Abstract
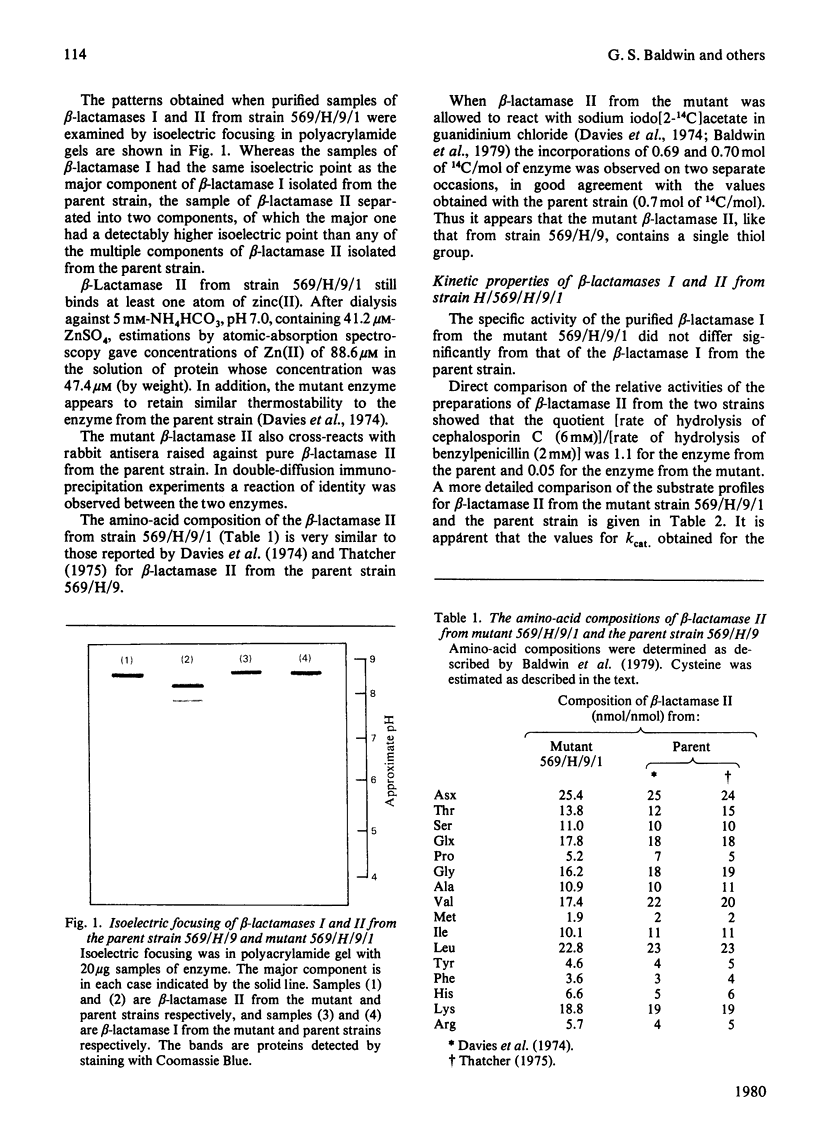
1. Mutants of Bacillus cereus 569/H/9 have been screened in a search for strains that synthesize variants of beta-lactamase II. 2. One of these mutants (strain 569/H/9/1) produces a beta-lactamase II-like enzyme that shows a selective decrease in cephalosporinase activity. 3. beta-Lactamase II from strain 569/H/9/1 has been purified to apparent homogeneity and its kinetic properties have been examined. This enzyme resembles the parent beta-lactamase II in its relative activity with benzylpenicillin as substrate when Zn(II) is replaced by other metal ions, but differs detectably from the parent enzyme in its isoelectric point.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Baldwin G. S., Galdes A., Hill H. A., Smith B. E., Waley S. G., Abraham E. P. Histidine residues of zinc ligands in beta-lactamase II. Biochem J. 1978 Nov 1;175(2):441–447. doi: 10.1042/bj1750441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin G. S., Waley S. G., Abraham E. P. Identification of histidine residues that act as zinc ligands in beta-lactamase II by differential tritium exchange. Biochem J. 1979 Jun 1;179(3):459–463. doi: 10.1042/bj1790459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Metal cofactor requirements of beta-lactamase II. Biochem J. 1974 Oct;143(1):129–135. doi: 10.1042/bj1430129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B., Abraham E. P. Separation, purification and properties of beta-lactamase I and beta-lactamase II from Bacillus cereus 569/H/9. Biochem J. 1974 Oct;143(1):115–127. doi: 10.1042/bj1430115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. B. Comparison of beta-lactamase II from Bacillus cereus 569/H/9 with a beta-lactamase from Bacillus cereus 5/B/6. Biochem J. 1975 Feb;145(2):409–411. doi: 10.1042/bj1450409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Knowles J. R. Directed selective pressure on a beta-lactamase to analyse molecular changes involved in development of enzyme function. Nature. 1976 Dec 23;264(5588):803–804. doi: 10.1038/264803a0. [DOI] [PubMed] [Google Scholar]
- Kuwabara S. Purification and properties of two extracellular beta-lactamases from Bacillus cereus 569-H. Biochem J. 1970 Jul;118(3):457–465. doi: 10.1042/bj1180457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSON E. E., POLLOCK M. R., TRIDGELL E. J. A comparison of the properties of penicillinase produced by Bacillus subtilis and Bacillus cereus with and without addition of penicillin. J Gen Microbiol. 1954 Dec;11(3):493–505. doi: 10.1099/00221287-11-3-493. [DOI] [PubMed] [Google Scholar]
- NEWTON G. G., ABRAHAM E. P. Isolation of cephalosporin C, a penicillin-like antibiotic containing D-alpha-aminoadipic acid. Biochem J. 1956 Apr;62(4):651–658. doi: 10.1042/bj0620651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. Penicillinase adaptation and fixation of penicillin sulphur by Bacillus cereus spores. J Gen Microbiol. 1953 Feb;8(1):186–194. doi: 10.1099/00221287-8-1-186. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. STIMULATING AND INHIBITING ANTIBODIES FOR BACTERIAL PENICILLINASE. Immunology. 1964 Nov;7:707–723. [PMC free article] [PubMed] [Google Scholar]
- Pollock M. R. The range and significance of variations amongst bacterial penicillinases. Ann N Y Acad Sci. 1968 Jun 14;151(1):502–515. doi: 10.1111/j.1749-6632.1968.tb11910.x. [DOI] [PubMed] [Google Scholar]
- Proceedings of the biochemical society. Biochem J. 1966 Jan;98(1):1–16P. [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. PURIFICATION AND PROPERTIES OF THE EXOPENICILLINASE FROM STAPHYLOCOCCUS AUREUS. Biochem J. 1963 Sep;88:452–459. doi: 10.1042/bj0880452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher D. R. Beta-lactamase (Bacillus cereus). Methods Enzymol. 1975;43:640–652. doi: 10.1016/0076-6879(75)43129-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



