Abstract
Upon activation, naive B cells exit their quiescent state and enter germinal center (GC) responses, a transition accompanied by increased protein synthesis. How protein translation efficiency is adequately adjusted to meet the increased demand requires further investigation. Here, we identify the methyltransferase METTL1 as a translational checkpoint during GC responses. Conditional knockout of Mettl1 in mouse B cells blocks GC entry and impairs GC formation, whereas conditional knock-in of Mettl1 promotes GC responses. Mechanistically, METTL1 catalyzes m7G modification in a specific subset of tRNAs to preferentially translate BCR signaling-related proteins, ensuring mitochondrial electron transporter chain activity and sufficient bioenergetics in B cells. Pathologically, METTL1-mediated tRNA m7G modification controls B-cell autoreactivity in SLE patients or lupus-prone mice, and deletion of Mettl1 alleviates dysregulated B-cell responses during autoimmune induction. Thus, these results support the function of METTL1 in orchestrating an effective B-cell response and reveal that aberrant METTL1-mediated tRNA m7G modification promotes autoreactive B cells in systemic autoimmunity.
Subject terms: B cells, Autoimmunity, Methylation
An effective B cell response requires a rapid increase in protein synthesis. Using multi-omics approaches, here the authors show that the methyltransferase METTL1 drives B cell activation via the tRNA m7G modification-dependent translation of BCR signaling and that aberrant METTL1 causes B cell autoreactivity in humans and mice, thus serving as a therapeutic target in systemic autoimmunity.
Introduction
B cells are at the center of the adaptive immune system, which is responsible for humoral immunity1. Upon antigen recognition, naive B cells undergo profound metabolic and transcriptional changes to exit their quiescent state2–4. Following interaction with CD4+ T cells to receive costimulatory signals, antigen-encountered B cells then enter germinal centers (GC). GC B cells give rise to long-lived memory B cells (MBC) and plasma cells (PC) that provide immune memory and protection against recurrent infections. Within GCs, GC B cells undergo clonal expansion, somatic mutation, and affinity maturation, where the demands of energy and protein synthesis are increased5–8. In addition to increasing mRNA transcription to synthesize more proteins, one of the major mechanisms is to increase protein translation efficiency (TE) to meet rapidly increasing demands9. However, how the regulation of TEs is involved in GC B-cell responses remains unclear. An aberrant GC B-cell response can lead to the generation of autoreactive B cells and the development of systemic autoimmunity10. It has not been yet reported whether dysregulated protein translation is involved in aberrant GC B-cell responses and the subsequent induction of autoimmunity.
The translation of messenger RNA (mRNA) into proteins in ribosomes is an important step in gene expression. Protein translation can be divided into initiation, extension, and termination11. Transfer RNAs (tRNA) are key adaptor molecules that decipher the genetic code, which is fundamentally important for mRNA translation. Posttranscriptional modifications strongly affect tRNA stability, tRNA structure, and mRNA translation12,13. tRNA-modifying enzymes are highly dysregulated in human cancers14, and aberrant modification of tRNAs is a novel regulator and initiator of human diseases and cancers15,16. In T cells, tRNA-m1A modification enhances TEs in T cells and enables T cells to synthesize essential amounts of protein for T-cell activation17. The function of tRNA modifications in B-cell responses is not known.
N7-methylguanosine (m7G) is one of the most prevalent tRNA modifications, and it is highly conserved in prokaryotes, eukaryotes, and some archaea18. In humans, m7G modification is catalyzed by the METTL1/WD repeat domain 4 (WDR4) complex. METTL1 is responsible for m7G catalysis, whereas WDR4 helps stabilize the methyltransferase complex. Mutation in WDR4 causes disorders in neurologic development19,20. In addition, METTL1/WDR4-mediated tRNA m7G modification is crucial for mRNA translation and embryonic stem cell self-renewal21. The upregulation of METTL1/WDR4-mediated tRNA m7G modification has been linked to increased tumorigenesis22–24. Recently, data showed that METTL1/WDR4-mediated tRNA m7G modification in cancer cells reshaped the tumor microenvironment by promoting the accumulation of polymorphonuclear myeloid-derived suppressor cells in the tumor microenvironment25. However, the function of METTL1 in immunity has not yet been reported.
Here, our data reveal the important role of METTL1-mediated m7G modification in B-cell activation. Specifically, METTL1 controls GC entry and GC formation, and deletion of Mettl1 in mouse B cells impairs GC responses significantly. We further identify that METTL1-mediated m7G preferentially controls the translation of B-cell receptor (BCR) signaling-related proteins to ensure mitochondrial respiration and bioenergetics generation. Importantly, autoreactive B-cell response is blunt when METTL1-mediated m7G is abolished in SLE patients or lupus-prone mice. Thus, this study provides evidence for the important function of METTL1-mediated m7G modification in B-cell-mediated immune responses. These data also suggest the aberrant METTL1-mediated m7G modification in the pathogenesis of B-cell-mediated systemic autoimmune diseases.
Results
METTL1-associated translation is enhanced in human B cells in response to vaccine immunization
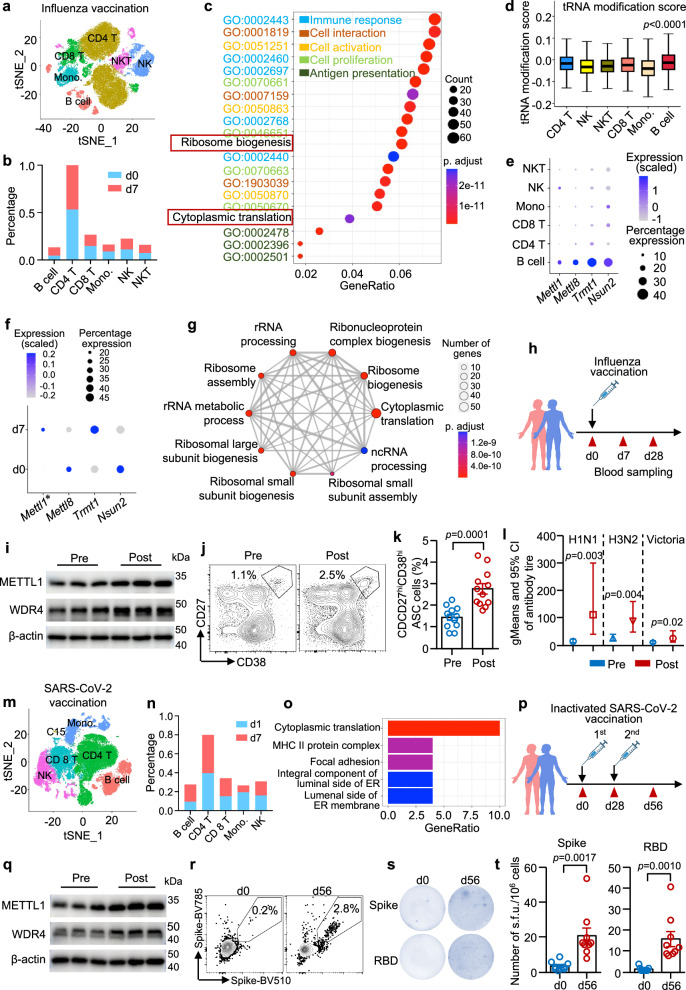
Upon activation, immune cells undergo fundamental changes in the translatomic profile and protein synthesis to cope with proliferation and differentiation26. To gain insight into the translational status of B cells after vaccination, we first accessed publicly available scRNA-seq data and performed bioinformatic analysis. The data revealed that the percentage of B cells was greater on d 7 than on d 0 (Fig. 1a, b; Supplementary Fig. 1a–c) after influenza vaccination. Gene Ontology (GO) enrichment analysis revealed that B cells from d 7 were enriched in translation and ribosome biogenesis compared with other immune cell compartments (Fig. 1c), suggesting that greater translation demands are needed to meet B-cell activation requirements during vaccination. tRNAs are key molecules that decode mRNA codons during translation in protein synthesis12. Bioinformatic analysis revealed that B cells present the highest score for tRNA modifications among subsets of immune cells in the blood, indicating high tRNA modification activity in B cells (Fig. 1d). METTL1 is the dominant tRNA-modifying enzyme that converts guanosine to m7G. Recent data show that METTL1/WDR4-mediated m7G tRNA modification is required for normal mRNA translation21,27. Methyltransferase genes involved in tRNA modification, including Mettl1, Mettl8, Trmt1, and Nsun2, were enriched in B cells (Fig. 1e). Although the average expression level and percentage expression of Mettl1 and Trmt1 were both greater in B cells from d 7 than in those from d 0, a significant change was observed only in Mettl1. In contrast, the change in Trmt1 did not reach statistical significance. In contrast, the expression of Mettl8 and Nsun2 was reduced on d 7 after immunization (Fig. 1f). These data suggest that METTL1 could represent the major methyltransferase for tRNA modification in B cells, indicating the important role of METTL1 in the B-cell response to immunization. Further analysis revealed that most of the upregulated differential expressed genes (DEG) in d 7 were enriched in translation and ribosome biogenesis when compared to d 0 B cells (Fig. 1g), suggesting the important role of translation control in B-cell responses. To further study the association between METTL1 and B-cell responses to immunization, individuals who received influenza vaccination were recruited, and blood samples were harvested (Fig. 1h). Western blot analysis revealed that the expression of both METTL1 and WDR4 was upregulated in B cells from d 7 after immunization (Fig. 1i). The upregulation of METTL1/WDR4 in B cells was accompanied by the expansion of antibody-secreting cells (ASC), as measured by flow cytometry on d 28 (Fig. 1j, k). Notably, the geometric titers of antibodies against the H1N1, H3N2, and Victoria influenza strains increased on d 28 after immunization (Fig. 1l).
Fig. 1. METTL1-associated translation is enhanced in B cells following vaccination.
a–g Single-cell RNA sequencing (scRNA-seq) analysis of PBMCs from donors before (d 0) and after (d 7) influenza vaccination (GSE211560). a t-SNE displaying cell clusters. b Bar graph displaying cell subset percentages before and after vaccination. c The top 20 terms upregulated in B cells compared with other clusters (adjusted p value via “clusterProfiler” GO analysis). d tRNA modification score (GO: 0006400) among cell clusters (4023 B cells, 29435 CD4 T cells, 7354 CD8 T cells, 4546 Monocytes, 6402 NK cells, 4613 NKT cells). The upper whisker is 75th percentile plus 1.5* interquartile range (IQR), and the lower is 25th percentile minus 1.5*IQR. The upper and lower bounds of box are 75th and 25th percentile, respectively, and the center is median. e Dot plot displaying differentially expressed genes (DEG) associated with common tRNA-modified enzymes. f Dot plot displaying Mettl1, Mettl8, Trmt1 and Nsun2 expression in B cells. g The top 10 GO-BP terms upregulated via GO analysis using upregulated DEGs on d 7 compared to d 0 B cells. h Experimental scheme of influenza vaccination. i METTL1 and WDR4 expression in B cells measured by western blotting. j, k The frequency of CD27hiCD38hi antibody-secreting cells (ASC) in PBMCs from donors receiving influenza vaccination (d 0 and d 28) measured by flow cytometry (n = 12). l H1N1, H3N2, and Victoria antibody titers in the sera of donors receiving influenza vaccination (d 0 and d 28), and n = 12. m–o scRNA-seq analysis of PBMCs from donors on the first day and 7 d after SARS-CoV-2 vaccination (GSE201534). m t-SNE displaying cell clusters from PBMCs. n Bar graph displaying cell subset percentages. o The top 5 terms upregulated in B cells compared with other clusters via GO analysis. p Experimental scheme of healthy donors receiving SARS-CoV-2 vaccination. q METTL1 and WDR4 expression in B cells measured by western blotting. r Representative flow cytometry plots of spike-specific B cells from the PBMCs of donors that received SARS-CoV-2 vaccination (d 0 and d 28). s, t Representative ELISpot and summary data showing spike- and receptor-binding domain (RBD)-specific IgG-secreting B cells from the PBMCs of donors who received SARS-CoV-2 vaccination (d 0 and d 28, n = 9). Biological independent samples for k, l, t. The data are presented as the mean ± SEM. A two-tailed unpaired Student’s t test in k, t, and Kruskal–Wallis test in l, d. Source data are provided as a Source Data file. h and p were created with BioRender.com.
To investigate the role of METTL1 in B-cell responses to immunization, scRNA-seq data from the SARS-CoV-2 vaccination cohort were acquired, and bioinformatic analysis was performed. We found that B cells were also expanded from individuals who received SARS-CoV-2 vaccination (Fig. 1m, n; Supplementary Fig. 1d, e). In line with influenza vaccination, cytoplasmic translation was notably enriched in B cells from individuals immunized with SARS-CoV-2 (Fig. 1o). Consistently, the expression of METTL1 and WDR4 was upregulated in B cells postimmunization, as determined by western blotting (Fig. 1p, q). The upregulation of METTL1/WDR4 was followed by the induction of spike-specific B cells, as measured by flow cytometry and ELISpot (Fig. 1r–t). Together, these data highlight the importance of METTL1-associated translation during B-cell responses to vaccination.
METTL1 is involved in GC responses
Secondary lymphoid organs (SLO) are sites where B cells recognize antigens captured by follicular dendritic cells28. Upon antigen recognition, naive B cells are activated, followed by the initiation of the GC response. The GC represents the epicenter for the formation of high-affinity antibodies through a somatic maturation mechanism29. Given the rapid and massive changes in response to antigen recognition by B cells during GC responses, we postulated the involvement of METTL1 in the early phase of B-cell activation in SLOs. To test this hypothesis, we first accessed the scRNA-seq data of the mouse spleen to obtain a broader picture of B-cell activation in response to immunization in SLOs. scRNA-seq analysis revealed that B cells from the spleens of NP-KLH-immunized mice could be divided into 7 clusters: erarlyGC-1, earlyGC-2, earlyGC-3, MBC, naive, PB and preGC (Supplementary Fig. 2a). Ribosome biogenesis scores among these clusters revealed that the earlyGC-3 cluster presents the highest activity of ribosome biogenesis (Supplementary Fig. 2b), which was further verified by GO analysis of DEGs upregulated in earlyGC-3 (Supplementary Fig. 2c). Moreover, the tRNA modification score indicated that the tRNA modification pathway was enriched in earlyGC-3 (Supplementary Fig. 2d). In line with our hypothesis, Mettl1 and Wdr4 expression were mostly upregulated in preGC and early GC B cells (Supplementary Fig. 2e, f), suggesting a role of METTL1/WDR4 in the early stage of B-cell activation and GC formation. To determine the involvement of METTL1 in B-cell responses, normal mice were immunized with NP-OVA, and the spleens were harvested for staining, and FACS analysis (Supplementary Fig. 2g). Immunofluorescence staining revealed that METTL1 was expressed in the mouse spleen, with the highest expression in PNA+ GC B cells (Supplementary Fig. 2h). Spleen samples were further analyzed by flow cytometry to measure METTL1 expression in B-cell subsets, and gating strategy was shown (Supplementary Fig. 2i). In line with the immunofluorescence staining, GC B cells presented the highest level of METTL1 in the spleen (Supplementary Fig. 2j, k). Moreover, METTL1 expression in B cells was notably upregulated after NP-OVA immunization, as determined by flow cytometry (Supplementary Fig. 2l, m). To further dissect the involvement of METTL1 in human B cells, surgically removed spleen samples were harvested and analyzed by flow cytometry (Supplementary Fig. 3a, b). Compared with other subsets, human GC B cells presented the highest level of METTL1, consistent with data from mice. Similar results were obtained for WDR4 expression in human B cells (Supplementary Fig. 3c, d). These data indicate the important role of METTL1 in GC responses.
METTL1 controls GC entry
To directly link the immune function of METTL1 to B-cell responses in vivo, we first generated conditional knockout (cKO) mice by crossing Cd19Cre mice with Mettl1flox/flox mice to obtain Cd19CreMettl1flox/flox cKO mice (Supplementary Fig. 4a). The deletion of Mettl1 was confirmed by western blotting (Fig. 2a). Concomitantly, the expression of WDR4 was dramatically downregulated in B cells when Mettl1 was deleted (Fig. 2a). We found that the composition of B cells across different developmental stages in the bone marrow was similar between cKO and WT mice (Supplementary Fig. 4b–d), suggesting that Mettl1 deletion does not disrupt bone marrow B-cell development. To evaluate the role of METTL1 in GC responses to immunization, we performed scRNA-seq to delineate the transcriptomic landscape to evaluate the impact of Mettl1 deletion on B-cell responses to vaccination at the single-cell level. Spleens were collected at d 14 after NP-OVA immunization, and B cells were processed for scRNA-seq (Fig. 2b, c). Spleen B cells could be clustered into 14 cell clusters, and cluster 13 was removed for fewer than 50 cells (Fig. 2d). Based on the signature gene expression profiles, these 13 clusters were defined into 7 cell subsets: naive, activated precursor (AP, similar to the GC precursor in Yazicioglu, Y.F.’s report30), early GC (highly similar to earlyGC-3 in Supplementary Fig. 3a), GC, GC (IFN), memory and PB (Fig. 2d; Supplementary Fig. 5a–h). By analyzing B-cell subsets in WT and cKO mice, we detected an accumulation of APs in the spleens of cKO mice. In contrast, early GCs, GCs and GCs (IFNs) were notably reduced in cKO mice (Fig. 2e). To determine the cell state of APs, we compared APs to other cell subsets, and DEGs were identified (Fig. 2f). Further GO analysis of the DEGs revealed an activation state of APs (Fig. 2g). It has been suggested that APs represent activated B cells and are doomed to enter GCs30, therefore we compared the gene signatures of GC B cells (upregulated genes compared with naive B cells in GSE4142) with those of naive, APs and early GC B-cell clusters via gene set enrichment analysis (GSEA). The GC B-cell signature was greater in APs than in naive GCs but lower in APs than in early GCs, suggesting a transitional state of APs from naive to GC B cells (Fig. 2h, i). Moreover, we performed Monocle pseudotime analysis on these cell subsets to further confirm the AP status after antigen recognition. Notably, naive B cells were found at the beginning of the trajectory, and early GC B cells were found at one terminal end, whereas APs were aligned along the trajectory between naive and early GCs (Fig. 2j). Therefore, we identified a differentiation trajectory from naive to APs and then early GC B cells. Additionally, we observed an increase in Mettl1 expression along the trajectory from APs to early GC (Fig. 2k, Supplementary Fig. 5i). A comparison of the trajectories of WT and cKO mice revealed retention in the AP state in cKO mice (Fig. 2l). Consistently, expansion of APs (IgD+GL-7+ B cells) was detected in cKO mice by flow cytometry (Fig. 2m, n). Given the accumulation of APs and the reduction in early GC B cells in cKO mice, we proposed that METTL1 is required for APs to enter GCs. To explore how Mettl1 deletion led to the accumulation of APs, we identified DEGs between WT and KO AP cells (Fig. 2o). Among these DEGs, Cd55 (also known as decay-accelerating factor), whose expression is repressed during GC formation31, was highly expressed in Mettl1-deleted AP. In contrast, the expression of Cd83, an important costimulatory molecule for B-cell activation and GC function32,33, was reduced in the Mettl1-deleted APs (Fig. 2o). Moreover, we detected higher levels of transcription factors crucial for GC formation in WT APs than in Mettl1-deleted APs (Fig. 2p). The expression of Foxp1, which is downregulated during GC formation, was found to be increased in Mettl1-deleted APs (Fig. 2p). GSEA revealed that compared with WT APs, Mettl1-deleted APs was less activated from the naive state to the GC B or early GC B state (Supplementary Fig. 5j, k). Taken together, these findings indicate that GC entry is halted when Mettl1 is deleted in B cells, suggesting that METTL1 controls GC entry during immunization.
Fig. 2. METTL1 controls germinal center (GC) B-cell entry.
a B cells were isolated from conditional knockout (cKO) mice or wild-type (WT) mice. Knockout of METTL1 in B cells was confirmed by western blotting. b–p Spleen B cells from NP-OVA-immunized mice were collected for scRNA-seq. b, c Experimental schemes were created with BioRender.com. d UMAP displaying B-cell clusters and cell cluster definitions. e Bar plots displaying the percentages of B-cell subsets in WT and cKO mice. f Volcano plot displaying DEGs in the activated precursor (AP) cell cluster compared with the other cell clusters (adjusted p value via “limma” R package). g The top 10 GO-BP terms enriched in the AP cell cluster compared with the other cell clusters (adjusted p value via “clusterProfiler” GO analysis). h Gene set enrichment analysis (GSEA) plot of the upregulated signature of germinal center (GC) B cells vs naive B cells (GO-C7 term: GSE4142_Naive_vs_GC_Bcell_DN) between the AP cell cluster and naive B-cell cluster (p value via “clusterProfiler” GSEA analysis). i GSEA plot of the upregulated signature in GC B cells vs naive B cells (GO-C7 term: GSE4142 naive vs GC B-cell DN) between the AP cell cluster and the GC B-cell cluster (p value via “clusterProfiler” GSEA analysis). j–l Pseudotime analysis of naive, AP and early GC cell clusters via Monocle2 analysis. j Pseudotime trajectory of naive, AP, and early GC cell clusters. k Mettl1 expression trajectory. l Cell trajectory in WT and cKO mice. m, n The frequencies of APs (IgD+GL7+B220+) in the spleens of cKO (n = 5 biological independent samples) or WT (n = 5 biological independent samples) mice immunized with NP-OVA were confirmed by flow cytometry, and representative plots are shown. A p value was calculated via a two-tailed unpaired Student’s t test. o Volcano plot displaying DEGs in the WT AP cell cluster compared with the cKO AP cell cluster (p value via “limma” R package). p Dot plot displaying the expression of important transcription factors involved in GC formation. The data are presented as the mean ± SEM. Source data are provided as a Source Data file.
METTL1 is required for GC responses
In the resting state, the size of the spleen and total number of splenocytes were similar between cKO and WT mice (Supplementary Fig. 6a). The percentages and numbers of total B220+ B cells were comparable between cKO and WT mice (Supplementary Fig. 6b, c). Furthermore, the percentages and numbers of B-cell subsets were also similar, except that the percentage and number of follicular B cells (FoB) were slightly reduced, and the percentage and number of marginal zone B cells (MZB) were increased in the spleens of cKO mice (Supplementary Fig. 6d–q). No significant changes were observed in the HE-stained spleen tissue (Supplementary Fig. 6r).
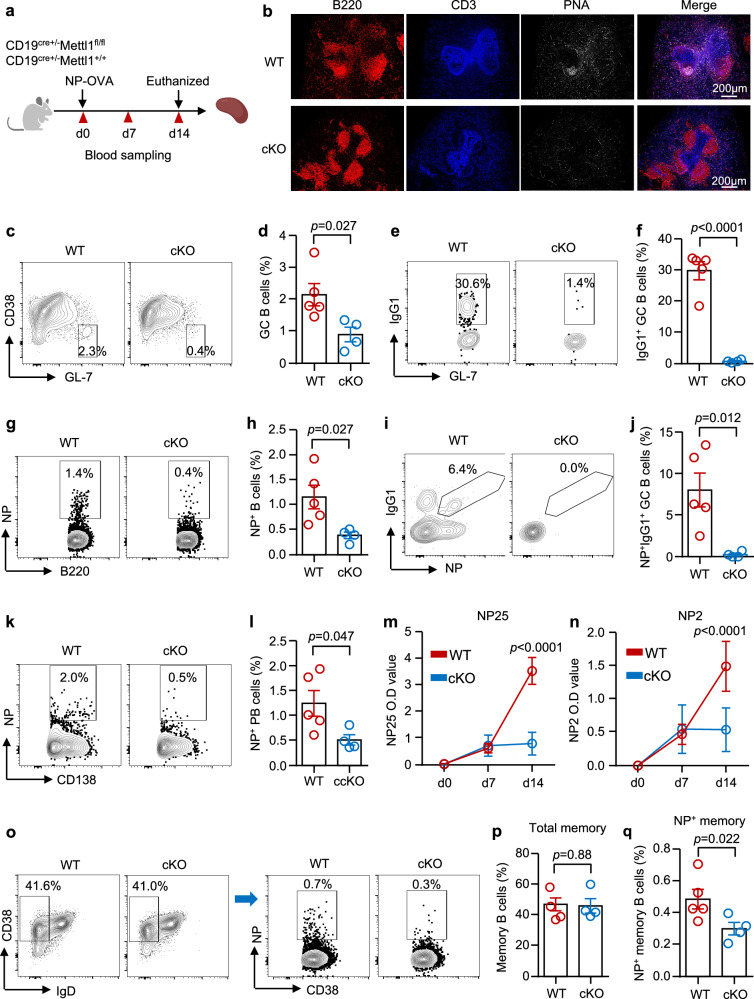
After NP-OVA immunization, the spleens and sera were harvested on d 14 to analyze B-cell responses and antibody affinity maturation (Fig. 3a). Immunofluorescence staining of spleen sections revealed a strong GC response after NP-OVA immunization in WT mice. However, GC staining was absent in the cKO mice (Fig. 3b). Furthermore, the percentage of GC B cells in cKO mice was notably lower than that in WT mice, as measured by flow cytometry (Fig. 3c, d). Moreover, class switching was impaired in cKO mice, and the number of IgG1-producing GC B cells was lower in cKO mice (Fig. 3e, f). To further assess the impact of Mettl1 deletion on B-cell responses to NP-OVA immunization, the cells were stained with fluorescence-labeled NPs and measured by flow cytometry. Our data revealed that the percentage of NP-specific B cells was reduced in the spleens of cKO mice (Fig. 3g, h). Surprisingly, NP-specific IgG1+ GC B cells were almost undetectable in the spleens of cKO mice (Fig. 3i, j). The percentage of NP-specific CD138+ cells was notably lower in the spleens of cKO mice than in those of WT mice (Fig. 3k, l). NP2-BSA and NP25-BSA were used to measure high-affinity IgG and total low-affinity IgG to evaluate affinity maturation by ELISA. Our data revealed that the level of IgG NP25 was significantly lower in the sera of cKO mice (Fig. 3m). In addition to impaired IgG production, METTL1 deficiency led to reduced affinity maturation efficiency, as demonstrated by the notably lower level of NP2 IgG in cKO mice (Fig. 3n). For the FoB/MZB subsets, we observed a greater proportion of MZB cells and a lower proportion of FoB cells in the cKO mice (Supplementary Fig. 7a, b). Of note, our data revealed that the percentages of T-cell subsets and the frequency of TFH cells were not changed in cKO mice (Supplementary Fig. 7c–f), which was consistent with the recent study34.
Fig. 3. METTL1 is required for GC B-cell responses.
a Experimental scheme, which was created with BioRender.com. b Representative images of immunofluorescence staining of spleen sections. B220 (red), CD3 (blue), and PNA (white). c, d Representative flow cytometry plots and frequencies of GL7+CD38− GC B cells in the spleens of WT and cKO mice. e, f Representative flow plots and frequencies of IgG1+ GC B cells among the GC B cells of the WT and cKO groups. g, h Representative flow cytometry plots and frequencies of NP+ B cells in WT and cKO mice. i, j Representative flow cytometry plots and frequencies of NP+IgG1+ GC B cells in WT and cKO mice. k, l Representative flow cytometry plots, and frequencies of NP+ plasmablasts (PB) in WT and cKO mice. m, n Anti-NP25 and anti-NP2 IgG antibodies in the serum were measured via ELISA. o–q Representative flow cytometry plots and frequencies of total memory B cells (p) and NP+ memory B cells (q) in WT and cKO mice. WT = 5 biological independent samples, cKO = 4 biological independent samples. The data are presented as the mean ± SEM. Two-tailed unpaired Student’s t test was used in d, f, h, j, l–n, p, q. Source data are provided as a Source Data file.
In addition to the generation of PCs, the GC response results in the production of MBCs6,35. Although the percentage of total MBCs was similar, the percentage of NP-specific MBCs was significantly lower in cKO mice (Fig. 3o–q). To further explore whether METTL1 plays a role in the formation of MBCs, we immunized mice with OVA repetitively after primary immunization and analyzed isotype-switched OVA-specific B cells 2 weeks later (Supplementary Fig. 8a). Notably, GC responses and the formation of PCs were reduced in cKO mice (Supplementary Fig. 8b–f). The number of anti-OVA IgG+ B cells in the spleen was reduced in cKO mice, as determined by ELISpot (Supplementary Fig. 8g, h). In line with the observed MBC responses, the production of OVA-specific IgG in the serum was lower in the cKO mice than in the control mice (Supplementary Fig. 8i). Despite the presence of MBCs in the spleen, repetitive immunization did not elicit a response as strong as the WT control did (Supplementary Fig. 8), suggesting that METTL1 is required for the formation of the MBCs and the recall response. Taken together, these data suggest that METTL1 is essential for B-cell responses.
METTL1 regulates extrafollicular B-cell responses
To test whether METTL1 plays a role in extrafollicular T-cell-independent (TI) responses, mice were immunized with NP-Ficoll (Supplementary Fig. 9a). Consistently, deletion of METTL1 led to a reduction in FoB cells and an increase in MZB cells (Supplementary Fig. 9b, c). Immunofluorescence staining revealed that NP-Ficoll failed to induce GC responses (Supplementary Fig. 9d). Although the frequency of PBs did not change, the percentage of PCs was lower in cKO mice than in WT mice, as measured by flow cytometry (Supplementary Fig. 9e, f). Additionally, we did not observe differences in NP-specific B cells or NP-specific PBs between the cKO and WT controls (Supplementary Fig. 9g–j). NP-specific PCs were reduced in cKO mice (Supplementary Fig. 9k, l). Extrafollicular TI B-cell responses are dominated by IgM and IgG3 antibodies36,37. Despite the increased frequency of MZ B cells, NP-specific IgM and NP-specific IgG3 antibodies were notably lower in cKO mice than in WT control mice (Supplementary Fig. 9m, n), suggesting a role for METTL1 in extrafollicular B-cell responses.
METTL1 controls GC responses via BCR signaling
The METTL1/WDR4 complex catalyzes the m7G modification of tRNAs in cancer cells and stem cells21,22. We thus performed a northwestern blot assay to assess the m7G modification level in Mettl1-deleted B cells and WT control cells. Notably, m7G modification was reduced in Mettl1-deleted B cells (Fig. 4a, Supplementary Fig. 10a). We employed TRAC-seq to profile the global m7G modifications in tRNAs in B cells (Fig. 4b). Using TRAC-seq, we identified 22 tRNAs that contain m7G modifications at the “AGGTC” motif sequence (Fig. 4c, d). Knockout of METTL1 significantly reduced cleavage scores (Fig. 4e, f). m7G signals in the identified tRNAs were significantly reduced in Mettl1-deleted B cells (Fig. 4g, h). To validate the METTL1-regulated tRNA components, we performed METTL1-RIP sequencing in 293 T cells overexpressing Mettl1. We found that the tRNAs identified by METTL1-RIP sequencing were highly consistent with tRNAs regulated by m7G modification identified by TRAC-seq (Fig. 4i–k, Supplementary Fig. 10b). Given the important function of m7G modification in mRNA translation, we verified translation impairment in Mettl1-deleted B cells via a puromycin uptake assay (Fig. 4l, m; Supplementary Fig. 10c, d). Taken together, these data reveal the importance of METTL1 in the regulation of tRNA m7G modification and mRNA translation in B cells.
Fig. 4. METTL1 regulates m7G tRNA modification in B cells.
a Northwestern blot showing m7G modification in B cells from WT and cKO mice. Representative bands are shown. b Experimental scheme of TRAC-seq for calculating cleavage scores. c List of m7G-modified tRNAs identified by TRAC-seq in spleen B cells from WT and cKO mice. d The motif sequence “AGGTC” at the m7G site was identified by TRAC-seq in B cells. e Representative image showing the different cleavage scores at the motif sequence. f Quantitative comparison of cleavage scores between B cells from WT and cKO mice (n = 1 mouse for each group). The upper whisker is the maxima, and the lower whisker is the minima. The upper and lower bounds of the box are the 75th percentile and 25th percentile, respectively. The center of the box is the median. g, h Expression profile of the 22 m7G-modified tRNAs based on TRAC-seq. The relative expression of each tRNA type was calculated from the combined expression of all tRNA genes belonging to the same tRNA type. The expression of the indicated tRNA type was then normalized by its overall average level in both groups and transformed by log2 (n = 1 mouse for each group). The upper whisker is the maxima, and the lower whisker is the minima. The upper and lower bounds of the box are the 75th percentile and 25th percentile, respectively. The center of the box is the median. i–k METTL1-IP tRNA experiments. i Western blots showing METTL1 overexpression in 293 T cells. Representative bands were shown. j Scatter plot displaying METTL1-IP tRNAs recognized by the tRNA library-seq. k Venn diagram displaying overlap of METTL1-RIP tRNA-seq and TRAC-seq data. l, m A puromycin intake assay was performed via flow cytometry, and representative histograms of B cells from WT (n = 5 biological independent samples) and cKO (n = 4 biological independent samples) mice are shown. The data are presented as the mean ± SEM. A two-tailed Mann–Whitney test was used in f, h, and a two-tailed unpaired Student’s t test was used in m. Source data are provided as a Source Data file.
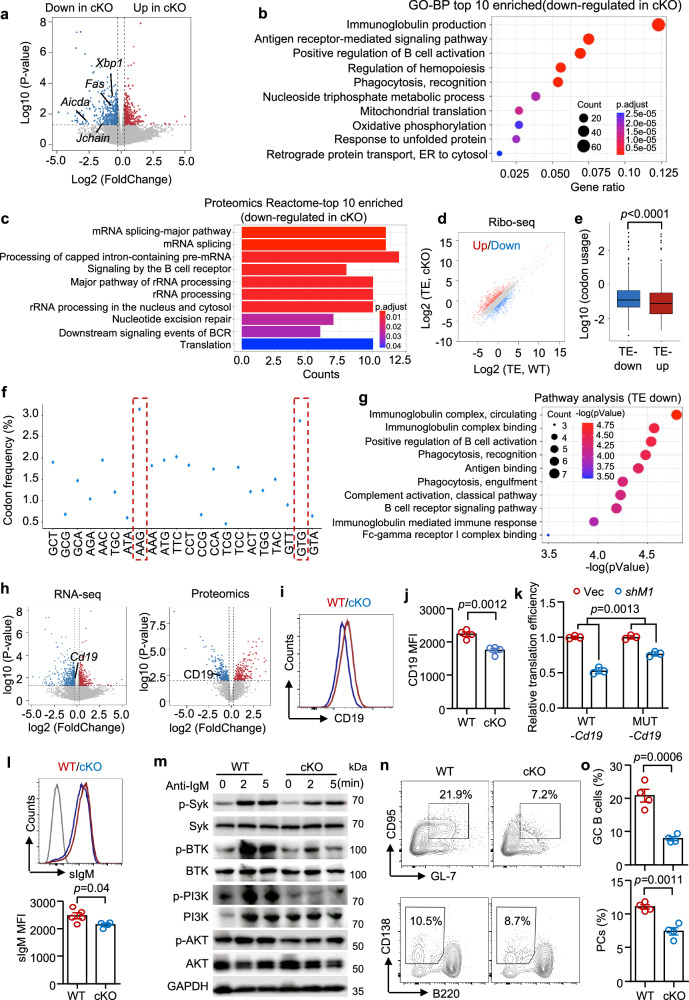
We combined RNA-seq and proteomics to screen for affected genes and pathways in Mettl1-deleted B cells. The RNA-seq data revealed 563 downregulated and 381 upregulated DEGs (Fig. 5a). Specifically, Aicda, Fas, Xbp1 and Jchain, which are activation markers of GC B cells and PCs, were downregulated in Mettl1-deleted B cells compared with WT B cells (Fig. 5a). Compared with those in Mettl1-deleted B cells, mitochondrion-, ribosome- and immunoglobulin-related pathways in WT B cells were enriched according to KEGG and GO analyses (Fig. 5b; Supplementary Fig. 11a, b). Proteomic data suggested enrichment of the BCR pathway, and ribosome- and mitochondrion-related pathways in WT B cells compared with Mettl1-deleted B cells (Fig. 5c, Supplementary Fig. 11c). To link the differences found by RNA-seq and proteomics, we further performed Ribo-seq to determine the precise mRNAs read by the ribosome and the transcripts associated with the translation machinery. By calculating the TEs of genes, 902 genes with downregulated TEs and 1331 genes with upregulated TEs were found in Mettl1-deleted B cells (Fig. 5d). Codon usage analysis revealed that mRNAs with decreased TEs (TE-down) had significantly greater usage of codons decoded by m7G-modified tRNAs (Fig. 5e). To identify the enriched codons regulated by METTL1, we compared codon frequency in TE-down genes in WT samples and found that AAG and GTG, which were decoded by LysCTT and ValCAC respectively, were the top 2 enriched codons regulated by METTL1 (Fig. 5f). Overall, these data suggest that decreased tRNA m7G modification selectively impaired the translation of mRNAs with a relatively high frequency of m7G-tRNA-decoded codons in B cells. To perform pathway enrichment analysis, we chose TE-downregulated (TE-down) mRNAs for which the fpkm of the WT sample was greater than 5. GO analysis revealed that most of these genes were involved in the BCR signaling pathway and B-cell responses (Fig. 5g).
Fig. 5. METTL1 controls the GC B-cell responses via BCR signaling.
a Volcano plot displaying DEGs between cKO B cells and WT control B cells through RNA-seq (p value via “limma” R package). b Top 10 terms enriched with downregulated mRNA in Mettl1-deleted B cells (adjusted p value via “clusterProfiler” GO-BP analysis). c Top 10 terms enriched with downregulated proteins in Mettl1-deleted B cells via Reactome pathway analysis (adjusted p value via “clusterProfiler” Reactome analysis). d–g Ribo-seq analysis of WT and cKO spleen B cells. d Scatterplots displaying the translation efficiency (TE) of genes in B cells from WT and cKO mice. e Frequency of m7G tRNA-decoded codon usage in genes with increased TEs (TE-up) and decreased TEs (TE-down) in Mettl1-deleted B cells (n = 1 mouse for each group). The upper whisker is 75th percentile plus 1.5*IQR, and the lower whisker is 25th percentile minus 1.5*IQR. The upper and lower bounds of box are 75th and 25th percentile, respectively, the center is median, and points show outliers. f Codon frequency of m7G tRNA-decoded codons in TE-down genes. g GO terms enriched with downregulated TE genes in Mettl1-deleted B cells (p value via “clusterProfiler” R package). h Volcano plot displaying nonsignificant changes in Cd19 expression via RNA-seq and significant downregulation of CD19 in Mettl1-deleted B cells via proteomic data (p value via “limma” R package). i, j Representative flow plot and summarized data showing CD19 expression levels in WT or Mettl1-deleted B cells (n = 4). k Bar plots displaying the relative TE changes after the transfection of WT-Cd19 or MUT-Cd19 with base mutations in specific codon plasmids into Mettl1-knockdown 293 T cells and control 293 T cells (n = 3). l Representative flow plot and summary data showing surface IgM (sIgM) expression levels in WT or Mettl1-deleted B cells (WT = 5 and cKO = 4). m The expression of p-SYK, p-BTK, p-PI3K, and p-AKT in WT or Mettl1-deleted B cells was measured via western blot and representative bands. n, o GC B cells or PCs were induced in vitro (n = 4). Representative plots are shown. Biological independent samples for j–l, o. The data are presented as the mean ± SEM. A two-tailed Mann–Whitney test was used in e, and a two-tailed unpaired Student’s t-test was used in j–l, o. Source data are provided as a Source Data file.
CD19 is a B-cell-specific transmembrane protein that functions as a coreceptor of BCR. Following BCR crosslinking and CD19 phosphorylation, B-cell activation is initiated by the Src family, which then activates Igα/Igβ38. CD19 can amplify the function of Src family kinases and recruit PI3K, thereby promoting BTK and AKT phosphorylation. Activated BTK could induce calcium signaling for B-cell activation (Supplementary Fig. 12a)39,40. RNA-seq revealed that the transcript level of Cd19 was comparable between Mettl1-deleted and WT B cells (Fig. 5h). Intriguingly, proteomic data suggested a significantly lower level of CD19 in Mettl1-deleted B cells than in WT B cells (Fig. 5h). To validate these findings, we measured the expression of CD19 at the protein level by flow cytometry. Notably, deletion of Mettl1 led to reduced CD19 expression on the B-cell surface (Fig. 5i, j), further supporting the notion that METTL1 controls BCR signaling-related proteins at the posttranscriptional level. We then conducted ribosome nascent-chain complex-bound mRNA (RNC) analysis and reported decreased TE of Cd19 mRNA in Mettl1-deleted B cells (Supplementary Fig. 12b). To validate, we performed synonymous base mutations in TTC, GTG, GCT, ACT, and CCT codons in Cd19 mRNA expression plasmid, which was transfected into Mettl1-knockdown 293 T cells (Supplementary Fig. 12c). These five codons were selected as the related tRNAs exhibited relatively large expression differences between the control and cKO groups and had a higher frequency within the Cd19 mRNA compared to other codons. Moreover, synonymous mutations of these codons can be achieved because they have synonymous non-m7G codons. As we expected, after base mutations, the TE of Cd19 was rescued (Fig. 5k), suggesting that TTC, GTG, GCT, ACT, and CCT, which are encoded by 5 m7G-modified tRNAs (PheGAA, ValCAC, AlaAGC, ThrAGT and ProAGG), were specific codons regulated by METTL1. Moreover, surface IgM (sIgM) was reduced in Mettl1-deleted B cells, indicating impaired BCR aggregation (Fig. 5l). Both the RNA-seq and proteomic data revealed significantly lower levels of IgM in Mettl1-deleted B cells than in control B cells (Supplementary Fig. 12d). To compare the mRNA and protein abundance of BCR signaling pathway-related genes, we performed heatmap analysis using RNA-seq and proteomic data in Mettl1-deleted B cells and control B cells. The data revealed that mRNA of BCR signaling pathway-related genes were comparable between the two groups, while the protein level of these genes was mostly lower in Mettl1-deleted B cells (Supplementary Fig. 12e). SYK, an important member of the Src family and a key molecule in the BCR signaling pathway39,40, whose phosphorylation level was reduced when Mettl1 was deleted in B cells, as measured by western blotting, and further confirmed by flow cytometry (Fig. 5m; Supplementary Fig. 12f, g), resulting in decreased levels of phosphorylated BTK in Mettl1-deleted B cells (Fig. 5m). CD19 is the key coreceptor in BCR signaling and is essential for PI3K-AKT signaling pathway activation39. Notably, the phosphorylation of PI3K was lower in Mettl1-deleted B cells than in WT control B cells, as measured by western blotting (Fig. 5m). Downstream PI3K signaling was further assessed, revealing that the level of phosphorylated AKT was reduced in Mettl1-deleted B cells (Fig. 5m, Supplementary Fig. 12h, i). BCR (sIgM) signaling capacity was further determined by measuring Ca2+ influx following stimulation with soluble goat F(ab’)2 anti-mouse IgM. Notably, calcium influx was lower in the Mettl1-deleted B cells than in the WT control cells (Supplementary Fig. 12j). Finally, we linked METTL1/WDR4-mediated TEs involved in BCR signaling to B-cell responses. Our data revealed that the B-cell proliferation and differentiation of GC B cells and PC cells were profoundly suppressed in Mettl1-deleted B cells (Fig. 5n, o; Supplementary Fig. 12k, l). Together, these data suggest that METTL1 promotes B-cell responses by enhancing BCR signaling through translation.
To explore the function of METTL1 in human B cells, we knocked down Mettl1 in human B cells sorted from healthy donors via a lentivirus. In vitro experiments revealed that Mettl1-knockdown B cells presented impaired proliferation and were likelier to remain in the G0 and G1 phases (Supplementary Fig. 13a–d). However, apoptosis was not affected by the knocking down of Mettl1 (Supplementary Fig. 13e, f). Moreover, impaired ASC differentiation and a class switch to IgG were found in Mettl1-knockdown B cells (Supplementary Fig. 13g–j).
METTL1 regulates ETC activity in B cells
Consistent with the enrichment of mitochondrion-related pathways in WT B cells compared with Mettl1-deleted B cells (Supplementary Fig. 11), the scRNA-seq data revealed that a series of genes related to the ETC were upregulated in WT B cells (Fig. 6a). KEGG analysis and GO-CC analysis revealed significant enrichment of pathways related to mitochondria (Fig. 6b, c). When the OXPHOS levels in B-cell subsets were scored, the early GC-3 cluster presented the highest OXPHOS score (Fig. 6d). After calculating the DEGs between WT early GC B cells and Mettl1-deleted early GC B cells, we performed GO analysis and detected enrichment of mitochondria-related pathways in WT early GC B cells (Fig. 6e, Supplementary Fig. 14a, b). The copy numbers of Ndufa1, Ndufa4, and Ndufa12 for the ETC complex (C) C I, Sdhb, and Uqcrq for C II, Cyc1 for C III and Cox6c, and Cox7b for C IV were notably reduced when Mettl1 was deleted in B cells, as confirmed by qPCR (Fig. 6f). On the other hand, ribo-seq data revealed that TEs of most of the key components of C I, II, III and IV in the ETC were not affected by Mettl1 deletion (Fig. 6g), suggesting that the expression of ETC proteins by METTL1 is regulated at the transcriptional level. Sdhb in C I and Ndufa12 in C II were among the most downregulated genes in Mettl1-deleted B cells. We further confirmed the downregulation of SDHB and NDUFA12 at the protein level by western blotting (Fig. 6h). Transmission electronic microscopy studies of B cells and flow cytometry revealed that Mettl1 deletion led to reduced mitochondrial mass (Fig. 6i, Supplementary Fig. 14c, d). To determine whether METTL1 is required for optimal mitochondrial function, a Seahorse assay was used to assess mitochondrial respiration in B cells. Notably, Mettl1 deletion led to impaired mitochondrial respiration in B cells. The OCR was reduced at the basal level. The maximal respiration capacity and ATP-linked production were lower in Mettl1-deleted B cells. The spare respiratory capacity, which represents the parameter for the mitochondrial reserve, was also decreased in Mettl1-deleted B cells (Fig. 6j, k).
Fig. 6. METTL1 controls mitochondrial electron transport train (ETC) activity in B cells.
a–e scRNA-seq analysis of spleen B cells from NP-OVA-immunized mice. a Volcano plot displaying DEGs between WT B cells and Mettl1-deleted B cells (adjusted p value via “limma” R package). The purple dots represent DEGs in the ETC. b The top 10 KEGG terms upregulated in WT B cells (adjusted p value via “clusterProfiler” KEGG analysis). c Network plot displaying the top 10 GO-CC terms upregulated in WT B cells. d Box plot displaying the OXPHOS score among different B-cell clusters (n = 1 mouse in WT group and n = 2 mice in cKO group). The upper whisker is 75th percentile plus 1.5*IQR, and the lower whisker is 25th percentile minus 1.5*IQR. The upper and lower bounds of box are 75th and 25th percentile respectively. The center of box is the median. e The top 10 terms upregulated in the WT early GC B-cell cluster compared with the Mettl1-deleted early GC B-cell cluster (adjusted p value via “clusterProfiler” GO-BP analysis). f Heatmap displaying the qPCR results for genes in the ETC (n = 4 biological independent samples). g Scatterplot displaying the TEs of genes in the ETC via Ribo-seq data analysis. h SDHB and NDUFA12 expression in spleen B cells from WT and cKO mice measured by western blot. i Transmission electronic microscopy studies displaying the mitochondria of B cells from WT and cKO mice and representative images were shown. j, k Seahorse Mito Stress assay was used to measure the mitochondrial respiration of WT and Mettl1-deleted B cells. Curves for the oxygen consumption rate (OCR) are displayed in j, and the data are summarized in k (n = 5 biological independent samples). A two-tailed unpaired Student’s t-test was used in k. The data (mean ± SEM) are shown. ns, not significant. Oligo oligomycin, FCCP carbonyl cyanide-p-trifluoromethoxyphenylhydrazone, R + A: rotenone + antimycin. Source data are provided as a Source Data file.
We further confirmed the role of METTL1 in the ETC in human B cells. Mettl1 was knocked down in human B cells via shRNA. We found that knockdown of Mettl1 also led to impaired mitochondrial respiration in human B cells (Supplementary Fig. 14e, f). ETC couples oxidative OXPHOS with ATP synthase to drive the generation of ATP in mitochondria. Disruption of the ETC assembly leads to mitochondrial dysfunction41. These data indicate that METTL1 is important for mitochondrial ETC activity in B cells.
METTL1 controls mitochondrial ETC activity through BCR signaling
BCR stimulation is involved in increased mitochondrial OXPHOS in B cells42,43. Mettl1 deletion had no effect on the translation of ETC proteins, suggesting that METTL1 might control mitochondrial OXPHOS through BCR signaling. To study the regulation of the BCR signaling-ETC axis in B-cell responses, we performed a gain-of-function study to investigate the function of METTL1-mediated BCR signaling in B-cell responses in vitro and in vivo. We first generated Mettl1 conditional knock-in (cKI) mice by crossing Cd19Cre mice with Rosa26-CAG-LSL-Mettl1 mice to obtain Cd19CreMettl1cKI mice (Supplementary Fig. 15a). The overexpression of Mettl1 in B cells was confirmed by western blotting (Fig. 7a). As expected, overexpression of Mettl1 resulted in increased WDR4 expression in B cells (Fig. 7a). No autoreactive B-cell responses were observed in these Cd19CreMettl1cKI mice (Supplementary Fig. 15b–f). Our data above revealed that METTL1 controls the TEs of BCR signaling-related genes and ETC activities (Figs. 5 and 6). A previous study showed that energy for early events in B cells following antigen recognition by BCRs is provided primarily by OXPHOS44. We thus hypothesized that METTL1 promotes B-cell responses by enhancing BCR signaling-mediated mitochondrial ETC activity. We first assessed BCR signaling in Mettl1-KI B cells to evaluate the role of METTL1 in BCR signaling. Our data revealed that surface IgM-BCR levels were greater in Mettl1-KI B cells than in WT control cells (Supplementary Fig. 16a, b). Concomitantly, SYK phosphorylation was increased in Mettl1-KI B cells stimulated with the anti-IgM antibody (Fig. 7b–d). Notably, the phosphorylation level of BTK was increased in Mettl1-KI B cells. Additionally, the phosphorylation of both PI3K and AKT was increased in Mettl1-KI B cells, as measured by western blotting (Fig. 7d). These data further confirmed the function of METTL1 in controlling BCR signaling in B cells. Consistently, overexpression of Mettl1 in B cells led to increased mitochondrial ETC activity. Transmission electronic microscopy studies revealed increased mitochondrial mass in Mettl1-KI B cells, which was reversed by the SYK inhibitor R406 (Fig. 7e). The key components of the ETC complex were upregulated in Mettl1-KI B cells, as measured by qPCR, and were normalized to the control level by a SYK inhibitor (Fig. 7f). In line with the qPCR data, the protein expression of SDHB and NDUFA12 was notably increased in Mettl1-KI B cells, which could also be normalized to the level of the WT control by inhibiting BCR signaling via a SYK inhibitor (Fig. 7g). Furthermore, BCR stimulation by anti-IgM led to increased mitochondrial mass in Mettl1-KI B cells, as measured by flow cytometry, and this effect was reversed by the SYK inhibitor (Supplementary Fig. 16c, d). Functionally, overexpression of Mettl1 increased mitochondrial respiration in B cells, which was consistently normalized to the level in control cells by a SYK inhibitor (Supplementary Fig. 16e, f). The induction of GC B-cell differentiation was increased in Mettl1-KI B cells, which was counteracted by BCR inhibition (Supplementary Fig. 16g, h).
Fig. 7. METTL1 regulates mitochondrial ETC activity through BCR signaling.
a METTL1 and WDR4 expression in B cells from Mettl1-cKI mice or WT mice was measured via western blotting. Representative bands are shown. b, c Representative flow cytometry plots for p-SYK stimulated with anti-mouse IgM. The data are summarized in c (n = 4 biological independent samples). d The expression of p-PI3K, p-AKT, and p-BTK in B cells was measured by western blotting. Representative bands are shown. e Transmission electronic microscopy images showing the mitochondria of WT B cells, cKI B cells, and cKI B cells treated with R406. Representative images are shown. f The expression level of the ETC in WT B cells, cKI B cells, or cKI B cells treated with the SYK inhibitor R406 was measured by qPCR (n = 4 biological independent samples). g The expression of SDHB and NDUFA12 in WT B cells, cKI B cells, or cKI B cells treated with the SYK inhibitor R406 was measured via western blotting. Representative bands are shown. h Experimental scheme of NP-OVA immunization. i Frequencies of B cells in the spleens of WT or cKI mice (n = 6 biological independent samples). j, k Representative flow cytometry plots, and frequencies of GC B cells in WT and cKI mice (n = 6 biological independent samples). l–o OVA+ B cells (l, m) and OVA+ GC B cells (n, o) in WT and cKI mice were measured via flow cytometry (n = 6 biological independent samples). p, q Representative ELISpot wells and summary data for anti-NP25- and anti-NP2-specific B cells in the splenocytes of WT and cKI mice (n = 4 biological independent samples). r ELISA for anti-NP25 and anti-NP2 IgG antibodies in the sera of WT and cKI mice (n = 4 biological independent samples). The data are presented as the mean ± SEM. A two-tailed unpaired Student’s t test was used in c, i, k, m, o, q, r. Source data are provided as a Source Data file.
We then performed an in vivo study to further investigate the role of METTL1 in B-cell responses to immunization. WT or cKI mice were challenged with NP-OVA, and the mice were euthanized 14 d later (Fig. 7h). In line with the findings in cKO mice, the number of B cells in the spleen was not different between cKI mice and WT control mice (Fig. 7i). The percentage of GC B cells in the spleen was increased in cKI mice (Fig. 7j, k). We then analyzed antigen-specific B cells in cKI or WT mice. Notably, the frequencies of both OVA-specific total B cells and OVA-specific GC B cells were increased in the spleens of cKI mice (Fig. 7l–o). GC B cells rely on fatty acid oxidation to perform OXPHOS45, and elevated OXPHOS activity promotes B-cell clonal expansion and positive selection42. To investigate whether METTL1-controlled ETC activities and OXPHOS are involved in GC responses and antibody maturation, NP25-BSA and NP2-BSA were used to measure low-affinity IgG NP25 and high-affinity IgG NP2, respectively, by ELISpot and ELISA. ELISpot revealed that the numbers of both NP25-specific B cells and NP2-specific B cells were increased in B cells from cKI mice (Fig. 7p, q). Notably, the concentrations of both IgG NP25 and the high-affinity antibody IgG NP2 were increased in the serum of cKI mice, as measured by ELISA (Fig. 7r), suggesting that the affinity maturation efficiency was increased in cKI mice. These data further confirm that METTL1 controls GC B-cell responses through BCR signaling-mediated mitochondrial ETC activities.
CD19 overexpression in Mettl1-deleted B cells rescues ETC activity during B-cell differentiation
For further validation, we overexpressed CD19 in Mettl1-deleted B cells (Fig. 8a). Similarly, total SYK expression was similar between WT and Mettl1-deleted B cells. CD19 overexpression also had no effect on total SYK expression in Mettl1-deleted B cells (Fig. 8b, c). Strikingly, the reduction in phosphorylated SYK (p-SYK) in Mettl1-deleted B cells stimulated with anti-IgM was reversed after CD19 was overexpressed (Fig. 8d, e). We then investigated whether the rescue of BCR signaling is accompanied by improved ETC function. The qPCR data revealed that the overexpression of CD19 in Mettl1-deleted B cells increased the transcript levels of genes that encode the ETC (Fig. 8f), which was confirmed by western blotting (Fig. 8g). A Seahorse assay was used to assess the actual activities of the ETC in Mettl1-deleted B cells after the overexpression of CD19. Consistently, CD19 overexpression rescued impaired mitochondrial respiration in Mettl1-deleted B cells (Fig. 8h, i). Importantly, the differentiation of GC B cells from Mettl1-deleted B cells was notably reversed when CD19 expression in Mettl1-deleted B cells was rescued (Fig. 8j, k). These data further confirm that METTL1 specifically controls the translation of CD19 in B cells.
Fig. 8. CD19 overexpression rescues ETC activity in Mettl1-deleted B cells.
a Expression level of CD19 in WT B cells, Mettl1-deleted B cells, and Mettl1-deleted B cells overexpressing Cd19 were measured by western blot. Representative bands were shown. b, c Representative histograms and MFI data of SYK expression were summarized (n = 3 biological independent samples). d, e Representative histograms and MFI data of p-SYK expression were summarized (n = 3 biological independent samples). f Heatmap displaying qPCR data of ETC in WT B cells, Mettl1-deleted B cells, and Mettl1-deleted B cells overexpressing Cd19 (n = 4 biological independent samples). g Western blots displaying protein levels of ETC in WT B cells, Mettl1-deleted B cells and Mettl1-deleted B cells overexpressing Cd19. Representative bands were shown. h, i Seahorse assay measuring mitochondrial respiration of WT B cells, Mettl1-deleted B cells, and Mettl1-deleted B cells overexpressing Cd19 (n = 4 biological independent samples). j, k Representative flow plots and summarized data displaying GC differentiation of WT B cells, Mettl1-deleted B cells, and Mettl1-deleted B cells overexpressing Cd19 (n = 4 biological independent samples). Data are shown as mean ± SEM. One-way ANOVA was used in c, e, i, k. Source data are provided as a Source Data file.
METTL1 promotes autoreactive B-cell responses
The data above revealed the essential role of METTL1/WDR4-mediated m7G modification in early B-cell activation and the response of GCs to immunization. We further investigated the role of METTL1 under disease conditions. In NZM2328 lupus model mice, METTL1 expression was significantly increased in B cells from pre-diseased mice compared with those from disease-free mice. METTL1 expression was further increased in B cells from diseased mice (Fig. 9a–c). Furthermore, the expression of METTL1 was greater in naive B cells and GC B cells from pre-diseased and diseased mice than in those from diseased free mice and was not detected in the compartments of the MBC and PC (Fig. 9d, e; Supplementary Fig. 17a–c). To rule out the effect of aging on METTL1 expression, we also measured METTL1 expression in normal B6 mice at comparable ages. We did not observe increased METTL1 expression in aged mice (Supplementary Fig. 17d–f). The upregulation of METTL1 in naive B cells in pre-diseased NZM2328 mice points to an important role of METTL1 in initiating early B-cell responses and disease development. Importantly, METTL1 expression was closely correlated with the number of anti-dsDNA+ B cells in the spleen, suggesting a role for METTL1 in promoting the autoreactive B-cell response in NZM2328 mice (Fig. 9f, g).
Fig. 9. METTL1 promotes autoreactive B-cell responses.
a–g Experiments on 3-month (3 m), 6 m and 9 m NZM238 mice (n = 4 biological independent samples). a Experimental scheme. b, c METTL1 expression in B cells from 3-, 6- and 9-month-old NZM2328 mice was measured via flow cytometry. Representative histograms and MFI data summarized in c. d, e Representative flow cytometry plots and MFIs of METTL1 expression in naive and GC B cells from 3 m, 6 m, and 9 m NZM2328 mice. f Representative ELISpot wells of anti-dsDNA IgG-secreting B cells in the splenocytes of 3 m, 6 m, and 9 m NZM2328 mice. g Linear correlation analysis between METTL1 MFI and anti-dsDNA IgG-secreting B cells in the spleens of NZM2328 mice (a simple linear regression analysis was used). h–s Autoimmune mouse models induced by apoptotic thymocytes were generated in WT and cKO mice (n = 6 biological independent samples). h Experimental scheme of the autoimmune mouse model induced by apoptotic thymocytes. i Immunofluorescence staining of spleen GCs in WT and cKO mice: B220 (red), CD3 (blue), and PNA (white). j, k Representative flow cytometry plots, and frequencies of GL7+CD38− GC B cells in the spleens of WT and cKO mice. l, m Representative flow cytometry plots and frequencies of PBs and PCs in the spleens of WT and cKO mice. n Representative ELISpot wells and summarized data showing anti-dsDNA IgG-secreting B cells in splenocytes from WT and cKO mice. o ELISA of anti-dsDNA IgG antibodies in the serum. p–r Representative anti-mouse IgG and C3 staining, and PAS staining of kidney sections and summary scores. s Proteinuria score curve of mice. The data are presented as the mean ± SEM. A two-tailed unpaired Student’s t test was used in k, m–o, q–s, and one-way ANOVA was used in c, e, f. Source data are provided as a Source Data file. a and h were created with BioRender.com.
To further study the function of METTL1 in the development of autoimmune diseases, autoimmunity was induced in cKO or WT control mice via apoptotic autologous thymocytes as previously described46 (Fig. 9h). Strikingly, immunofluorescence staining revealed that the GC response was almost absent in the spleens of cKO mice (Fig. 9i). Moreover, the percentages of GC B cells and CD138+ PC/PB cells were reduced in the spleens of cKO mice (Fig. 9j–m). ELISpot assays revealed that the number of anti-dsDNA+ B cells was decreased in cKO mice (Fig. 9n). Additionally, the concentration of anti-dsDNA antibodies was significantly lower in the serum of cKO control mice than in that of WT control mice (Fig. 9o). Notably, the immune complex deposition, cellular proliferation and basement membrane thickening in the glomeruli were reduced when Mettl1 was deleted in B cells (Fig. 9p–r). Importantly, the development of proteinuria was notably inhibited in Mettl1-KO mice (Fig. 9s). The percentage of TFH cells was not affected by Mettl1 deletion (Supplementary Fig. 17g). These data suggest that METTL1 promotes an autoreactive GC B-cell response to drive the development of systemic autoimmunity.
METTL1 promotes B-cell responses in systemic autoimmunity
SLE represents a prototypical autoimmune disease featuring the expansion of autoreactive B cells and the production of autoantibodies47. BCR signaling is enhanced in B cells from patients with SEL48,49. To link METTL1 to altered BCR signaling in patients with systemic autoimmunity, we first accessed a public database of scRNA-seq data from patients with SLE (Fig. 10a). scRNA-seq analysis revealed that the transcripts of both Mettl1 and Wdr4 were upregulated in B cells from patients with SLE compared with those from HCs (Fig. 10b). Among the B-cell subsets, ASC cells expressed the highest levels of Mettl1 and Wdr4 in patients with SLE (Fig. 10c), suggesting a role for METTL1/WDR4 in systemic autoimmunity. To confirm the involvement of METTL1/WDR4 in B-cell dysregulation in patients with SLE, blood samples were collected from SLE patients or HCs, and the expression of METTL1/WDR4 in B cells was measured by flow cytometry or western blotting. In line with the scRNA-seq data, the flow cytometry data revealed that ASC cells presented the highest level of METTL1 expression (Fig. 10d–f). Notably, METTL1 expression was upregulated in B cells from patients with SLE and correlated with disease activity in SLE patients (Fig. 10g, h). The expression of METTL1 and WDR4 in B cells from patients with SLE was further measured by western blotting. Similarly, both METTL1 and WDR4 were notably upregulated in B cells from patients with SLE (Fig. 10i). As a result, the level of m7G modification was also increased in B cells from patients with SLE (Fig. 10j, Supplementary Fig. 18). In accordance with the data from the mice, increased METTL1/WDR4-mediated m7G modification led to enhanced BCR signaling in B cells from patients with SLE, which could be inhibited and normalized to the control level when Mettl1 was knocked down in B cells by lentivirus (Fig. 10k). Similarly, compared with HCs, SLE B cells presented increased ETC activity. The expression of ETC components decreased when Mettl1 expression was knocked down in SLE B cells (Fig. 10l, m). To gain more insight into mitochondrial ETC activity in SLE B cells, mitochondrial respiration was measured by the Seahorse assay. The data revealed that SLE B cells presented elevated mitochondrial respiration and that knockdown of Mettl1 normalized mitochondrial respiration in SLE B cells (Fig. 10n, o). Importantly, the expansion of ASC cells in patients with SLE was counteracted by knocking down Mettl1 in SLE B cells (Fig. 10p–r). These data suggest that METTL1 promotes the dysregulation of autoreactive B cells through BCR signaling in human systemic autoimmunity.
Fig. 10. METTL1 promotes B-cell responses in systemic autoimmunity.
a–c scRNA-seq data analysis of B-cell subclusters from systemic lupus erythematosus (SLE) patients and HCs. a UMAP displaying B-cell clustering. b Dot plot displaying Mettl1 and Wdr4 expression in SLE patients and HCs. c Dot plot displaying Mettl1 and Wdr4 expression in B-cell clusters. d Gating strategy for B-cell subsets in PBMCs. e, f Representative flow cytometry plots and MFIs of METTL1 expression among SLE B-cell subsets gated on d (n = 12 biological independent samples). g MFIs of METTL1 expression in PBMC B cells (SLE = 12 biological independent samples, HCs = 10 biological independent samples). h Correlation between MFI of METTL1 expression in PBMC B cells and disease activity (SLEDAI) in SLE patients (simple linear regression analysis was used). i METTL1 and WDR4 expression in B cells from HCs and SLE patients was measured by western blotting. Representative bands were shown. j m7G levels in B cells from HCs and SLE patients were quantified by northern blotting. Representative bands were shown. k–r SLE B cells were transfected with shMettl1 or vector. k The phosphorylation of PI3K and AKT in HC B cells, SLE B cells, and SLE B cells treated with shMettl1 was measured by western blotting. Representative bands were shown. l Heatmap displaying the results of qPCR analysis of the ETC genes in HC B cells, SLE B cells, and SLE B cells treated with shMettl1 (n = 3 biological independent samples). m SDHB and NDUFA12 expression in HC B cells, SLE B cells, and SLE B cells treated with shMettl1 were measured by western blotting. Representative bands were shown. n, o Seahorse assay measuring mitochondrial respiration in HC B cells, SLE B cells, and SLE B cells treated with shMettl1 (n = 4 biological independent samples). The OCRs are displayed in n and summarized in o. p Representative flow cytometry plots of CD27hiCD38hi ASCs in HCs and SLE patients. q, r Representative flow cytometry plots and frequencies of ASC differentiation from SLE B cells (n = 8 biological independent samples). The data are presented as the mean ± SEM. A two-tailed unpaired Student’s t test in g and a two-tailed paired Student’s t test in r, paired one-way ANOVA in f, and one-way ANOVA in o. Source data are provided as a Source Data file.
Discussion
The GC response is essentially important for the generation of high-affinity antibodies. The formation of GCs is driven by activated naive B cells, which exit their naive state upon antigen recognition8,29. This complicated process involves early B-cell activation and GC B-cell responses, which require efficient protein translation and bioenergy. In the present work, we show that METTL1 is essential for fulfilling the rapid requirements of protein synthesis for effective B-cell responses. At the epigenetic translation level, we identify METTL1 as an important checkpoint in controlling early B-cell activation and GC responses, allowing selection and antigen-specific GC B-cell expansion. Pathologically, METTL1/WDR4-mediated tRNA m7G modification promotes autoreactive B-cell responses and drives the development of systemic autoimmunity in both mice and humans. Specifically, codon-dependent translation control by METTL1/WDR4-mediated tRNA m7G modification enables B cells to increase the translation of proteins involved in BCR signaling. Overall, this work highlights the importance of translational control in early B-cell responses and that targeting METTL1 could normalize autoreactive B-cell responses in systemic autoimmunity.
B-cell activation involves antigen recognition and T-cell costimulation2. BCR stimulation leads to upregulation of the translational machinery50. Here, we show that translation and ribosome biogenesis are notably upregulated in human and mouse B cells in response to immunization. m7G modification represents the most prevalent type of tRNA modification and actively participates in biological and pathological functions by controlling cellular TEs. METTL1/WDR4 functions as the dominant enzyme of tRNA m7G modification12. In line with previous studies in stem cells and cancers21,24, our data suggest that METTL1 is required for m7G modification in B cells. Moreover, METTL1/WDR4-mediated tRNA m7G modification is essential for maintaining TEs for highly demanding proteins during B-cell responses. Early B-cell activation is initiated by the ligation of BCRs with antigens captured by FDCs in the follicle. Upon activation, FO B cells undergo serial critical and complicated changes. First, antigen-experienced FO B cells migrate to the T:B border to receive costimulatory signals from TFH cells and then enter GCs to initiate GC responses2,29. Recently, data have shown that tRNA modification enables sufficient protein synthesis for early T-cell activation17. In accordance with data from T cells, our scRNA-seq data reveals that Mettl1 deletion leads to impaired formation of GCs, leading to the accumulation of GC progenitors in Mettl1-deficient mice. In addition, both the formation and high-affinity IgG production are reduced by deleting Mettl1, highlighting the importance of METTL1 in GC responses.
CD19 is a B-cell–specific coreceptor that is essential for normal antibody responses51. Altered CD19 signaling results in early GC B-cell differentiation52. CD19-/- mice are deficient in GC formation and antibody production, with no significant effect on the number of B-cell precursors in the bone marrow53. In humans, mutation of the Cd19 gene causes a defective response to antigenic stimulation of mature B cells and hypogammaglobinemia54. Here, we reveal that CD19 expression in B cells is controlled by tRNA m7G modification at the translational level. The protein level of CD19 is decreased by Mettl1 deletion without affecting the Cd19 mRNA level, indicating epigenetic translational control of CD19 by METTL1. CD19 functions as a coreceptor to enhance BCR signaling through the PI3K-AKT pathway39,51. Accordingly, decreased CD19 expression caused by Mettl1 deletion impairs BCR signaling. In addition, our Ribo-seq data reveals a broader regulatory effect of METTL1 on B cells in which the TEs of the BCR signaling pathway are downregulated in Mettl1-deleted B cells. It has been shown that increased BCR signaling strength reduces the number of MZ B cells55,56. Consistent with these reports, we reveal that Mettl1 deletion impairs BCR signaling and favors MZ B-cell development. Together, METTL1/WDR4-mediated tRNA m7G modification causes biased preferential translation of BCR signaling, which is required for early B-cell activation and GC B-cell responses.
Upon antigen ligation, B cells upregulate the phosphorylation of the downstream protein BTK through SYK57. Here, we show that tRNA m7G modification leads to enhanced BCR signaling in B cells and SYK, BTK phosphorylation. A previous study revealed that the overexpression of BTK in B cells causes spontaneous formation of GCs and increased PC numbers, leading to antinuclear autoantibody production and systemic lupus SLE-like autoimmune pathology58. Consistently, our data reveals that deletion of Mettl1 in B cells abolishes the induction of autoimmunity. The formation of autoreactive B cells and the production of autoantibodies are notably reduced. In addition, our data show that BCR signaling is important for mitochondrial ETC activity and that overexpression of Mettl1 enhances mitochondrial ETC activity. These data lead us to propose that METTL1/WDR4-mediated tRNA modification promotes autoreactive GC B-cell responses by enhancing BCR signaling-controlled mitochondrial ETC activities, which is in line with our previous finding that autoreactive B cells in SLE exhibit increased fatty acid oxidation and mitochondrial respiration in GC B cells46. Recently, data have shown that mitochondrial translation in B cells is important for the entry of activated GC precursors into the GC reaction30. Our Ribo-seq data reveals that the translation of the ETC in mitochondria is not affected by Mettl1 deletion, implying that the ETC is downstream of BCR signaling rather than the direct target of METTL1/WDR4-mediated tRNA m7G modification.
Our study reveals that METTL1 is required for effective B-cell responses. Furthermore, this study reveals a novel mechanism by which tRNA m7G modification by METTL1 allows essential protein translation via the BCR signaling pathway to promote early B-cell activation and GC entry (Supplementary Fig. 19). Systemic autoimmunity is characterized by overactivation of autoreactive B cells and the production of autoantibodies. B-cell depletion therapy has shown promising potential in the treatment of lupus. Rituximab, a monoclonal antibody targeting CD20, has been extensively studied and has demonstrated significant efficacy in reducing disease activity in lupus patients, particularly in those with refractory or severe disease59,60. Furthermore, more recent agents like belimumab, a monoclonal antibody that inhibits B-cell activating factor, show sustained improvements in disease outcomes and have been approved for the treatment of SLE61. Ongoing research into novel B-cell-targeted therapies such as new molecules and pathways, continues to advance the field, offering hope for more effective and personalized treatment options for lupus patients in the near future. Given the importance of translational control in B-cell activation, our study identified a key molecule in controlling autoreactive B-cell responses. METTL1 could serve as a therapeutic target to treat patients with systemic autoimmunity.
Methods
Mice
Mettl1flox/flox mice were generated as previously described23 and were crossed with Cd19Cre mice (#T003785, GemPharmatech, Nanjing, China) to generate Mettl1cKO mice. CD19 conditional knock-in (Cd19CreMettl1cKI) mice were generated by crossing Cd19Cre mice with Rosa26-CAG-LSL-Mettl1 mice23. Co-housed Cd19CreMettl1+/+ mice served as controls. All experimental mice were bred and maintained in specific pathogen-free conditions. The mice were housed under a 12-hour (h) light/12-h dark cycle at ~18–23 °C with 40–60% air humidity. Eight to 10-week-old mice of both sexes were used for the experiments. 9-month-old Cd19CreMettl1cKI and control mice were used for autoreactive response analysis. Female NZM2328 mice were generously provided by Professor Shu Man Fu, University of Virginia46. Wild-type (WT) C57BL/6 mice were obtained from the Model Organisms Center (#SM-001, Shanghai, China). All animal experiments were approved by the Ethics Committee of Sun Yat-sen University.
Human samples
For experiments on vaccination, peripheral blood samples were collected from healthy individuals before and after vaccination (influenza vaccine or inactivated SARS-CoV-2 vaccine). The baseline characteristics are listed in Supplementary Table 1. In addition, peripheral blood samples were collected from patients who fulfilled the American College of Rheumatology 1997 criteria for SLE62 at the First Affiliated Hospital, Sun Yat-sen University. The exclusion criteria were as follows: acute and chronic infections; malignancy; and pregnancy or lactation in females. Age- and sex-matched healthy controls (HC) were recruited accordingly. Patient demographics are summarized in Supplementary Table 2. Human spleen samples were collected as we described previously46. Informed consent and written consent forms were obtained prior to inclusion from all participants, with approval from the Institutional Ethical Committee of the First Affiliated Hospital, Sun Yat-sen University.
Cell lines
293 T cells were purchased from the American Type Culture Collection (ATCC). 293 T cells were cultured in DMEM containing 10% fetal bovine serum (FBS), 1% Penicillin, and 1% streptomycin in a water-saturated atmosphere under 5% CO2 at 37 °C in an incubator (Thermo Scientific, USA).
Mouse immunization
Age- and sex-matched Cd19CreMettl1flox/flox and Cd19CreMettl1+/+ mice aged 8-10 weeks were immunized with NP-OVA (100 µg/mouse, LGC, Cat#: N-5051-10), OVA (100 µg/mouse, Sigma-Aldrich, Cat#: A5503) or NP-Ficoll (100 µg/mouse, Biosearch, Cat#: F-1420-10) mixed with an equal volume of alum adjuvant (Sigma-Aldrich, Cat#:77161) intraperitoneally. Age- and sex-matched 8–10-week-old Cd19CreMettl1cKI and Cd19CreMettl1+/+ mice were immunized with NP-OVA (100 µg/mouse, LGC, Cat#: N-5051-10). Blood samples were collected on day (d) 7 after immunization, and the B-cell response to immunization in the spleen was analyzed 2 weeks after immunization. To measure the recall of MBs, the mice were immunized with OVA repetitively every 2 weeks 3 times, and B-cell responses in the spleen were analyzed. Mice were euthanatized using excessive 1% pentobarbital sodium anesthesia at the endpoint.
Autoimmune mouse model
An autoimmune mouse model was induced as described previously46. Briefly, thymocytes isolated from WT C57BL/6 J mice were irradiated with -irradiation (600 rads) for 1 h and cultured in a complete medium at 37 °C with 5% CO2 for 3 h. The apoptosis rate was confirmed by Annexin V and 7-aminoactinomycin D (7-AAD) staining (>95%). Age- and sex-matched Cd19CreMettl1flox/flox and Cd19CreMettl1+/+ female 8-10-week-old mice were injected with apoptotic thymocytes (1107 cells/mouse/week, for 4 weeks in total) intravenously. Mice were euthanatized using excessive 1% pentobarbital sodium anesthesia at the endpoint.
B-cell isolation
For mouse B-cell isolation, mouse spleens were first smashed into single-cell suspensions, and red blood cells were removed by red blood cell lysis buffer, after which B cells were isolated with an EasySep Mouse B-Cell Isolation Kit (STEMCELL Technologies, Cat#: 19854) from splenocytes. For mouse naive B-cell isolation, purified B cells were subsequently stained with FITC-conjugated anti-mouse CD23 and isolated with an EasySep mouse FITC-Positive Selection Kit (STEMCELL Technologies, Cat#: 17668). For human B-cell isolation, peripheral blood mononuclear cells (PBMC) were freshly isolated by density gradient centrifugation and then isolated with an EasySep Human B-Cell Isolation Kit (STEMCELL Technologies, Cat#: 19054).
B-cell culture
For mouse B-cell activation, the cells were cultured in RPMI 1640 containing 10% FBS and 1% penicillin‒streptomycin (complete medium) in the presence of anti-mouse CD40 antibodies (5 µg/ml, BioLegend, Cat#: 102802), anti-mouse IgM (10 µg/ml, Jackson ImmunoRsearch, Cat#: 115-001-020) and IL-2 (20 ng/ml, Sino Biological, Cat#: 510661-MNAE) for 48 h. GC B-cell differentiation was induced as described previously34,63. The cells were cultured in a complete medium in the presence of anti-mouse CD40 antibodies (5 µg/ml), anti-mouse IgM (10 µg/ml), IL-4 (20 ng/ml, PeproTech, Cat#: 214-14-5), and IL-2 (20 ng/ml) for 4 d. For PC differentiation, the cells were cultured in a complete medium supplemented with anti-mouse CD40 antibodies (5 µg/ml), anti-mouse IgM (10 µg/ml), IL-21 (50 ng/ml, Sino Biological, Cat#: 50137-MNAE) and IL-2 (20 ng/ml) for 7 d. SYK inhibitors (R406, Selleck, Cat#: S2194) were added for specific experiments as indicated. The cells were cultured in a humidified atmosphere at 37 °C with 5% CO2. For human B-cell differentiation, the cells were cultured in complete medium supplemented with CD40L (5 µg/ml, Sino Biological, Cat#: 10239-H01H), anti-human IgM (5 µg/ml, Sigma-Aldrich, Cat#: I0759), IL-21 (50 ng/ml, Sino Biological, Cat#: GMP-10584-HNAE-20) and IL-2 (20 ng/ml, PeproTech, Cat#: 200-02-10) for 7 d.
Public single-cell RNA sequencing (scRNA-seq) data acquisition
Public scRNA-seq data (GSE211560, GSE201534, GSE189819, and GSE163121) were acquired from the GEO database (http://www.ncbi.nlm.nih.gov/geo/).
Tissue dissociation and preparation of single-cell suspensions and sample tag labeling for scRNA-seq
The spleens were cut into small pieces and smashed gently to obtain single-cell suspensions. After B-cell isolation, the single-cell suspensions were subjected to centrifugation for 5 min (4 °C, 500 × g). The supernatant was discarded, and 200 µL of cold sample buffer (BD Biosciences) was added to the cell mixture. After careful and gentle pipetting, the cell suspension for single-cell experiments was prepared. The samples were labeled with sample tags (BD Biosciences, Cat#: 633793).
Cell capture, scRNA library preparation, and sequencing
For cell capture and library preparation for scRNA-seq, a BD Rhapsody system (BD Biosciences) was used on the basis of the manufacturer’s protocols. In brief, 1 µL of calcein AM (2 mM; Thermo Fisher, Cat#: C1430) and 1 µL of DRAQ7 (0.3 mM; Thermo Fisher, Cat#: 564904) were added to a 200 µL cell suspension (at a 1:200 dilution). Then, the mixture was pipetted gently and incubated at 37 °C in the dark for 5 min. Subsequently, the cell viability and concentration of the suspension were measured with a hemocytometer (BD Bioscience, Cat#: 633703). The single-cell suspension was then loaded onto a BD Rhapsody cartridge (BD Bioscience, Cat#: 400000847). Thereafter, the cell capture beads were loaded into the microwells and thoroughly washed to ensure that a single magnetic bead bonded with only one cell in each microwell. The lysis mixture was subsequently added and incubated at room temperature (RT) for 2 min. The cell capture beads were subsequently retrieved for subsequent steps, including complementary DNA synthesis, exonuclease I digestion, and multiplex PCR-based library construction. The sequencing libraries were prepared via whole-transcriptome analysis index PCR, and the PCR products were purified to enrich the 3’ ends of the transcripts, which were linked with the cell label and molecular indices. Finally, quality checks of the indexed libraries were performed with a Qubit fluorometer by the Qubit dsDNA HS Assay. The sequencing of the libraries was conducted on an Illumina NovaSeq 6000 system.
scRNA-seq data preprocessing
The raw sequencing reads were trimmed to keep the first 75 bases. The trimmed reads were then quality filtered via fastp. The BD Rhapsody whole-transcriptome official analysis pipeline was applied under the default settings to obtain a cell–gene expression matrix and a quality control report for each sample. The mouse reference genome was used for read alignment.
scRNA-seq data analysis
The R package “Seurat” (version: 4.4.0) was used to convert the scRNA-seq data to Seurat objects. First, we performed quality control of the scRNA-seq data by removing cells with fewer than 200 genes, more than 5000 genes, or more than 20% mitochondrial genes. After data filtering, the data were normalized, and cells expressing high levels of Cd3e, Cd4, and Cst3 were excluded from the matrix. The top 2,000 highly variable genes across cells were subjected to principal component analysis (PCA). For cell clustering, in the scRNA-seq data of the mice that received NP-OVA, the top 35 significant principal components (PC) were used for t-SNE clustering, and clusters containing fewer than 50 cells were removed. In the public scRNA-seq data, the top 20 significant PCs were used for t-SNE clustering. DEGs among different clusters were defined by a fold change >0.25 and adjusted P < 0.05. DEGs between WT and KO were defined by a fold change >0.10 and P < 0.05. GO and KEGG analyses were performed via the R package “clusterProfiler” (version: 4.0.2). GSEA was performed to assess related pathways and molecular mechanisms between the 2 groups via “GSEA” function in “clusterProfiler”. The pseudotime trajectories were generated with the “Monocle2” package (version: 2.20.0).
Western blot
B cells from PBMCs or splenocytes were lysed with RIPA lysis buffer, and the concentrations were detected with a BCA protein assay kit (Thermo Fisher, Cat#: 23227). Proteins were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS‒PAGE) gels and then transferred to PVDF membranes. The membranes were blocked with 5% bovine serum albumin (BSA) in TBST buffer for 1 h and incubated with primary antibodies against METTL1 (1:1000, Abcam, Cat#: ab271063), WDR4 (1:1000, Novus Biological, Cat#: 15902), SDHB (1:1000, Abcam, Cat#: ab175225), NDUFA12 (1:1000, Abcam, Cat#: ab192617), SYK (1:1000, Cell Signaling Technology, Cat#: 2717), p-AKT (1:1000, Cell Signaling Technology, Cat#: 4060), AKT (1:1000, Cell Signaling Technology, Cat#: 4691 T), p-PI3K (1:3000, Cell Signaling Technology, Cat#: 17366 or Immunoway, Cat#: YP0224), PI3K (1:1000, Cell Signaling Technology, Cat#: 4257 T), p-BTK (1:3000, Cell Signaling Technology, Cat#: 87141), BTK (1:1000, Cell Signaling Technology, Cat#: 8547 T), and CD19 (1:1000, Cell Signaling Technology, Cat#: 3574S) at 4 °C overnight. Anti-β-actin (1:5000, Servicebio, Cat#: GB15003) or anti-GAPDH (1:5000, Cell Signaling Technology, Cat#: 2118) primary antibodies were used as internal controls. The membranes were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit (1:2000, Cell Signaling Technology, Cat#: 7074) or anti-mouse (1:2000, Cell Signaling Technology, Cat#: 7076) secondary antibodies. Protein expression was detected by enhanced chemiluminescence (Cytiva, Cat#: AI800).
Flow cytometry
For cell surface staining, the cells were stained with the following antibodies at RT for 25 min: APC-conjugated anti-mouse B220 (1:100, Cat#103212, BioLegend), FITC-conjugated anti-mouse CD19 (1:100, Cat#152404, BioLegend), Pacific Blue-conjugated anti-mouse CD38 (1:100, Cat#102720, BioLegend), PE-Cy7-conjugated anti-mouse GL7 (1:100, Cat#144620, BioLegend), Percp-conjugated anti-mouse IgD (1:100, Cat#405736, BioLegend), APC-Cy7-conjugated anti-mouse CD138 (1:100, Cat#142530, BioLegend), Pacific Blue-conjugated anti-mouse CD21 (1:100, Cat#123414, BioLegend), APC-Cy7-conjugated anti-mouse CD23 (1:100, Cat#101630, BioLegend), AF647-conjugated anti-mouse IgM (1:100, Cat#406526, BioLegend), AF488-conjugated IgG1 (1:100, Cat#406626, BioLegend), APC-conjugated anti-mouse CXCR5 (1:100, Cat#145506, BioLegend), Percp/cy5.5-conjugated anti-mouse PD-1 (1:100, Cat#135208, BioLegend), PE-conjugated anti-mouse CD3 (1:100, Cat#100205, BioLegend), and PB-conjugated anti-mouse CD8 (1:100, Cat#100725, BioLegend). For antigen-specific mouse B-cell staining, the cells were stained with AF555-conjugated OVA (1:2000, Thermo Fisher, Cat#: O34782), AF647-conjugated OVA (1:2000, Thermo Fisher, Cat#: O34785) or NP-PE (1:100, Biosearch, Cat#: N-5070-1) at 4 °C for 25 min.
For surface staining of human cells, the cells were stained with the following antibodies at RT for 25 min: Zombie Red, PE-conjugated anti-human CD19 (1:100, Cat#363004, BioLegend), BV421-conjugated anti-human CD138 (1:100, Cat#356516, BioLegend), FITC-conjugated anti-human IgD (1:100, Cat#348206, BioLegend), AF700-conjugated anti-human CD27 (1:100, Cat#356416, BioLegend), BV650-conjugated anti-human CD38 (1:100, Cat#356620, BioLegend), PE/Dazzle594-conjugated anti-human CD24 (1:100, Cat#311134, BioLegend), and BV605-conjugated anti-human IgM (1:100, Cat#314524, BioLegend). For spike-specific B-cell staining, the biotinylated spike protein was tetramerized by the addition of either BV510-conjugated streptavidin (Cat#405233, BioLegend) or BV785-conjugated streptavidin (Cat#405249, BioLegend) at a protein:streptavidin molar ratio of 6:1 at 4 °C for 1 h to make 2 tetramers: [spike]4-BV510 and [spike]4-BV785. The cells were stained with tetramers at 4 °C for 30 min64.
For intracellular staining, the cells were permeabilized and fixed with the eBioscience™ Foxp3/Transcription Factor Staining Buffer Set (Invitrogen, Cat#: 00-5523-00) at 4 °C for 30 min, followed by incubation with a primary antibody against METTL1 (1:500, Abcam, Cat#: ab271063) at 4 °C for 30 min and incubation with AF488-conjugated anti-rabbit secondary antibodies (1:500, Thermo Fisher, Cat#: A-11008) at 4 °C for 30 min. For BCR signaling pathway staining, the cells were first stimulated with anti-mouse IgM (10 µg/ml) for the indicated time points. After surface staining, the cells were permeabilized and fixed with a Cytofix/Cytoperm Fixation and Permeabilization Kit (BD Biosciences, Cat#: 554722) and stained with primary antibodies against SYK (1:400, Cell Signaling Technology, Cat#: 13198), p-SYK (1:400, Cell Signaling Technology, Cat#: 2717), and p-AKT (1:400, Cell Signaling Technology, Cat#: 4060), followed by an AF488-conjugated anti-rabbit secondary antibody (1:500). All the antibodies used for flow cytometry are listed in Supplementary Data 1.
Serum H1N1, H3N2, and Victoria antibody titer measurements
The antibody titers against H1N1, H3N2, or Victoria was measured by a hemagglutination inhibition (HAI) assay as described previously65.
Ex vivo ELISpot
Mouse IgG secretion ELISpot was performed as we described previously46. ELISpot plates (Mabtech, Cat#: 3654-TP-10) were precoated with calf thymus DNA (100 µg/ml, MCE, Cat#: HY-109517), OVA (10 µg/ml, Sigma‒Aldrich, Cat#: A5503), NP25-BSA (5 µg/ml, Biosearch, Cat#: N-5050XL-10) or NP2-BSA (5 µg/ml, Biosearch, Cat#: N-5050H-10) at 4 °C overnight. After being blocked with RPMI 1640 containing 10% FBS at RT for 1 h, 5105 splenocytes/well were plated on ELISpot plates in the presence of R848 (1 µg/ml, Tocris, Cat#: 4536/10) and IL-2 (20 ng/ml, Sino Biological, Cat#: 51061-MNAE). The plates were incubated at 37 °C with 5% CO2 for 20 h, followed by incubation with anti-mouse IgG-HRP (1:2000, Huabio) at RT for 2 h. For human spike/RBD-specific IgG-secreting B cells, ELISpot plates were precoated with recombinant protein spike (50 µg/ml, Sino Biological, Cat#: 40589-V08B1) or spike RBD (50 µg/ml, Sino Biological, Cat#: 40592-VNAH) at 4 °C overnight. After blockage, 5 × 105 PBMCs (prestimulated with R848 and IL-2 for 72 h) were plated on ELISpot plates in the presence of R848 (1 µg/ml, Tocris, Cat#: 4536/10) or IL-2 (20 ng/ml, PeproTech, Cat#: 200-02-10). The plates were incubated at 37 °C with 5% CO2 for 20 h, followed by incubation with biotin anti-human IgG (1:1000, MabTech, Cat#: 3850-6-250) at RT for 2 h and HRP-avidin (1:1000, vector, Cat#: A-2004-5) at RT for 1 h. An AEC coloring system (DAKEWE, Cat#: 2030613) was used to detect antigen-specific IgG-secreting B cells. Spots were captured and counted by an ImmunoSpot Analyzer.
Immunofluorescence staining and imaging
Spleen paraffin blocks were cut into 4 μm sections. The slides were deparaffinized in xylene and then rehydrated with a graded alcohol series. Antigen retrieval was performed by a microwave with citrate-antigen retrieval buffer for 15 min. Slides were then washed with distilled water for 1 min and TBST for 2 min, followed by blocking with 10% normal goat serum at RT for 30 min and then incubation with the following primary antibodies: anti-mouse B220 (1:200, Santa Cruz, Cat#: sc-19597), PNA (1:500, Vector, Cat#: B-1075-5) and anti-mouse CD3 (1:100, Abcam, Cat#: ab5690) or anti-mouse METTL1 (1:1000) at 4 °C overnight. After 5 washes with TBST, the slides were incubated with the following secondary antibodies: AF488-conjugated anti-rabbit secondary antibody (1:500, Thermo Fisher, Cat#: A-11008), CY5-conjugated anti-rat secondary antibody (1:200, Jackson ImmunoResearch, Cat#: 712-175-153) and DyLight 594-conjugated streptavidin (1:200, Vector, Cat#: SA-5594-1). The stained slides were scanned by a fluorescence microscope (Olympus).
Kidney tissues were embedded in optimal cutting temperature (OCT) compound (SAKURA, Cat#: 4583) and frozen in liquid nitrogen. The tissue blocks were sectioned into 5 μm slides, followed by fixation in precooled acetone for 10 min. After 10 min of washing with PBST and 3 washes with PBS, the slides were incubated with 10% normal goat serum at RT for 1 h, followed by staining with AF488-conjugated anti-mouse IgG (1:500, Thermo Fisher, Cat#: A10680) or a FITC-conjugated anti-mouse complement component 3 (C3) antibody (1:150, Thermo Fisher, Cat#: PA1-29718) at 4 °C overnight. The slides were washed and stained with DAPI. The fluorescence signals were examined by a fluorescence microscope (Olympus), and the mean intensity of fluorescence was scored from 0-4 in a blinded manner as we described previously66.
RNA isolation and quantitative real-time PCR (qPCR)
RNA was extracted from cells by an RNA Quick Purification Kit (ESscience) or TRIzol. cDNA was generated by Evo M-MLV RT Master Mix (AG), and the amplification of target genes was accomplished by SYBR Green Pro Taq HS (AG) kits. The amplification protocol was as follows: denaturation for 30 s at 95 °C, followed by 40 cycles of denaturation for 5 s at 95 °C and annealing/extension for 30 s at 60 °C. The data are expressed as the expression relative to that of GAPDH. The sequences of primers used in this study are listed in Supplementary Table 3.
Northwestern blot
Northwestern blotting was conducted as previously described21. Briefly, 2 µg of total RNA was heat denatured at 70 °C for 5 min and loaded on a 15% polyacrylamide Tris-borate-EDTA (TBE) urea gel to separate the RNA. The RNAs were subsequently transferred onto a positively charged nylon membrane and crosslinked with a UVP crosslinker (Analytik Jena, USA) for 3 min on each side. RNAs with m7G modification were detected by western blotting with an anti-m7G antibody (MBL International, Cat#: RN017M).
TAE agarose gel electrophoresis
Two micrograms of total RNA were mixed with 6× DNA loading dye (Biosharp) and loaded onto a 2% agarose gel prepared with TAE buffer. Electrophoresis was performed at 140 V for 35 minutes to separate the RNA molecules on the basis of their size. After the run, the RNA bands were visualized under UV light.
Ribosomal-nascent chain (RNC) extraction
RNC extraction was performed as previously described67. Sorted B cells were cultured with anti-CD40 (5 µg/ml), anti-mouse IgG (10 µg/ml) and IL-2 (20 ng/ml) for 48 h. After pretreatment with 100 µg/ml cycloheximide (MedChemExpress, Cat#: HY-12320) for 15 min, the cells were washed with prechilled PBS and treated with 2 ml of cell lysis buffer (1% Triton X-100 in ribosome buffer (RB buffer) [20 mM HEPES-KOH (pH 7.4), 15 mM MgCl2, 200 mM KCl, 100 µg/ml cycloheximide and 2 mM dithiothreitol]). After 30 min of lysis on ice, the cell lysates were transferred into prechilled Eppendorf tubes for centrifugation at 16,200 × g at 4 °C for 10 min. Then, 10% of the supernatants were separated as input samples for subsequent qPCR, and the remaining samples were transferred to the surface of 30% sucrose buffer (30% sucrose in RB buffer). After 5 h of ultracentrifugation at 18,250 × g and 4 °C, the RNCs were extracted for subsequent qPCR.
RNA sequencing and data processing
Total RNA from the sorted B cells was isolated and purified via the TRIzol reagent. The RNA amount and purity were quantified, and the RNA integrity was assessed via a Bioanalyzer 2100 (Agilent) with an RNA integrity number (RIN) > 7.0 and confirmed by electrophoresis on a denaturing agarose gel. The RNA fragments were reverse transcribed into cDNA with SuperScript™ II Reverse Transcriptase (Invitrogen, cat. #1896649). After library construction, the average insert size for the final cDNA library was 300 ± 50 bp. Finally, we performed 2×150 bp paired-end sequencing (PE150) on an Illumina NovaSeq™ 6000 (LC-Bio Technology Co., Ltd., Hangzhou, China) following the vendor’s recommended protocol. DEGs were analyzed by Limma package (v3.54.2) and defined as p < 0.05 and |log2Foldchange | >0.25. Pathway analysis was performed using ClusterProfiler package (v4.6.2).
Proteomics
The sorted B cells were centrifuged at 500 × g for 5 min. After that, 50 µl lysis buffer (8 M urea, 1% protease inhibitor cocktail) was added to the cell sediment, followed by sonication three times on ice using a high-intensity ultrasonic processor (Scientz). The remaining debris was removed by centrifugation at 12,000 × g at 4 °C for 10 min. Finally, the supernatant was collected, and the protein concentration was determined with BCA kit according to the manufacturer’s instructions. For digestion, the protein solution was reduced with 5 mM dithiothreitol for 30 min at 56 °C and alkylated with 11 mM iodoacetamide for 15 min at RT in darkness. The protein sample was then diluted by adding 100 mM TEAB to urea concentration less than 2 M. Finally, trypsin was added at 1:50 trypsin-to-protein mass ratio for the first digestion overnight and 1:100 trypsin-to-protein mass ratio for a second 4 h-digestion. Finally, the peptides were desalted by C18 SPE column. The tryptic peptides were dissolved in solvent A (0.1% formic acid, 2% acetonitrile/in water) and loaded to Mass Spectrometer (ThermoFisher Orbitrap MS) for MS analysis after drying. The resulting MS/MS data were processed using ProteinDiscovery search engine (v2.5). Differential expressed proteins (DEP) were analyzed by Limma package (v3.54.2) and defined as p < 0.01 and |log2Foldchange|>0.25. Pathway analysis was performed using ClusterProfiler package (v4.6.2).
Ribosome profiling sequencing (Ribo-seq)
The ribosomal profiling technique was carried out as previously reported68 with minor modifications, as described below. Isolated B cells were incubated with a cell culture medium containing cycloheximide (100 µg/ml) for 2 min to halt translation. The cell extracts were treated with RNase H and DNase I and subsequently subjected to selection via size exclusion columns (Illustra MicroSpin S-400 HR Columns, GE Healthcare, Cat#: 27-5140-01). An RNA Clean and Concentrator-25 kit (ZYMO Research, Cat#: R1017) was used for ribosome footprint fragment isolation, and magnetic beads (Vazyme) were used for purification. Next, Ribo-seq libraries were constructed via the NEBNext Multiple Small RNA Library Prep Set for Illumina (New England Biolabs, Cat#: E7300L), followed by reverse transcription and PCR amplification. The 140–160 bp PCR products were enriched to generate a cDNA library and sequenced via the Illumina Nova6000 platform.
Ribo-seq data analysis
Read filtering, reference genome alignment and quantification of gene abundance were performed on the ribo-seq data. After differentially translated gene analysis and comparisons of differences between translation and transcription, GO and KEGG enrichment analyses were performed.
Transmission electron microscopy (TEM)
B cells were centrifuged and fixed in 2.5% glutaraldehyde and 2% paraformaldehyde (wt/vol) in 0.1 M phosphate buffer (pH 7.4). The cell sediment was then processed for electron microscopy and examined with a Tecnai G2 Spirt Twin electron microscopy as we described before69.
Seahorse assay
B-cell mitochondrial respiration was detected with an XF Mitochondria Stress Test Kit (Agilent, Cat#: 103015-100) following the manufacturers’ protocols. Mouse or human B cells were harvested, counted and replated in a Cell-tak (22.4 µg/ml, Corning) precoated XF96 microplate with 5105 B cells/well. To accelerate the attachment of the cells to the plate, the plate was centrifuged at 200 × g for 1 min. The plate was then incubated at 37 °C without CO2 for 45-60 min before analysis by a Seahorse XF96 analyzer. The data were analyzed by Agilent Seahorse Analytics software.
ELISA
To measure the level of anti-OVA/NP25/NP2/dsDNA antibodies in mouse sera, OVA (10 µg/ml), NP25 (5 µg/ml), NP2 (5 µg/ml) or calf thymus DNA (10 µg/ml) was precoated on 96-well plates at 4 °C overnight. The wells were blocked with PBS containing 3% BSA at 37 °C for 2 h, followed by incubation with diluted mouse serum in PBS (1:3200) at 37 °C for 2 h. The plates were washed three times with PBST and then incubated with goat anti-mouse IgG-HRP (1:5000) at 37 °C for 1 h. For anti-mouse IgM or anti-mouse IgG3 antibody measurement, the plates were incubated with biotin anti-mouse IgM (1:500, BioLegend, Cat#: 406503) or biotin anti-mouse IgG3 (1:1000, BioLegend, Cat#: 406803) followed by HRP-avidin (1:1000, Vector, Cat#: A-2004-5). A TMB coloring system (Proteintech, Cat#: B200051) was used to detect the O.D. value at 450 nm.
Calcium flux assay
Fresh splenocytes were incubated with Fluo-3 AM (4 µM, Sigma‒Aldrich, Cat#: 39294) in Ca2+-free buffer for 30 min at 37 °C in the dark. After 3 washes, the cells were stained with APC-conjugated anti-mouse B220 and suspended in a buffer containing Ca2+ to detect Ca2+ influx by flow cytometry. During the process, the basal level of Ca2+ was detected for 90 s, and anti-mouse IgM (10 µg/ml) was added to stimulate Ca2+ influx. The level of Ca2+ influx was recorded by flow cytometry for 5 min.
Hematoxylin-eosin (HE) staining
Tissue samples embedded in paraffin were cut into 5 µm thick sections, which were subsequently deparaffinized with xylene, rehydrated with a descending gradient of ethanol, stained with hematoxylin and eosin, dehydrated with an elevated gradient of ethanol and made transparent with xylene. Finally, the sections were scanned under a microscope to observe the spleen morphology.
Periodic acid-schiff (PAS) staining
Mouse Kidneys were fixed in 10% neutral formalin, dehydrated, and embedded with paraffin. Tissue blocks were cut into 4 µm thick sections and followed by PAS staining. Histopathological evaluation was performed on a scale of 0–3 according to cell proliferation and cell infiltration by two observers blind to the protocol as previously described66.
Puromycin intake assay
Cells were incubated with puromycin (10 µg/ml, MedChemExpress, Cat#: HY-K1057-1) at 37 °C for 30 min, permeabilized and fixed for intracellular staining with AF488-conjugated anti-puromycin (1:100, BioLegend, Cat#: 381505). Puromycin intake levels were detected by flow cytometry.
tRNA reduction and cleavage (TRAC-seq)
TRAC-seq was conducted as previously described70. First, total RNA was extracted from mouse B cells with or without METTL1 deletion via TRIzol reagent and then subjected to small RNA purification via the miRNA Isolation Kit (mirVana, Cat#: AM1561). The isolated small RNAs were demethylated with recombinant wild-type AlkB and D135S AlkB proteins. The demethylated small RNAs were treated with 0.2 M NaBH4 on ice for 30 min in the dark and then purified via the Oligo Clean & Concentrator Kit (Zymo Research, USA, Cat#: D4060). The NaBH4-treated small RNAs were further incubated with 100 mL of cleavage buffer (H2O: glacial acid: aniline=7:3:1) at RT in the dark for 2 h. After purification via the Oligo Clean & Concentrator Kit, the small RNAs were subjected to cDNA library construction via the NEBNext Multiplex Small RNA Library Prep Set for Illumina Kit. High-throughput sequencing was performed on these libraries, and the obtained results were analyzed as previously described70.
METTL1 knockdown in B cells
Lentiviral vectors expressing pLKO.1 shRNA targeting GFP (shGFP) or Mettl1 (shM1) was purchased from Horizon Discovery. For lentivirus production, the lentiviral vectors, packaging vector pCMV-ΔR8.9, and enveloped vector pCMV-VSVG were cotransfected into 293 T cells with Lipofectamine 3000 reagent (Invitrogen). The packaged viruses were collected 48 h after transfection and used for infection of B cells with 10 µg/ml polybrene (Yeasen). The cells were centrifuged at 800 × g for 1 h. After infection, the supernatant was discarded, and the medium was replaced with a fresh, complete medium. Puromycin (2.5 µg/ml; MedChemExpress, Cat# HY-K1057) was used to select the infected cells for 48 h.
Plasmid construction
The Cd19 overexpression plasmid was constructed by cloning the mouse Cd19 gene into pcDNA3.1(+). Briefly, mRNA was isolated from mouse splenocytes to generate cDNA, and Cd19 was then cloned with forward primer (5’- cttggtaccgagctcggatccGCCACCATGCCATCTCCTCTCCCTGTCTC-3’) and reverse primer (5’- aacgggccctctagactcgagTCACGTGGTTCCCCAAGTCC-3’), using 2× Phanta Flash Master Mix (P510-01, Vazyme Biotech, China). After digested by BamHI and XhoI restriction endonucleases (TransGen Biotech, China), the Cd19 overexpression plasmid pcDNA3.1(+)-Cd19 was constructed using a MultiS One Step Cloning kit (C113-01, Vazyme Biotech, China) according to the manufacturer’s instructions. After linearization, plasmid vectors and insert fragments were recombined by recombinase Exnase MultiS, then pcDNA3.1-Cd19 was transformed into DH5α and selected by ampicillin. The recombinant plasmids were extracted using an Endofree Maxi Plasmid Kit (TIANGEN DP117, China) and confirmed by sequencing (BGI, China). Isolated mouse B cells were transfected with pcDNA3.1-Cd19 plasmid using a Nucleofector Kit (Lonza) according to the manufacturer’s protocols. Cells were then let to rest overnight to recover from the electroporation. Efficiency was confirmed by Western blot.
Cd19 luciferase reporter construct
The coding sequences of wild-type Cd19 (WT-Cd19) and a mutant Cd19 (MUT-Cd19) featuring synonymous mutations were cloned and inserted into the pmirGLO plasmid vector between the ATG start codon and the coding sequence of the firefly luciferase (F-luc) reporter gene. Specifically, the following synonymous mutations in the coding sequence were introduced: TTC codons were replaced with TTT (15 sites), GTG codons were replaced with GTC (13 sites), GCT codons were replaced with GCC (7 sites), ACT codons were replaced with ACG (6 sites), and CCT codons were replaced with CCC (16 sites). All mutations do not alter the encoded amino acids. The plasmid constructs were then transfected into 293 T cells. The relative TEs of the WT- and MUT-Cd19 sequences were determined by quantifying the F-luc luciferase activity and normalizing it to the activity of the Renilla luciferase (R-luc) reporter.
METTL1-RIP-sequencing
293 T cells were transfected with either an exogenous METTL1 overexpression plasmid or an empty vector control plasmid. At 48 h post-transfection, the cells were subjected to UV crosslinking at 4000 × 100 µJ/cm2. The cells were then lysed, and anti-FLAG magnetic beads were used for immunoprecipitation of crosslinked RNA‒protein complexes at 4 °C for 4 h with rotation. RNA was subsequently extracted from the immunoprecipitated material by TRIzol reagent following the manufacturer’s instructions. Recombinant WT AlkB and D135S AlkB MUT proteins were employed to remove dominant methylations from the isolated RNA samples, facilitating efficient reverse transcription of tRNAs. The demethylated RNA samples were then subjected to cDNA library construction via the Multiplex Small RNA Library Prep Set for Illumina (New England Biolabs, USA) according to the manufacturer’s instructions for high-throughput sequencing. The cDNA libraries were sequenced with an Illumina NextSeq 500.
Statistical analysis and reproducibility
The data are presented as the means ± SEMs. Statistical analysis was performed by Prism 9.0. Comparisons were assessed by two-tailed unpaired Student’s t test, paired Student’s t test, or one-way ANOVA with repeated measurements followed by multiple comparison post-test. Statistical methods are indicated in the figure legends for each panel. For blots and microscopy image studies, three times, each experiment was repeated independently, and representative images were shown. In western/northwestern blot studies, the samples were derived from the same experiment, and blots were processed in parallel.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Source data
Acknowledgements
This work is supported by the National Natural Science Foundation of China (82471764, 82071819) and the Fundamental Research Funds for the Central Universities (22hytd11) to H.Z., National Natural Science Foundation of China (82171770) to N.Y.
Author contributions
H.Z. conceived and designed the study. B.C. and X.Y. recruited patients and processed patient samples. J.L. and Y.L. helped breed and maintain the experimental animals. S.W. performed the in vitro and in vivo experiments with help from H.H., Y.Q., X.R., Z.L., M.L., and J.W. H.H. and Z.W. performed and analyzed the TRAC-seq data. H.Z., S.W., N.Y., and S.L. analyzed and interpreted the data. H.Z. and S.W. wrote the manuscript, with all the authors providing corrections.
Peer review
Peer review information
Nature Communications thanks George Tsokos and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated or analyzed during this study are included in this article and its supplementary information files and are available from the corresponding author upon request. Sequencing data generated in this study have been deposited in Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa/). scRNA-seq data have been deposited under the CRA014155. Ribo-seq data have been deposited under the CRA014160. TRAC-seq have been deposited under the CRA018131. RNA-seq have been deposited under the CRA017868. METTL1-RIP tRNA library-seq have been deposited under the HRA008135. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD057426. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuyi Wang, Hui Han.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54941-4.
References
- 1.Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol.15, 160–171 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Cyster, J. G. & Allen, C. D. C. B cell responses: cell interaction dynamics and decisions. Cell177, 524–540 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jellusova, J. The role of metabolic checkpoint regulators in B cell survival and transformation. Immunol. Rev.295, 39–53 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Klein, U. et al. Transcriptional analysis of the B cell germinal center reaction. Proc. Natl Acad. Sci. USA100, 2639–2644 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boothby, M. & Rickert, R. C. Metabolic regulation of the immune humoral response. Immunity46, 743–755 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suan, D., Sundling, C. & Brink, R. Plasma cell and memory B cell differentiation from the germinal center. Curr. Opin. Immunol.45, 97–102 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Young, C. & Brink, R. The unique biology of germinal center B cells. Immunity54, 1652–1664 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu Rev. Immunol.40, 413–442 (2022). [DOI] [PubMed] [Google Scholar]
- 9.Salerno, F. et al. An integrated proteome and transcriptome of B cell maturation defines poised activation states of transitional and mature B cells. Nat. Commun.14, 5116 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brink, R. & Phan, T. G. Self-reactive B cells in the germinal center reaction. Annu. Rev. Immunol.36, 339–357 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Silvera, D., Formenti, S. C. & Schneider, R. J. Translational control in cancer. Nat. Rev. Cancer10, 254–266 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Orellana, E. A., Siegal, E. & Gregory, R. I. tRNA dysregulation and disease. Nat. Rev. Genet.23, 651–664 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou, H. J., Donnard, E., Gustafsson, H. T., Garber, M. & Rando, O. J. Transcriptome-wide Analysis of Roles for tRNA Modifications in Translational Regulation. Mol. Cell68, 978–992.e974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang, Z. et al. Global analysis of tRNA and translation factor expression reveals a dynamic landscape of translational regulation in human cancers. Commun. Biol.1, 234 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol.22, 375–392 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Wang Y. et al. tRNA modifications: insights into their role in human cancers. Trends Cell Biol. 33, 1035–1048 (2023). [DOI] [PubMed]
- 17.Liu, Y. et al. tRNA-m(1)A modification promotes T cell expansion via efficient MYC protein synthesis. Nat. Immunol.23, 1433–1444 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Alexandrov, A., Martzen, M. R. & Phizicky, E. M. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA8, 1253–1266 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, X. et al. Speech and language delay in a patient with WDR4 mutations. Eur. J. Med. Genet.61, 468–472 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Braun, D. A. et al. Mutations in WDR4 as a new cause of Galloway-Mowat syndrome. Am. J. Med. Genet. A176, 2460–2465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, S. et al. Mettl1/Wdr4-mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell71, 244–255.e245 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai, Z. et al. N(7)-Methylguanosine tRNA modification enhances oncogenic mRNA translation and promotes intrahepatic cholangiocarcinoma progression. Mol. Cell81, 3339–3355.e3338 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Han, H. et al. N(7)-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat. Commun.13, 1478 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orellana, E. A. et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol. Cell81, 3323–3338.e3314 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, H. et al. Targeting tumour-intrinsic N(7)-methylguanosine tRNA modification inhibits MDSC recruitment and improves anti-PD-1 efficacy. Gut72, 1555–1567 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Piccirillo, C. A., Bjur, E., Topisirovic, I., Sonenberg, N. & Larsson, O. Translational control of immune responses: from transcripts to translatomes. Nat. Immunol.15, 503–511 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Boulias, K. & Greer, E. L. Put the pedal to the METTL1: adding internal m(7)G increases mRNA translation efficiency and augments miRNA processing. Mol. Cell74, 1105–1107 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Suzuki, K., Grigorova, I., Phan, T. G., Kelly, L. M. & Cyster, J. G. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J. Exp. Med.206, 1485–1493 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol.15, 137–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yazicioglu, Y. F. et al. Dynamic mitochondrial transcription and translation in B cells control germinal center entry and lymphomagenesis. Nat. Immunol.24, 991–1006 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cumpelik, A. et al. Dynamic regulation of B cell complement signaling is integral to germinal center responses. Nat. Immunol.22, 757–768 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHeyzer-Williams, L. J., Milpied, P. J., Okitsu, S. L. & McHeyzer-Williams, M. G. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat. Immunol.16, 296–305 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krzyzak, L. et al. CD83 modulates B cell activation and germinal center responses. J. Immunol.196, 3581–3594 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Luo, W. et al. SREBP signaling is essential for effective B cell responses. Nat. Immunol.24, 337–348 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suan, D. et al. CCR6 defines memory B cell precursors in mouse and human germinal centers, revealing light-zone location and predominant low antigen affinity. Immunity47, 1142–1153.e1144 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Swanson, C. L. et al. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J. Exp. Med.207, 1485–1500 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shih, T. A., Roederer, M. & Nussenzweig, M. C. Role of antigen receptor affinity in T cell-independent antibody responses in vivo. Nat. Immunol.3, 399–406 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Saouaf, S. J. et al. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc. Natl. Acad. Sci. USA91, 9524–9528 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Depoil, D. et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat. Immunol.9, 63–72 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Xu, Y. et al. No receptor stands alone: IgG B-cell receptor intrinsic and extrinsic mechanisms contribute to antibody memory. Cell Res.24, 651–664 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vercellino, I. & Sazanov, L. A. The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol.23, 141–161 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Chen, D. et al. Coupled analysis of transcriptome and BCR mutations reveals role of OXPHOS in affinity maturation. Nat. Immunol.22, 904–913 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Urbanczyk, S. et al. Mitochondrial respiration in B lymphocytes is essential for humoral immunity by controlling the flux of the TCA cycle. Cell Rep.39, 110912 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Akkaya, M. et al. Second signals rescue B cells from activation-induced mitochondrial dysfunction and death. Nat. Immunol.19, 871–884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisel, F. J. et al. Germinal center B cells selectively oxidize fatty acids for energy while conducting minimal glycolysis. Nat. Immunol.21, 331–342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng, Q. et al. Spleen fibroblastic reticular cell-derived acetylcholine promotes lipid metabolism to drive autoreactive B cell responses. Cell Metab.35, 837–854.e838 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Caielli, S., Wan, Z. & Pascual, V. Systemic lupus erythematosus pathogenesis: interferon and beyond. Annu. Rev. Immunol.41, 533–560 (2023). [DOI] [PubMed] [Google Scholar]
- 48.Liossis, S. N., Kovacs, B., Dennis, G., Kammer, G. M. & Tsokos, G. C. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J. Clin. Invest.98, 2549–2557 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jenks, S. A. & Sanz, I. Altered B cell receptor signaling in human systemic lupus erythematosus. Autoimmun. Rev.8, 209–213 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yeomans, A. et al. Engagement of the B-cell receptor of chronic lymphocytic leukemia cells drives global and MYC-specific mRNA translation. Blood127, 449–457 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Fearon, D. T. & Carroll, M. C. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol.18, 393–422 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Wang, Y. & Carter, R. H. CD19 regulates B cell maturation, proliferation, and positive selection in the FDC zone of murine splenic germinal centers. Immunity22, 749–761 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Wang, Y. et al. The physiologic role of CD19 cytoplasmic tyrosines. Immunity17, 501–514 (2002). [DOI] [PubMed] [Google Scholar]
- 54.van Zelm, M. C. et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N. Engl. J. Med.354, 1901–1912 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Cariappa, A. et al. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity14, 603–615 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Samardzic, T. et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol.32, 561–567 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Nemazee, D. Mechanisms of central tolerance for B cells. Nat. Rev. Immunol.17, 281–294 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kil, L. P. et al. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood119, 3744–3756 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Liu, Z. & Davidson, A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat. Med.18, 871–882 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merrill, J. T. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum.62, 222–233 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Furie, R. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum.63, 3918–3930 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum.40, 1725 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez-Figueroa, P. et al. Follicular regulatory T cells produce neuritin to regulate B cells. Cell184, 1775–1789.e1719 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Liu, Y. et al. Robust induction of B cell and T cell responses by a third dose of inactivated SARS-CoV-2 vaccine. Cell Discov.8, 10 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu, Y. et al. The impact of circadian rhythms on the immune response to influenza vaccination in middle-aged and older adults (IMPROVE): a randomised controlled trial. Immun. Ageing19, 46 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li, X. et al. CRAC channel controls the differentiation of pathogenic B cells in lupus nephritis. Front. Immunol.12, 779560 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li, M. et al. NAMPT is a metabolic checkpoint of IFNgamma-producing CD4(+) T cells in lupus nephritis. Mol. Ther.31, 193–210 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ingolia, N. T., Brar, G. A., Rouskin, S., McGeachy, A. M. & Weissman, J. S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc.7, 1534–1550 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fu, R. et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol.69, 1636–1646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin, S., Liu, Q., Jiang, Y. Z. & Gregory, R. I. Nucleotide resolution profiling of m(7)G tRNA modification by TRAC-Seq. Nat. Protoc.14, 3220–3242 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files and are available from the corresponding author upon request. Sequencing data generated in this study have been deposited in Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa/). scRNA-seq data have been deposited under the CRA014155. Ribo-seq data have been deposited under the CRA014160. TRAC-seq have been deposited under the CRA018131. RNA-seq have been deposited under the CRA017868. METTL1-RIP tRNA library-seq have been deposited under the HRA008135. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD057426. Source data are provided with this paper.