248/135.899 (2020). [Google Scholar]
- 46.Melo, R. F. R., Gondim, N. H. B., Santiago, A. L. C. M. A. & Maia, L. C. Miller, N. A. Coprophilous fungi from Brazil: updated identification keys to all recorded species. Phytotaxa436, 104–124. 10.11646/phytotaxa.436.2.2 (2020). [Google Scholar]
- 47.Castle, M. E. & MacDaid, E. The decomposition of cattle dung and its effect on pasture. J. Br. Grassld Soc.27, 133–137 (1972). [Google Scholar]
- 48.Masunga, G. S., Andresen, Ø., Taylor, J. E. & Dhillion, S. S. Elephant dung decomposition and coprophilous fungi in two habitats of semi-arid Botswana. Mycol. Res.110, 1214–1226. 10.1016/j.mycres.2006.07.004 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Freymann, B. P., Buitenwerf, R., de Souza, O. & Olff, H. The importance of termites (Isoptera) for the recycling of herbivore dung in tropical ecosystems: a review. J. Entomol.105, 165–173 (2008). [Google Scholar]
- 50.Eghball, B., Wienhold, B. J., Gilley, J. E. & Eigenberg, R. A. Mineralization of manure nutrients. J. Soil. Water Conserv.57, 470–473 (2002). [Google Scholar]
- 51.Aarons, S. R., O’Connor, C. R. & Gourley, C. J. P. Dung decomposition in temperate dairy pastures I. Changes in soil chemical properties. Aust. J. Soil Res.42, 107–114 (2004). [Google Scholar]
- 52.Webster, J. Presidential address. Coprophilous fungi. Trans. Br. Mycol. Soc.54, 161–180 (1970). [Google Scholar]
- 53.Pecl, G. T. et al. Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science. 355(6332), 1–9. 10.1126/science.aai9214 (2017). [DOI] [PubMed]
- 54.Felfili, J. M. et al. Projeto Biogeografia do bioma Cerrado: vegetação e solos. Cadernos De Geociências do IBGE. 12, 75–166 (1994). [Google Scholar]
- 55.Cardoso, M. R. D., Marcuzzo, F. F. N. & Barros, J. R. Classificação climática De Köppen-Geiger para o Estado De Goiás E Distrito Federal. ACTA Geográfica. 8, 40–55. 10.5654/acta.v8i16.1384 (2014). [Google Scholar]
- 56.Silva, D. J. & Queiroz, A. C. Análises De Alimentos: Métodos Químicos e Biológicos (UFV, Viçosa, 2006).
- 57.Tedesco, M. J., Gianello, C., Bissani, C. A., Bohnen, H. & Volkweiss, S. J. Análise de solo, plantas e outros materiais. Universidade Federal do Rio Grande do Sul, Departamento de Solos, Boletim Técnico 5 (1995).
- 58.Miyazawa, M., Pavan, M. A., Muraoka, T., Carmo, C. A. F. S. & Mello, W. J. Análise química De Tecido Vegetal. In Manual de Análise Químicas de Solos, Plantas e Fertilizantes (ed Silva, J. C.) (EMBRAPA, Brasília, 1999).
- 59.Souza, L. D. S., Azevedo, D. D. O., de Carvalho, A. J. A., Simões, W. L. & Voltolini, T. V. Qualidade Nutricional De Plantas forrageiras de ocorrência natural na caatinga. Enciclopédia Biosfera. 9, 178–185 (2013). [Google Scholar]
- 60.Karberg, N. J., Scott, N. A. & Giardina, C. P. Methods for estimating litter decomposition. In Field measurements for forest carbon monitoring Hoover, C. M. (ed) (Springer Dordrecht, 2008). 10.1007/978-1-4020-8506-2_8) .
- 61.Hammer, Ø. et al. Past 4.x—PAleontological STatistics (Natural History Museum, University of Oslo, 2021).
- 62.Olson, J. S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology44, 322–331. 10.2307/1932179 (1963). [Google Scholar]
- 63.Alban, D. H. & Pastor, J. Decomposition of aspen, spruce, and pine boles on two sites in Minnesota. Can. J. for. Res.23, 1744–1749. 10.1139/x93-220 (1993). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the conduction of the study are available from the corresponding author upon reasonable request. Data is provided within the manuscript or supplementary information files.