Significance
Feeding mice with a high-sucrose diet (HSD) resulted in colon inflammation and impaired gut barrier tight junction, as well as decreased colon level of 5(6)-epoxyeicosatrienoic acid. This is associated with an increase in its metabolic enzyme, soluble epoxide hydrolase (sEH). Reduction of sEH by both chemical intervention and intestinal-specific knockout of sEH significantly attenuated HSD-mediated gut barrier dysfunction. In vitro studies revealed that treatment with high sucrose led to inflammation in the intestinal epithelia and impaired tight junction. Treatment with 5(6)-epoxyeicosatrienoic acid ablated high sucrose-mediated cellular inflammation and improved tight junctions impaired by high sucrose. The present study gains insights into the pathology and pharmacology of HSD-caused gut barrier dysfunction.
Keywords: Claudins, epoxyeicosatrienoic acid, high sucrose diet, metabolomics, soluble epoxide hydrolase
Abstract
Highsucrose diet (HSD) was reported as a causative factor for multiorgan injuries. The underlying mechanisms and therapeutic strategies remain largely uncharted. In the present study, by using a metabolomics approach, we identified the soluble epoxide hydrolase (sEH) as a therapeutic target for HSD-mediated gut barrier dysfunction. Specifically, 16-week feeding on an HSD caused gut barrier dysfunction, such as colon inflammation and tight junction impairment in a murine model. A metabolomics analysis of mouse colon tissue showed a decrease in the 5(6)-epoxyeicosatrienoic acid [5(6)-EET] level and an increase in soluble epoxide hydrolase, which is related to HSD-mediated injuries to the gut barrier. The mice treated with a chemical inhibitor of sEH and the mice with genetic intervention by intestinal-specific knockout of the sEH gene significantly attenuated HSD-caused intestinal injuries by reducing HSD-mediated colon inflammation and improving the impaired tight junction caused by an HSD. Further, in vitro studies showed that treatment with 5(6)-EET, but not its hydrolytic product 5,6-dihydroxyeicosatrienoic acid (5,6-DiHET), significantly ablated high sucrose-caused intestinal epithelial inflammation and impaired tight junction. Additionally, 5(6)-EET is anti-inflammatory and improves gut epithelial tight junction while 5,6-DiHET cannot do so. This study presents an underlying mechanism of and a therapeutic strategy for the gut barrier dysfunction caused by an HSD.
The impact of diets on health has been gaining increasing attention worldwide (1–5). High sucrose (HS), together with high fat, is a typical feature of the Western diet. High-sucrose diet (HSD), including food and beverages rich in sucrose, has been extensively found as a risk factor for a variety of illnesses, such as obesity (6, 7), insulin resistance and glucose intolerance (8, 9), fatty liver (10), systemic and cerebral inflammation (11), and many other chronic diseases (7). In addition, HSD usually leads to additive or synergetic detrimental effects of a high-fat diet mainly because the liberated fructose stimulates the de novo synthesis of fatty acids (12). However, the molecular mechanisms underlying HSD-mediated organ injuries remain largely unknown. In the past four decades, while HSD exposure in high-income countries kept slowly increasing, HSD exposure in low-income and middle-income countries has been markedly increasing along with the accrual export of Western economy, culture, and lifestyles (7). Therefore, there is an urgent need to explore the mechanisms underlying HSD-mediated multiorgan injuries and potential intervention targets. Accordingly, metabolomics, proteomics, and transcriptomics, as well as other innovative techniques, have been employed in the above-mentioned studies (13–16).
Metabolomics is an effective approach to investigate the changes in metabolome caused by multiple endogenous factors and various exogenous factors, including but not limited to genetic mutations, epigenetic modifications, aging, environmental exposure, diet and nutrients, diseases, drugs, and lifestyles (17, 18). Metabolomics has been widely used to investigate pathogenic mechanisms, diagnostic markers, and intervention targets (19–22). Recently, by adopting some advanced techniques, metabolic flux, single-cell metabolomics, and spatial metabolomics were developed as a new generation of metabolomics techniques to enhance the power and performance of traditional metabolomics (23–26). Notably, we have used both nontargeted metabolomics and targeted metabolomics methods to investigate the potential intervention targets or biomarkers for acute kidney disease, obesity-related kidney injury, chronic kidney disease, gouty arthritis, and colon cancers (17, 27–30). In particular, a targeted metabolomics approach to the metabolism of polyunsaturated fatty acids (PUFAs), including linoleic acid, alpha-linolenic acid, arachidonic acid, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), was extensively employed to explore the mechanisms underlying PUFAs metabolism-driven pathogenic and pharmacological process (27–30). Most organ and tissue injuries are associated with inflammation, which is closely associated with the metabolism of PUFAs and particularly the metabolism of arachidonic acid. Therefore, in the present study, we used the established targeted metabolomics approach to investigate the role of PUFAs’s metabolism in HSD-mediated gut barrier injury. Here, we report that soluble epoxide hydrolase (sEH), the metabolic enzyme mediating the transformation of epoxide fatty acids (EpFAs) derived from PUFAs by cytochrome P450, to form conversely or less active vicinal diols, is a prospective intervention target for prophylactic and therapeutic treatments of HSD-mediated gut barrier dysfunction.
Results
HSD did not Cause an Excess Gain of Body Weight in a Murine Model.
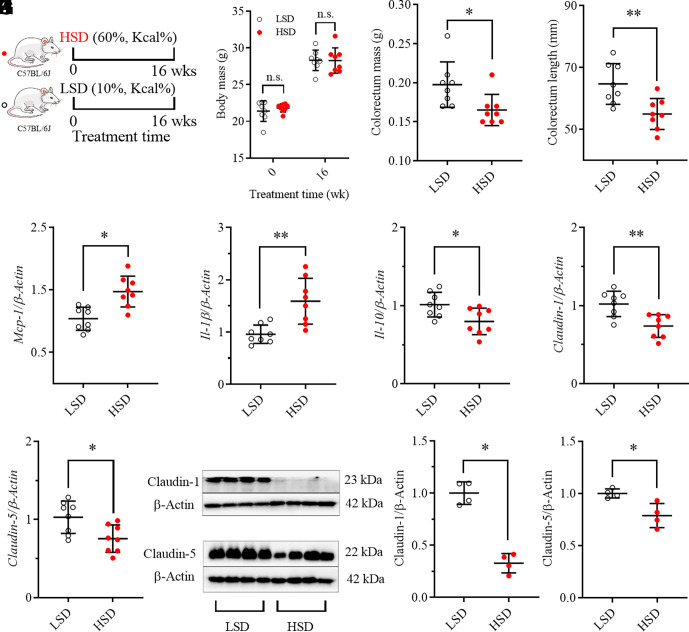
As illustrated in Fig. 1B, the mice fed with an HSD did not experience excess weight gain after 16 wks of feeding when compared with the ones fed with a low sucrose diet (LSD).
Fig. 1.
HSD caused colorectum inflammation and injury to gut barrier integrity in a murine model. (A) A brief scheme of the experimental design; mice fed with an HSD caused no excess weight gain (B) but significantly decreased colorectum mass (C) and length (D). Mice fed with an HSD increased inflammatory Mcp-1 (E) and Il-1β (F) but decreased anti-inflammatory Il-10 (G), as well as decreased Claudin-1 and Claudin-5 at mRNA (H and I) and protein levels (J–L). Data represent mean ± SD (N = 8 or 4). Statistical difference was determined by a two-tailed noncompartmental test with the Mann–Whitney test (*0.01 < P ≤ 0.05, **0.001 < P ≤ 0.01).
HSD Caused Colorectal Inflammation in a Murine Model.
Feeding the mice with an HSD led to significant decreases in colorectum mass (Fig. 1C) and length (Fig. 1D) when compared with mice fed with an LSD. In addition, HSD feeding to mice resulted in significant increases in inflammatory cytokines Mcp-1 (Fig. 1E) and Il-1β (Fig. 1F), and significant reduction in the anti-inflammatory Il-10 (Fig. 1G) at mRNA levels in colorectum tissue when compared with an LSD feeding. In addition, pathological analysis revealed that the mice fed with HSD resulted in obvious inflammatory cell infiltration (SI Appendix, Fig. S1).
HSD Caused Injury to Gut Barrier Dysfunction in a Murine Model.
HSD feeding to mice for 16 wks caused significantly lower Claudin-1 and Claudin-5, the markers of intestine permeability, at the mRNA (Fig. 1 H and I) and protein (Fig. 1 J–L and SI Appendix, Fig. S2) levels in colorectum tissue than an LSD did.
Targeted Metabolomics Revealed the Upregulation of Colorectum sEH in the Mice Fed on an HSD.
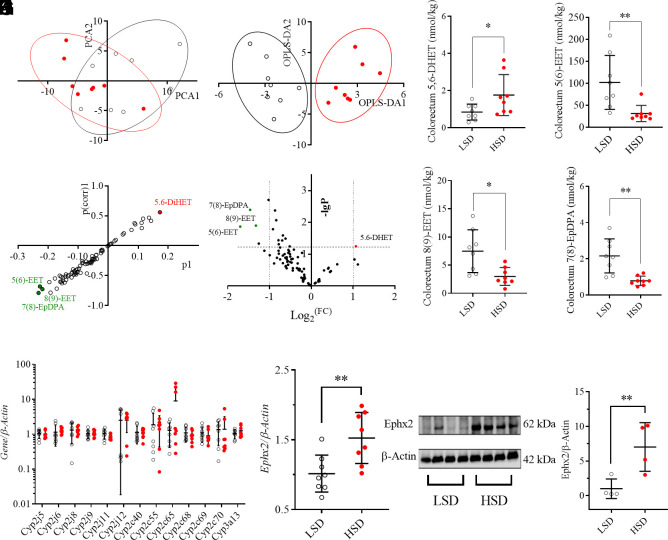
An established targeted metabolomics analysis of murine colorectum tissue from the mice fed on an HSD and LSD recorded 85 oxylipins (SI Appendix, Table S1) in the analyzed samples. An unsupervised multivariate method principal component analysis (PCA) was employed to process the recorded concentration, resulting in a visual difference between the mice fed on an HSD from those with LSD (Fig. 2A), by which the first three components of the PCA took 70.6% of the variation of the data. Then orthogonal partial least squares-discriminant analysis (OPLS-DA), a supervised multivariate method, was employed to process the data. Two-dimensional score plot, constructed by one predictive component and two orthogonal components model, led to an obvious visual separation of the mice fed on an HSD from the ones on an LSD (Fig. 2B). Interestingly, both S-plot (Fig. 2C) and a volcano plot (Fig. 2D) identified the decreases in three EpFAs, including 5(6)-EET, 8(9)-EET, and 7(8)-EpDPA, and increase in 5,6-DiHET in the colorectum tissue from the mice fed on an HSD as the major factors leading to the separation of the mice fed on an HSD from the ones with an LSD. The significant differences of these four oxylipins between the two groups were further supported by a noncompartmental test with the Mann–Whitney test (Fig. 2 E–H). Because EpFAs were the products of PUFAs in the presence of Cyp2cs and Cyp2js and were further metabolized by sEH, colorectum expression of these genes at mRNA levels was quantitatively analyzed. As illustrated in Fig. 2I, HSD feeding caused nonsignificant changes in Cyp2cs, Cyp2js and Cyp3a13. It should be noted that data of Cyp2c29/37/38/39/44 are not presented because they are expressed over 10-fold lower than the illustrated ones. However, sEH (coding gene, Ephx2) was up-regulated in the colorectum tissue of the mice fed on an HSD when compared with those of LSD at both mRNA (Fig. 2J) and protein (Fig. 2 K and L) levels.
Fig. 2.
HSD caused upregulation of soluble epoxide hydrolase (sEH, coding gene Ephx2). (A) Two-dimensional PCA score plots show visual difference of the mice fed with HSD (red dots) from the controls (LSD, unfilled cycles), R2X=0.706, Q2=0.413; (B) Two-dimensional OPLS-DA score plots show visual difference of the mice fed with HSD (red dots) from the controls (LSD, unfilled cycles), R2X = 0.605, Q2 = 0.527; The S-plots (C) and volcano plot (D) of the OPLS-DA model for the processed data; Colorectum levels of 5,6-DiHET (E), 5(6)-EET (F), 8(9)-EET (G), and 7(8)-EpDPA (H) for the mice fed with HSD and LSD; HSD feeding caused slight modification of Cyp2cs, Cyp3a13, and Cyp2js (I) but a significant increase in Ephx2 at mRNA (J) and protein (K and L) levels. Data represent mean ± SD (N = 8 or 4). Statistical difference was determined by a two-tailed noncompartmental test with the Mann–Whitney test (*0.01 < P ≤ 0.05, **0.001 < P ≤ 0.01).
Intestinal sEH Is Involved in the Metabolism of 5(6)-EET and 14(15)-EET.
To test whether intestinal sEH is involved in the metabolism of 5(6)-EET, we tested the degradation of 5(6)-EET and 14(15)-EET in cell culture media (without enzymes), normal LoVo cells (that express sEH), and LoVo cells with knockout of sEH. The knockout efficacy of sEH was supported by western blot analysis (SI Appendix, Fig. S3 A and B). As shown in SI Appendix, Fig. S3 C–E, when in the media with no enzyme, unlike 14(15)-EET being stable through the tested time (10 h), 5(6)-EET was degraded rapidly. However, in the presence of a cellular system with enzymes, knockout of sEH reduced the degradation significantly of 5(6)-EET and 14(15)-EET as well.
Treatment with an sEH Inhibitor Attenuated HSD-Mediated Colorectal Inflammation and Gut Barrier Dysfunction.
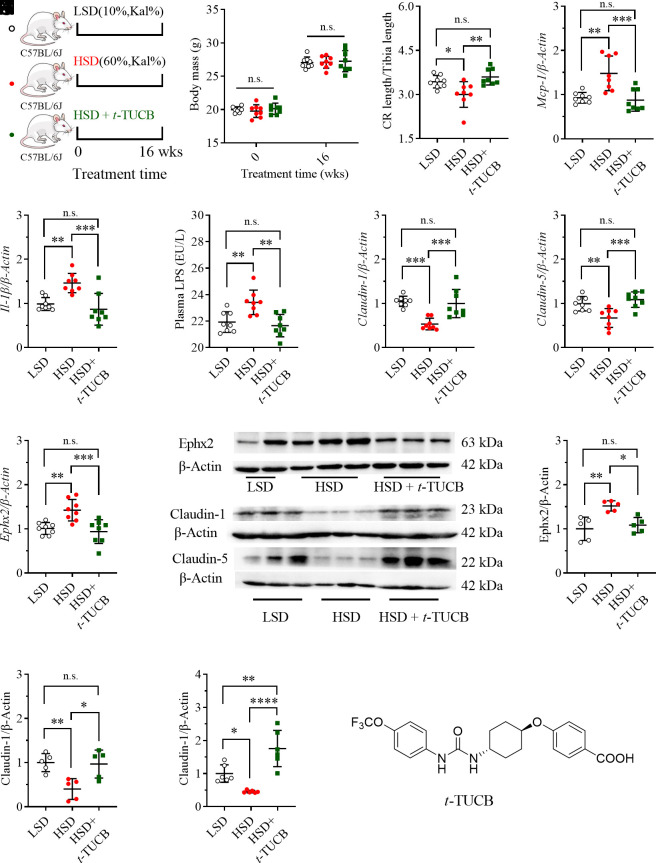
To test whether sEH is an intervention target for HSD-caused colorectal inflammation and injury, a sEH inhibitor t-TUCB (structure shown as Fig. 3N) was provided in drinking water to the mice fed with an FSD (Fig. 3A). We used t-TUCB in the present study based on its potent inhibitory activity against murine sEH and a satisfactory pharmacokinetic profile in mice (31). As expected, the mice treated with an HSD with or without t-TUCB slightly changed the mouse’s body mass (Fig. 3B). The treatment with t-TUCB significantly reversed HSD-mediated decrease in relative colorectal length (Fig. 3C), and inflammatory Mcp-1 (Fig. 3D) and Il-1β (Fig. 3E) in colorectal tissue. Feeding the mice with HSD led to a significantly increased plasma level of LPS, which was significantly reduced by t-TUCB treatment (Fig. 3F). HSD-mediated decreases in colorectal Claudin-1 and Claudin-5 at mRNA and protein levels were significantly up-regulated by t-TUCB treatment (Fig. 3 G, H, J, L, and M and SI Appendix, Fig. S6). The action of t-TUCB against sEH at both mRNA and protein levels is shown in Fig. 3 I–K, which was also supported by the increase in the sum of colorectum EET levels by TPPU treatment (SI Appendix, Fig. S4). In addition, pathological analysis revealed that treatment of t-TUCB attenuated HSD-mediated inflammatory cell infiltration in the colon tissue (SI Appendix, Fig. S5).
Fig. 3.
Inhibition of sEH with an sEH inhibitor attenuated HSD-mediated injury to gut barrier integrity. (A) A brief scheme of the experimental design; (B) Mice fed with an HSD with or without t-TUCB did not cause excessive body gain; Treatment with the sEH inhibitor t-TUCB attenuated HSD-mediated reduction in the ratio of colorectal length to tibia length (C), increase in plasma LPS level (D), increases in colon Mcp-1 (E) and Il-1β (F), decreases in colon claudin-1 and claudin-5 and increase in colon sEH at mRNA levels (G–I) and corresponding changes at protein levels (J, K, L, and M), as well as increase in colon sEH at mRNA (I) and protein levels (J and K); (N) Structure of t-TUCB, an inhibitor of sEH. Data represent mean ± SD (N = 8 or 5 to 6). Statistical difference was determined by ANOVA followed by a Bonferroni’s (variance homogeneity) or Dunnett’s (variance heterogeneity) post hoc comparison test (* 0.01 < P ≤ 0.05, **0.001< P ≤ 0.01, ***0.0001< P ≤ 0.001, **** P ≤ 0.0001).
Intestinal-Specific Knockout of sEH Attenuated HSD-Mediated Colorectal Inflammation and Gut Barrier Dysfunction.
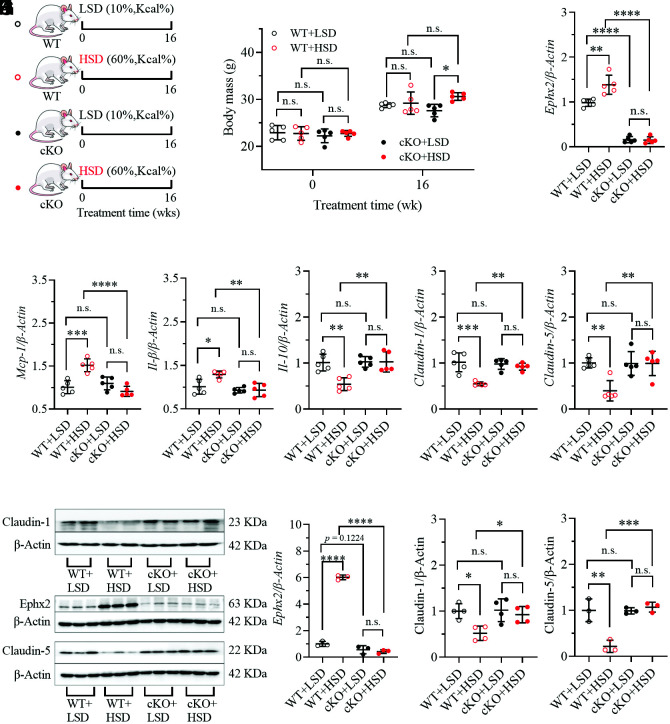
Conditional (intestinal-specific) knockout (cKO) of sEH was identified by PCR analysis of the mouse toe tips (SI Appendix, Fig. S7), which was further supported by the lower mRNA and protein levels of sEH in intestinal tissue from cKO mice fed on an LSD than the WT mice on an LSD (Fig. 4 C, I, and J). Similar to t-TUCB treatment, cKO mice fed with an HSD resulted in a dramatic decrease in mRNA and protein levels of sEH when compared with WT mice fed with an HSD (Fig. 4 C, I, and J). Therefore, it could be expected that cKO of sEH fed with an HSD or LSD led to nonsignificant change in mice body weight when compared with the WT mice treated with an LSD or HSD, exactly as shown in Fig. 4B. However, cKO of sEH significantly attenuated HSD-mediated increase in intestinal Mcp-1 (Fig. 4D), Il-1β (Fig. 4E), and decrease in intestinal Il-10 (Fig. 4F) in WT mice. Again, cKO of sEH significantly up-regulated the HSD-mediated decrease in intestinal Claudin-1 and Claudin-5 at mRNA and protein levels (Fig. 4 G, H–L and SI Appendix, Fig. S9). Histological analyses of colon tissue also indicated that cKO of sEH attenuated HSD-mediated colon inflammation (SI Appendix, Fig. S8).
Fig. 4.
Intestinal epithelial-specific knockout (cKO) of sEH attenuates HSD-mediated injury to the gut barrier. (A) A brief scheme of the experimental design; (B) Both cKO mice and their wildtype (WT) littermates fed on an HSD did not cause excessive body gain; (C) An HSD feeding resulted in upregulation of intestinal Ephx2 in WT mice while slight change in Ephx2 cKO mice; An HSD feeding resulted in significance increases in intestinal Mcp-1 (D) and IL-1β (E), as well as significant decreases in intestinal IL-10 (F), Claudin-1 (G), and Claudin-5 (H) in WT mice. However, these significant changes in the intestine caused by an HSD feeding were reduced by conditional knockout of Ephx2 in the intestine; (I) Conditional knockout of Ephx2 in the intestine diminished the significant increase in Ephx2 and decreases in Claudin-1 and Claudin-5 caused by an HSD feeding in the WT littermates; The quantitative analyses of band density were presented as (J–L). Data represent mean ± SD (N = 3 to 5). Statistical difference was determined by ANOVA followed by a Bonferroni’s (variance homogeneity) or Dunnett’s (variance heterogeneity) post hoc comparison test (*0.01 < P ≤ 0.05, **0.001 < P ≤ 0.01, ***0.0001 < P ≤ 0.001, ****P ≤ 0.0001).
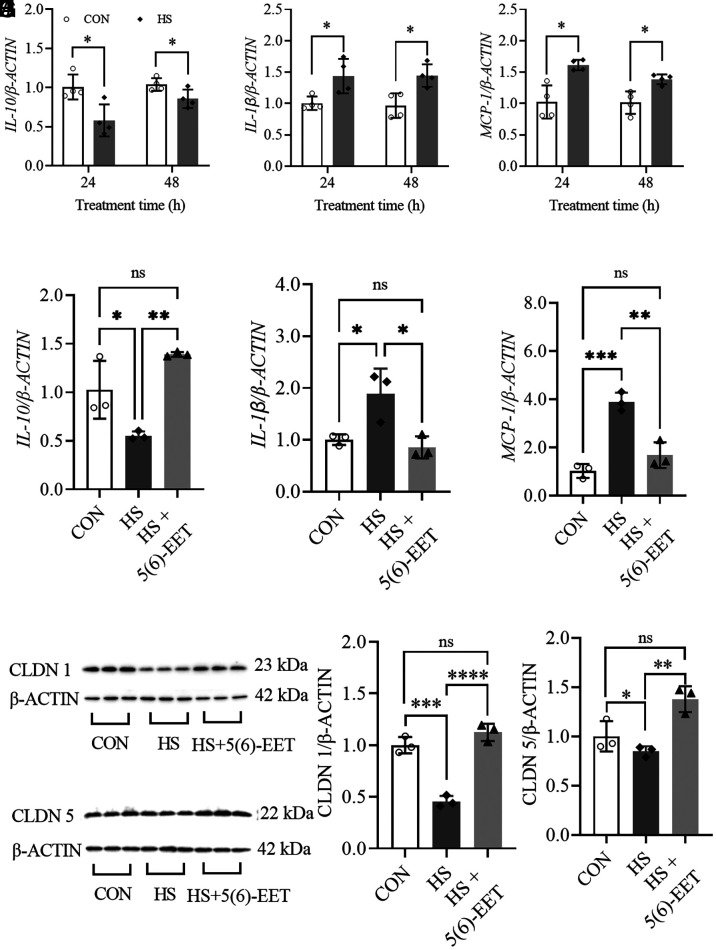
Treatment with High Sucrose (HS) Caused Injury to Intestinal Epithelial Cells.
Treatment of human colon epithelial cells (LoVo) with high sucrose (HS) resulted in a significant decrease in cell IL-10 (Fig. 5A), and a significant increase in cell IL-1β (Fig. 5B) and MCP-1 (Fig. 5C) at mRNA levels. It should be noted that, at each treatment time, we took the control cells as the baseline for the corresponding HS-treated cells, although the mRNA levels of all three cytokines increased along with the treatment time. In addition, treatment of HS also caused a significant decrease in CLAUDIN 1 (CLDN1) and CLAUDIN 5 (CLDN5) (Fig. 5 G–I) at the protein level.
Fig. 5.
5(6)-EET attenuates high-sucrose (HS)-mediated injury to intestinal epithelial cells. The treatment of HS resulted decrease in IL-10 (A), and an increase in IL-1β (B) and MCP-1 (C) in LoVo cells in a time-relevant manner. The treatment of 5(6)-EET (100 nM) significantly reversed the HS-mediated decrease in IL-10 (D), and increase in IL-1β (E) and MCP-1 (F). The treatment of 5(6)-EET (100 nM) significantly ablated HS-mediated decrease in Claudin-1 and 5 (G–J). (H) and (J) are the quantitative analysis results of band density presented in (G) and (I), respectively. Data represent mean ± SD (N = 3 to 4). The statistical difference between the two groups was determined by a two-tailed noncompartmental test with the Mann–Whitney test, while the statistical difference among three groups was determined by ANOVA followed by a Bonferroni’s (variance homogeneity) or Dunnett’s (variance heterogeneity) post hoc comparison test (*0.01 < P ≤ 0.05, **0.001 < P ≤ 0.01, ***0.0001< P ≤ 0.001, ****P ≤ 0.0001).
5(6)-EET but not 5,6-DiHET Attenuated HS-Mediated Injury to Intestinal Epithelial Cells.
As illustrated in Fig. 5 D–F, treatment of 5(6)-EET significantly reversed the HS-mediated decrease in cell IL-10 and an increase in IL-1β and MCP-1 at mRNA levels. In addition, 5(6)-EET treatment increased the HS-mediated decrease in cell CLAUDIN-1 and 5 at the protein level (Fig. 5 G–J). However, treatment of 5,6-DiHET at the same concentration of 5(6)-DiHET failed in reversing HS-induced damages to the tested cells (SI Appendix, Fig. S10).
5(6)-EET but not 5,6-DiHET Is Anti-Inflammatory and Increases Tight Junction for Intestinal Epithelial Cells.
Treatment of the human colon epithelial cells (NCM460) with 5(6)EET resulted in a decrease in cell IL-1β and CLDN1 (SI Appendix, Figs. S11A, and S11B) at mRNA levels in a time-dependent manner. In addition, 5(6)-EET treatment resulted in a time-dependent increase in cell CLDN1 (SI Appendix, Fig. S11 E–G) at protein levels. In contrast, the treatment of NCM460 cells with the same dose of 5,6-DiHET, the sEH-mediated product of 5(6)-EET, slightly modified the cell mRNA levels of IL-1β and CLDN1 (SI Appendix, Figs. S11C and S8D), and protein levels of CLDN1 (SI Appendix, Fig. S11H). These changes have been shown to correlate 5(6)-EET with the resolution of inflammation.
Discussion
This study disclosed that even under healthy conditions, continuous consumption of an HSD resulted in injuries to the intestinal barrier, including intestinal inflammation and impaired gut barrier tight junction, together with an upregulation of sEH, which was subsequently showed to be a potential therapeutic target for HSD-mediated gut injuries. HSD-mediated intestinal inflammation was evidenced by HSD-caused increase in proinflammatory cytokine IL-1β and Mcp-1 and decrease in anti-inflammatory IL-10 at mRNA levels (Fig. 1 E–G). As expected, HSD-caused upregulation of Mcp-1 and IL-1β was also observed in the other two animal experiments (Figs. 3 D and E and 4 D–E). Unfortunately, HSD feeding led to a slight decrease in IL-10 in the mice fed with an HSD compared with the ones with an LSD in the second animal experiment (data now shown), perhaps due to the different batches of the mice in the two experiments. However, a significant decrease in IL-10 was consistently observed in WT mice feeding with an HSD in the third animal experiment (Fig. 4F). In addition, colorectal inflammation caused by an HSD feeding is also supported by shortened colon length caused by HSD consumption (Fig. 1D). Meanwhile, decrease in colorectal Claudin-1 and Claudin-5 at both mRNA and protein levels were consistently observed in the mice fed with an HSD compared with the mice with an LSD in all three animal experiments (Figs. 1 H–L, 3 G, H, J, L, and M, 4 G–I, K, and L), indicative of the reduced gut barrier tight junction. In addition, the impaired intestinal barrier in the mice fed with a HSD was also evidenced by increased plasma level of LPS, which is a marker of intestinal permeability (Fig. 3F). These results collectively support that long-term feeding of HSD caused gut barrier dysfunction. The gut barrier dysfunction caused by an HSD in this study is also supported by several previously reported studies (32–34). In addition, sucrose will be hydrolyzed to form equivalent fructose and glucose in the mammal body in the presence of sucrase-isomaltase. Many scientists directly investigated the side effects of excessive consumption of fructose and/or glucose. Consistently, both a high-fructose diet and a high-glucose diet were reported to induce impaired gut barrier (35–37).
A targeted metabolomics study of colon oxylipins demonstrated the critical role of CYP/sEH-mediated EpFAs and corresponding diols [5(6)-EET, 8(9)-EET, 7(8)-EpDPA, and 5,6-DiHET] in HSD-mediated gut barrier dysfunction (Fig. 2 A–D). CYPs were excluded as the causative factors for the changes of EpFAs and diols because they were slightly modified by HSD feeding (Fig. 2I). In contrast, sEH was found to be increased in colon tissue at both mRNA and protein levels (Fig. 2 J–L), which was in agreement with the HSD-caused increase in sEH in the latter two animal experiments as shown in Figs. 3 and 4, respectively. Subsequently, sEH was found an intervention target for HSD-mediated gut barrier dysfunction because inhibition of sEH with a chemical inhibitor significantly ameliorated HSD-caused reduced gut barrier tight junction and colon inflammation (Fig. 3 C–M), which was further in accordance with the findings obtained from feeding the mice with a conditional knockout of sEH in the gut epithelia with an HSD (Fig. 4). In addition, a sEH was also reported a therapeutic target for high-fat diet-caused colonic inflammation and gut barrier dysfunction (38), which further supports the findings in this study.
To explore whether reduced colon levels of EpFAs are the causative factor for gut barrier dysfunction, we selected 5(6)-EET and 5,6-DiHET for further study because they were key mediators contributing to the separation of the mice treated with an HSD and LSD (Fig. 2 B–D). Treatment of HS-induced LoVo cells with 5(6)-EET or 5,6-DiHET demonstrated that 5(6)-EET is more active in reversing HS-induced injury to tested cells (Fig. 5 and SI Appendix, Fig. S10), which was further consistent with the findings from the treatment of human gut epithelia (NM460) with 5(6)-EET and 5,6-DiHET (SI Appendix, Fig. S11). Taken together, these in vitro results explain the decreased colon level of 5(6)-EET and increased colon level of 5,6-DiHET contributed greatly to the gut barrier dysfunction caused by HSD feeding. Administration of DiHETs to mice resulted in murine colon inflammation and a decreased colon level of Claudin-5 (39). This study disclosed HSD feeding caused an increase in 5,6-DiHET, in the murine colon, which also supports that the increase in 5,6-DiHET could cause injury to the gut barrier. However, the increased colon levels caused by HSD were only found for 5,6-DiHET but not 14,15-, 11,12-, and 8,9-DiHET in this study, indicating the complexity of the CYP/sEH axis in mediating the metabolism of AA because DiHETs could be further metabolized (28).
One could concern that 5(6)-EET is chemically metabolized to form 5,6-DiHET lactone directly without the involvement of sEH (40). As shown in SI Appendix, Fig. S3, when in cell culture media without enzymes, 14(15)-EET was stable while 5(6)-EET degraded rapidly, indicating that 5(6)-EET is degraded at least in part nonenzymatically, which is consistent with the previously reported study about 5(6)-EET (40). However, in a cellular system containing sEH, the degradation of 5(6)-EET, was similar to that of 14(15)-EET, was slowed down in the knockout of sEH, indicating 5(6)-EET, similar to 14(15)-EET, could be also metabolized by sEH. Our study revealed that 5(6)-EET could be metabolized through sEH, in parallel to the predominantly nonenzymatic pathways.
In addition, increased plasma level of LPS in the mice fed on a HSD convinced impaired gut barrier function by increased gut barrier permeability. Because gut microbiota is the major source of LPS, an HSD feeding may cause gut dysbiosis to drive the impaired gut barrier and up-regulate sEH (41), the details of which need further investigation. We also knew that sugar consumption varies between individuals and throughout the world. Sucrose is the most common sugar in the diets and beverages with the major source of cane sugar. Corn syrup is another common sugar used in diets and beverages, which contains varying amounts of glucose, fructose, maltose, and many oligosaccharides. A sucrose can be broken to form equal a fructose and a glucose in the body. Sucrose, glucose, and fructose have similar effects on health, but not always the same (42, 43). This study focused solely on sucrose instead of a comparison study of the three common sugars.
In brief, this study provides pathological insights into HSD-mediated organ injuries, but also proposes an intervention method to attenuate HSD-mediated gut barrier dysfunction. In addition, this study extends our understanding of the potential application of sEH inhibitors, perhaps a diet rich in natural sEH inhibitors, for example, capsaicin, macamides, and their source materials (44, 45), could benefit people with a fondness for an HSD.
Materials and Methods
All animal experiments were conducted in line with the protocols approved by the Animal Use and Care Committee of Chongqing Medical University. The mice accessed to LSD or HSD and water ad-lib for 16 wk. LSD (10% kcal sucrose, AIN93G) and HSD (60% kcal sucrose, D12329) were purchased from Wuxi Fanbo Biotechnology Co., Ltd. (Wuxi, China), which were manufactured according to the formula and protocols of Research Diets, Inc. (New Brunswick, NJ). t-TUCB was administered in drinking water (10 mg/L). The Ephx2flox/flox mice and Villin-Cre mice were commercially acquired from the Shanghai Model Organisms Center, Inc (Shanghai, China). An Ephx2 flox heterozygous mouse was crossed with a Villin-Cre mouse to generate Ephx2 flox/flox;Villin-Cre (cKO) mice for further experiment. The group information is presented in Figs. 1–4. The full information of materials, animal and cellular protocols, and analytical protocols is provided in online SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This study was supported by a National Key Research and Development Program of China (2022YFE0131300), and the National Natural Science Foundation of China (NSFC) Grant (82273408), as well as NIEHS Grants (1R35ES030443 and R01 ES02710), and NIEHS Superfund Grant (P42 ES04699). We thank Dr. C. Morisseau, UC Davis, for constructive discussions.
Author contributions
J.-Y.L. designed research; A.-Z.L., X.F., Q.J., X.Z., H.-H.Y., K.-D.N., Q.-J.P., X.H., L.-T.Z., Y.-W.M., and Y.-N.L. performed research; S.H.H. and B.D.H. contributed new reagents/analytic tools; A.-Z.L., X.F., Q.J., X.Z., and J.-Y.L. analyzed data; and B.D.H. and J.-Y.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: E.A.D., University of Massachusetts-Amherst; D.D., Georgia Tech; R.S., Onkologics; and G.Y., Northwestern University Feinberg School of Medicine.
Contributor Information
Bruce D. Hammock, Email: bdhammock@ucdavis.edu.
Jun-Yan Liu, Email: jyliu@cqmu.edu.cn.
Data, Materials, and Software Availability
All data and materials are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Forouhi N. G., Unwin N., Global diet and health: Old questions, fresh evidence, and new horizons. Lancet 393, 1916–1918 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Fresan U., Sabate J., Vegetarian diets: Planetary health and its alignment with human health. Adv. Nutr. 10, S380–S388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hampton T., Parental diet may affect the cardiovascular health of several generations. Circulation 140, 1353–1353 (2019). [Google Scholar]
- 4.Lawrence M. A., McNaughton S. A., Vegetarian diets and health. BMJ 366 (2019). [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan A., Metabolic cross-talk between diet and health. Nat. Food 1, 398–399 (2020). [Google Scholar]
- 6.Kouno T., et al. , Ghrelin O-acyltransferase knockout mice show resistance to obesity when fed high-sucrose diet. J. Endocrinol. 228, 115–125 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Malik V. S., Hu F. B., The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 18, 205–218 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaspar J. M., Coelho I. C., Macedo M. P., Aging and high sucrose diet-induced insulin resistance increases the expression of GLUT12 in skeletal muscle. Diabetes 62, A510–A510 (2013). [Google Scholar]
- 9.Sakamoto E., et al. , Ingestion of a moderate high-sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon-like peptide-1 secretion. J Diabetes Invest 3, 432–440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun S. M., et al. , High sucrose diet-induced dysbiosis of gut microbiota promotes fatty liver and hyperlipidemia in rats. J. Nutr. Biochem. 93, 108621 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Patkar O. L., et al. , A binge high sucrose diet provokes systemic and cerebral inflammation in rats without inducing obesity. Sci. Rep.-UK 11, 11252 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ter Horst K. W., Serlie M. J., Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients 9, 981 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song S. Y., et al. , Myocardial Rev-erb-mediated diurnal metabolic rhythm and obesity paradox. Circulation 145, 448–464 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Dam E., et al. , Sugar-induced obesity and insulin resistance are uncoupled from shortened survival in Drosophila. Cell Metab. 31, 710–725 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis H. M., Mirzaei M., Pardey M. C., Haynes P. A., Cornish J. L., Proteomic analysis of the dorsal and ventral hippocampus of rats maintained on a high fat and refined sugar diet. Proteomics 13, 3076–3091 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Yang Z., et al. , Multi-omics comparison of the spontaneous diabetes mellitus and diet-induced prediabetic macaque models. Front. Pharmacol. 12, 784231 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu D. Y., et al. , Metabolomics analysis of human plasma reveals decreased production of trimethylamine N-oxide retards the progression of chronic kidney disease. Br. J. Pharmacol. 179, 4344–4359 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Li K. F., Tong H. H. Y., Chen Y. W., Sun Y. Z., Wang J., The emerging roles of next-generation metabolomics in critical care nutrition. Crit. Rev. Food Sci. 62, 2113761 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Kim H., Rebholz C. M., Metabolomic biomarkers of healthy dietary patterns and cardiovascular outcomes. Curr. Atheroscler. Rep. 23, 26 (2021). [DOI] [PubMed] [Google Scholar]
- 20.Fraga-Corral M., et al. , Analytical metabolomics and applications in health, environmental and food science. Crit. Rev. Anal. Chem. 52, 712–734 (2022). [DOI] [PubMed] [Google Scholar]
- 21.Masoodi M., et al. , Metabolomics and lipidomics in NAFLD: Biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastro. Hepat. 18, 835–856 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Panyard D. J., Yu B., Snyder M. P., The metabolomics of human aging: Advances, challenges, and opportunities. Sci. Adv. 8, eadd6155 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Islam M., Chen B., Spraggins J. M., Kelly R. T., Lau K. S., Use of single-cell -omic technologies to study the gastrointestinal tract and diseases, from single cell identities to patient features. Gastroenterology 159, 453–466 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang R. J., Chen B. W., Zhang H., Tu L. Y., Luan T. A., Stable isotope-based metabolic flux analysis: A robust tool for revealing toxicity pathways of emerging contaminants. Trac-Trend Anal Chem 159, 116909 (2023). [Google Scholar]
- 25.Bartman C. R., TeSlaa T., Rabinowitz J. D., Quantitative flux analysis in mammals. Nat. Metab. 3, 896–908 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali A., et al. , Single cell metabolism: Current and future trends. Metabolomics 18, 77 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng B. Q., et al. , Epoxide metabolites of arachidonate and docosahexaenoate function conversely in acute kidney injury involved in GSK3 beta signaling. Proc. Natl. Acad. Sci. U.S.A. 114, 12608–12613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y., et al. , Inhibition of soluble epoxide hydrolase attenuates a high-fat diet-mediated renal injury by activating PAX2 and AMPK. Proc. Natl. Acad. Sci. U.S.A. 116, 5154–5159 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong C., et al. , Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome P450/epoxyoctadecenoic acid axis via TLR4/Keapl/NRF2 signaling. Cancer Res. 81, 4485–4498 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Wang W. C., et al. , Targeted metabolomics identifies the cytochrome P450 monooxygenase eicosanoid pathway as a novel therapeutic target of colon tumorigenesis. Cancer Res. 79, 1822–1830 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu J. Y., et al. , Substituted phenyl groups improve the pharmacokinetic profile and anti-inflammatory effect of urea-based soluble epoxide hydrolase inhibitors in murine models. Eur. J. Pharm. Sci. 48, 619–627 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laffin M., et al. , A high-sugar diet rapidly enhances susceptibility to colitis via depletion of luminal short-chain fatty acids in mice. Sci. Rep. 9, 12294 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell S. B., et al. , Sucrose-induced hyperglycemia dysregulates intestinal zinc metabolism and integrity: Risk factors for chronic diseases. Front. Nutr. 10, e1220533 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burr A. H. P., et al. , Excess dietary sugar alters colonocyte metabolism and impairs the proliferative response to damage. Cell Mol. Gastroenterol. Hepatol. 16, 287–316 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J., et al. , Disruption of the intestinal mucosal barrier induced by high fructose and restraint stress is regulated by the intestinal microbiota and microbiota metabolites. Microbiol. Spectr. 11, e0469822 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todoric J., et al. , Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat. Metab. 2, 1034–1045 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Do M. H., Lee E., Oh M. J., Kim Y., Park H. Y., High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10, 761 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W. C., et al. , Lipidomic profiling reveals soluble epoxide hydrolase as a therapeutic target of obesity-induced colonic inflammation. Proc. Natl. Acad. Sci. U.S.A. 115, 5283–5288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y., et al. , Soluble epoxide hydrolase is an endogenous regulator of obesity-induced intestinal barrier dysfunction and bacterial translocation. Proc. Natl. Acad. Sci. U.S.A. 117, 8431–8436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulton D., Falck J. R., McGiff J. C., Carroll M. A., Quilley J., A method for the determination of 5,6-EET using the lactone as an intermediate in the formation of the diol. J. Lipid. Res. 39, 1713–1721 (1998). [PubMed] [Google Scholar]
- 41.Fu Y., et al. , High sucrose diet-induced abnormal lipid metabolism in mice is related to the dysbiosis of gut microbiota. Clin. Nutr. ESPEN 63, 491–500 (2024). [DOI] [PubMed] [Google Scholar]
- 42.Geidl-Flueck B., et al. , Fructose- and sucrose- but not glucose-sweetened beverages promote hepatic de novo lipogenesis: A randomized controlled trial. J. Hepatol. 75, 46–54 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Fattore E., Botta F., Bosetti C., Effect of fructose instead of glucose or sucrose on cardiometabolic markers: A systematic review and meta-analysis of isoenergetic intervention trials. Nutr Rev 79, 209–226 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Kim J. H., Jo Y. D., Jin C. H., Isolation of soluble epoxide hydrolase inhibitor of capsaicin analogs from Capsicum chinense Jacq. cv. Habanero. Int. J. Biol. Macromol. 135, 1202–1207 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Singh N., et al. , N-Benzyl-linoleamide, a constituent of Lepidium meyenii (Maca), is an orally bioavailable soluble epoxide hydrolase inhibitor that alleviates inflammatory pain. J. Nat. Prod. 83, 3689–3697 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All data and materials are included in the article and/or SI Appendix.