Abstract
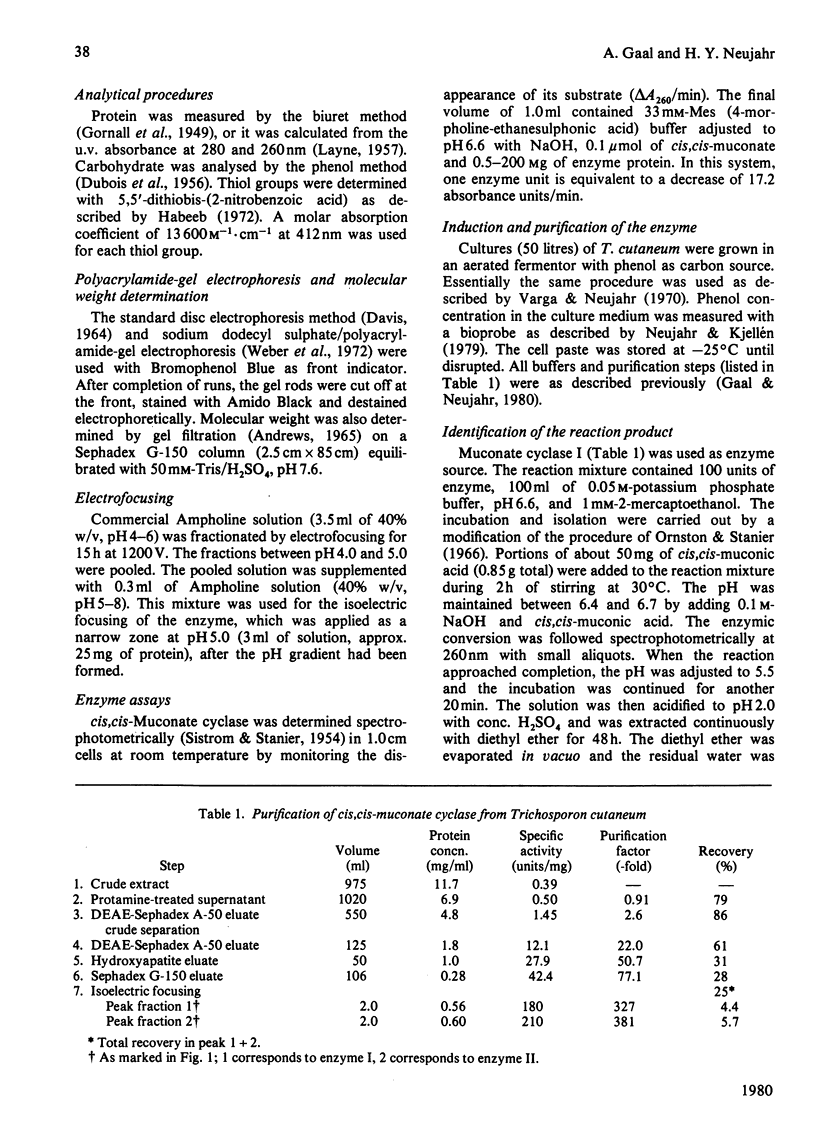
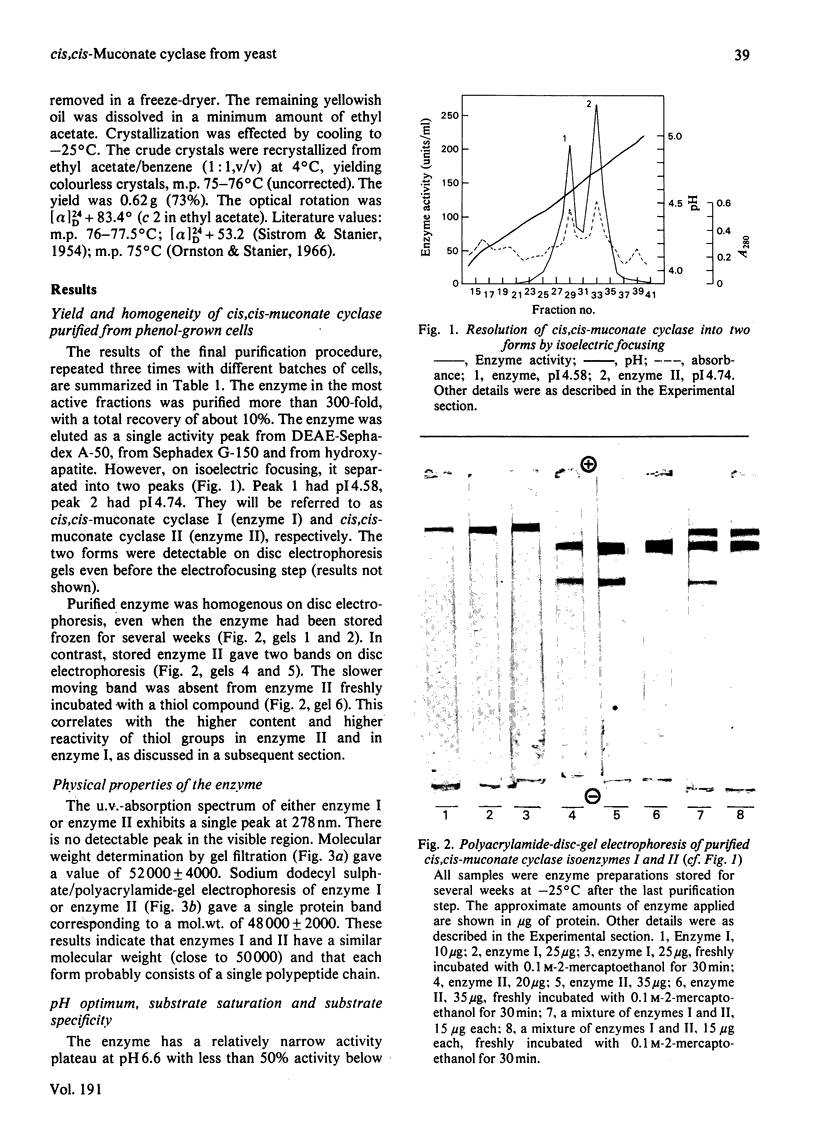
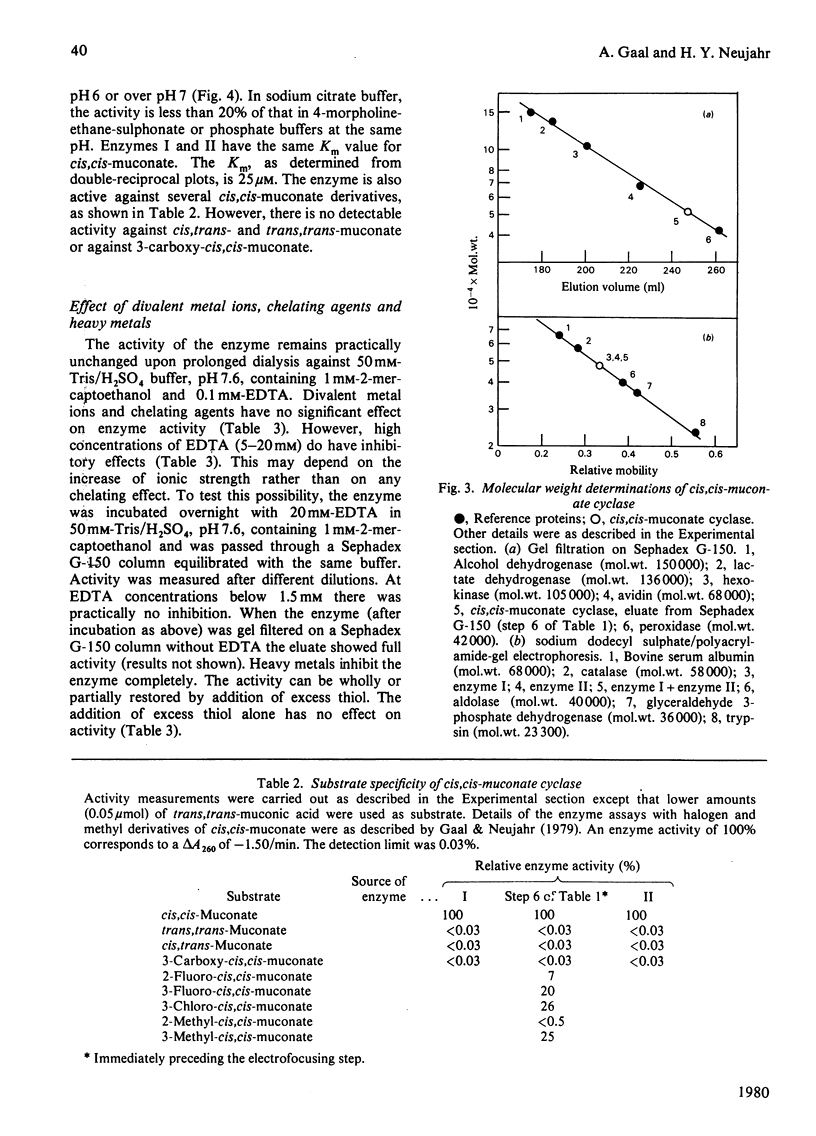
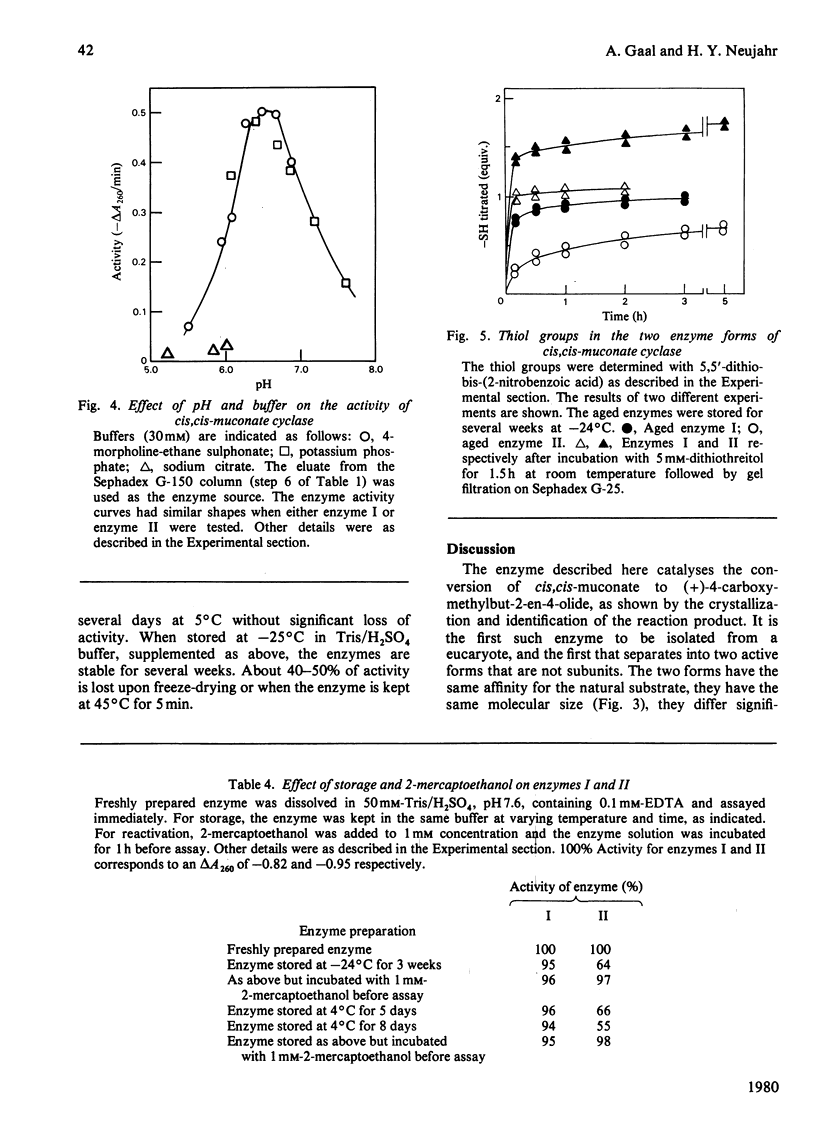
The inducible enzyme catalysing the conversion of cis,cis-muconate to (+)-muconolactone was purified 300-fold from the yeast Trichosporon cutaneum, grown on phenol. The enzyme has a sharp pH optimum at pH 6.6. It reacts also with several monohalogen derivatives and with one monomethyl derivative of cis,cis-muconate, but not with cis,trans- or trans,trans-muconate or 3-carboxy-cis,cis-muconate. In contrast with the corresponding enzymes in bacteria, the yeast enzyme does not require added divalent metal ions for activity and is not inhibited by EDTA. The purified enzyme can be resolved into two peaks by isoelectric focusing. The two forms have pI 4.58 (cis,cis-muconate cyclase I) and pI 4.74 (cis, cis-muconate cyclase II), respectively. Each of these is homogenous on polyacrylamide-gel electrophoresis in the absence or presence of sodium dodecyl sulphate. The two enzyme forms have the same molecular weight (50000) as determined by gel filtration and by sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. They have the same Km value (25 microM) for cis,cis-muconate. They differ with respect to their content of free thiol groups. cis, cis-Muconate cyclase I contains one thiol group, essential for activity, but relatively stable upon storage. cis, cis-Muconate cyclase II contains two thiol groups that are readily oxidized during storage with concomitant loss of activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain R. B., Bilton R. F., Darrah J. A. The metabolism of aromatic acids by micro-organisms. Metabolic pathways in the fungi. Biochem J. 1968 Aug;108(5):797–828. doi: 10.1042/bj1080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. A., Cain R. B. Regulation of aromatic metabolism in the fungi: metabolic control of the 3-oxoadipate pathway in the yeast Rhodotorula mucilaginosa. J Gen Microbiol. 1974 Nov;85(1):37–50. doi: 10.1099/00221287-85-1-37. [DOI] [PubMed] [Google Scholar]
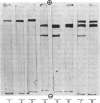
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gaal A. B., Neujahr H. Y. Maleylacetate reductase from Trichosporon cutaneum. Biochem J. 1980 Mar 1;185(3):783–786. doi: 10.1042/bj1850783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal A., Neujahr H. Y. Metabolism of phenol and resorcinol in Trichosporon cutaneum. J Bacteriol. 1979 Jan;137(1):13–21. doi: 10.1128/jb.137.1.13-21.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber T. J., Street J. R., Bull A. T., Cook K. A., Cain R. B. Aromatic metabolism in the fungi. Growth of Rhodotorula mucilaginosa in p-hydroxybenzoate-limited chemostats and the effects of growth rate on the synthesis of enzymes of the 3-oxoadipate pathway. Arch Microbiol. 1975;102(2):139–144. doi: 10.1007/BF00428358. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. I. Properties of cis,cis-muconate-lactonizing enzyme and muconolactone isomerase from Pseudomonas putida. Biochemistry. 1973 Aug 28;12(18):3523–3530. doi: 10.1021/bi00742a027. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Gaal A. Phenol hydroxylase from yeast. Purification and properties of the enzyme from Trichosporon cutaneum. Eur J Biochem. 1973 Jun;35(2):386–400. doi: 10.1111/j.1432-1033.1973.tb02851.x. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Gaal A. Phenol hydroxylase from yeast. Sulfhydryl groups in phenol hydroxylase from Trichosporon cutaneum. Eur J Biochem. 1975 Oct 15;58(2):351–357. doi: 10.1111/j.1432-1033.1975.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Kjellén K. G. Phenol hydroxylase from yeast. Reaction with phenol derivatives. J Biol Chem. 1978 Dec 25;253(24):8835–8841. [PubMed] [Google Scholar]
- Neujahr H. Y., Lindsjö S., Varga J. M. Oxidation of phenols by cells and cell-free enzymes from Candida tropicalis. Antonie Van Leeuwenhoek. 1974;40(2):209–216. doi: 10.1007/BF00394378. [DOI] [PubMed] [Google Scholar]
- Neujahr H. Y., Varga J. M. Degradation of phenols by intact cells and cell-free preparations of Trichosporon cutaneum. Eur J Biochem. 1970 Mar 1;13(1):37–44. doi: 10.1111/j.1432-1033.1970.tb00896.x. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Patel R. N., Meagher R. B., Ornston L. N. Relationships among enzymes of the beta-ketoadipate pathway. II. Properties of crystalline beta-carboxy-cis,cis-muconate-lactonizing enzyme from Pseudomonas putida. Biochemistry. 1973 Aug 28;12(18):3531–3537. doi: 10.1021/bi00742a028. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of formation of beta-ketoadipic acid by bacteria. J Biol Chem. 1954 Oct;210(2):821–836. [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Thatcher D. R., Cain R. B. Metabolism of aromatic compounds by fungi. 1. Purification and physical properties of 3-carboxy-cis-cis-muconate cyclase from Aspergillus niger. Eur J Biochem. 1974 Oct 2;48(2):549–556. doi: 10.1111/j.1432-1033.1974.tb03796.x. [DOI] [PubMed] [Google Scholar]
- Thatcher D. R., Cain R. B. Metabolism of aromatic compounds by fungi. 2. Subunit structure of the 3-carboxy-cis-cis-muconate cyclase of Aspergillus niger. Eur J Biochem. 1974 Oct 2;48(2):557–562. doi: 10.1111/j.1432-1033.1974.tb03797.x. [DOI] [PubMed] [Google Scholar]
- Varga J. M., Neujahr H. Y. Purification and properties of catechol 1,2-oxygenase from Trichosporon cutaneum. Eur J Biochem. 1970 Feb;12(3):427–434. doi: 10.1111/j.1432-1033.1970.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]