Main text
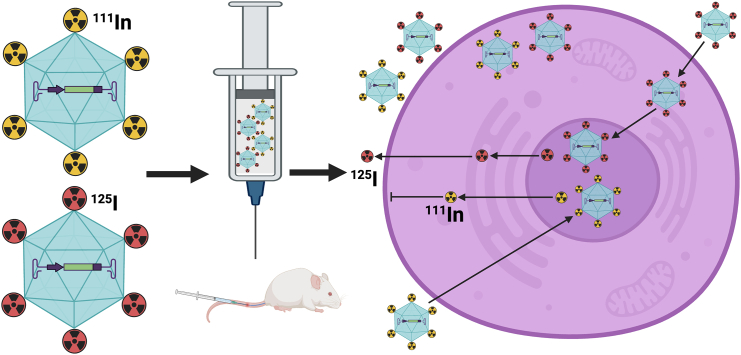
Recombinant adeno-associate virus (rAAV) gene therapy is approved for the clinical treatment of a growing number of diverse genetic diseases, including ocular, neuronal, and hematological disorders.1 Most of the rAAV capsids used in current gene therapy trials have a strong tropism for the liver after systemic delivery, which curtails the ability to effectively target non-hepatic tissues. The success of rAAV gene therapy has resulted in the isolation and engineering of many new rAAV capsid serotypes to create rAAVs that target the liver and a variety of non-hepatic tissue types.2,3 In a study published in this issue of Molecular Therapy Methods and Clinical Development, Wang et al. describe a dual capsid radiolabeling method (Figure 1) in combination with quantitative PCR (qPCR) and a reporter cassette to determine the biodistribution and pharmacokinetics of both the rAAV capsid and vector genome. This approach could be used to better characterize new and existing rAAV capsids.4 Radiolabeling of the rAAV capsid with a single isotope has been used to determine biodistribution, but this method cannot differentiate whether the vectors are intra- or extracellular.5 The dual capsid labeling this study describes was adapted from a dual labeling approach used successfully to track antibody internalization.6 The dual radiolabeling strategy utilizes the co-delivery of 125I (non-residualizing) and 111In (residualizing) radionuclide conjugated rAAVs to allow for the quantification, by single-photon emission computed tomography (SPECT), of both rAAV capsids that are cellularly internalized (degraded, 111In-125I) and those that reside in the extracellular matrix (intact,125I).
Figure 1.
rAAV capsid is labeled with either 111In or 125I tracer isotope and mixed at equal proportions prior to systemic injection into mice
The isotopes on the rAAV capsid that gain nuclear entry are degraded and the isotopes are released. The rAAV-labeled capsid that is cellularly internalized is degraded to 111In or 125I. The 111In released after degradation cannot escape the cell membrane; however, 125I can cross the cell membrane to extracellular matrix and is excreted.
In this study, the authors first demonstrated that their radioactive labeling method produces rAAV vectors with a high radioactive specificity without adversely affecting capsid integrity. Next, they demonstrated that cellular uptake and transgene expression were unaltered by labeling using an in vitro assay with two different cell lines and seven different rAAV serotypes relative to unlabeled rAAV. Lastly, the method was applied in vitro by systemically injecting mice with either dual-labeled AAV9 or AAV-PHP.eB that were radiolabeled to determine the capsid and vector biodistribution in multiple harvested organs over time. The biodistribution results observed in mice following systemic delivery indicate that rAAVs, with rapid uptake from systemic circulation, were mostly located in the extracellular matrix of the liver and spleen at early time points, which resulted in differences in the quantifications of the capsid and the vector genome. Importantly, the characterization determined by the dual labeling method agreed with historical data from these well-characterized rAAV vectors, AAV9, a naturally occurring capsid, and AAV-PHP.eB, an engineer capsid, confirming the validity of this approach.7,8
Having accurate biodistribution and transduction information for rAAV vectors is critical for the appropriate selection of the best rAAV capsid capable of targeting the affected tissue and/or cell type(s) for a specific disease. Currently, most rAAV biodistribution studies rely on the extraction of DNA and total RNA from a tissue type, followed by qPCR or digital droplet PCR of the rAAV genome and transgene mRNA to characterize an AAV vector. This approach only allows for the quantification of the total amount of rAAV genomes and mRNA transcripts in a particular tissue; it does not determine whether the rAAV vector in the tissue is intracellular or extracellular. Several other approaches have been used to characterize rAAV biodistribution and transduction, such as reporter rAAV vectors, tdTomato fluorescence/Cre-mediated recombination reporter mice, in situ hybridization with probes to detect the rAAV genomes and transcripts, and fluorescence labeling of rAAV capsids.9 To have a therapeutic effect, an rAAV needs to hone to the target tissue(s), cross the cell membrane, traffic to the nucleus, and undergo complementary strand synthesis, transcription, and translation. Then, the translated protein needs to be trafficked to the location where it is functional.10 Therefore, to accurately and thoroughly characterize the pharmacokinetics and pharmacodynamics of an rAAV gene therapy, multiple methods of characterization will be required to determine the fate of the rAAV capsid, genome, transcript, and protein product. In diseases that are cell-autonomous or cell-type-specific, rAAV characterization methods which determine the number of cells transduced and/or the cell type transduced will be vital to the selection of a suitable AAV capsid. One drawback to the dual labeling approach is it cannot determine the rAAV location at the cellular scale due to insufficient resolution and, therefore, would not be appropriate for determining cell type transduced or intracellular trafficking studies.
While the current study only applied this approach in mice, translation to large animal models, such as non-human primates, which more accurately model rAAV distribution in humans, could be a fruitful application for the dual rAAV capsid radioisotope labeling approach. In addition, this labeling strategy could allow for integration with SPECT/CT imaging, providing a noninvasive in vivo imaging method for preclinical pharmacokinetic studies. Dual rAAV capsid radioisotope labeling allows for the characterization of the rAAV capsid biodistribution of the rAAV capsid, and in combination with other approaches, it could provide more comprehensive data to determine the pharmacokinetics and pharmacodynamics of an rAAV gene therapy vector, allowing for more precise capsid selection prior to advancement to clinical trials.
Acknowledgments
R.J.C. was supported by the Intramural Research Program of the NHGRI through 1ZIAHG200318-19.
Declaration of interests
R.J.C. has no conflicts of interest to declare.
References
- 1.Wang J.H., Gessler D.J., Zhan W., Gallagher T.L., Gao G. Adeno-associated virus as a delivery vector for gene therapy of human diseases. Signal Transduct. Targeted Ther. 2024;9:78. doi: 10.1038/s41392-024-01780-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buning H., Huber A., Zhang L., Meumann N., Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr. Opin. Pharmacol. 2015;24:94–104. doi: 10.1016/j.coph.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Gao G., Vandenberghe L.H., Alvira M.R., Lu Y., Calcedo R., Zhou X., Wilson J.M. Clades of Adeno-associated viruses are widely disseminated in human tissues. J. Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Li R., Sadekar S., Kamath A.V., Shen B.-Q. A novel approach to quantitate biodistribution and transduction of adeno-associated virus gene therapy using radiolabeled AAV vectors in mice. Mol. Ther. Methods Clin. Dev. 2024;32 doi: 10.1016/j.omtm.2024.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kothari P., De B.P., He B., Chen A., Chiuchiolo M.J., Kim D., Nikolopoulou A., Amor-Coarasa A., Dyke J.P., Voss H.U., et al. Radioiodinated Capsids Facilitate In Vivo Non-Invasive Tracking of Adeno-Associated Gene Transfer Vectors. Sci. Rep. 2017;7 doi: 10.1038/srep39594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney P.L., Rogers P.E., Johnson D.K. Dual isotope study of iodine-125 and indium-111-labeled antibody in athymic mice. J. Nucl. Med. 1989;30:374–384. [PubMed] [Google Scholar]
- 7.Jang S., Shen H.K., Ding X., Miles T.F., Gradinaru V. Structural basis of receptor usage by the engineered capsid AAV-PHP.eB. Mol. Ther. Methods Clin. Dev. 2022;26:343–354. doi: 10.1016/j.omtm.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manfredsson F.P., Rising A.C., Mandel R.J. AAV9: a potential blood-brain barrier buster. Mol. Ther. 2009;17:403–405. doi: 10.1038/mt.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Lim D.A., Lawlor M.W., Dimmock D., Vite C.H., Lester T., Tavakkoli F., Sadhu C., Prasad S., Gray S.J. Biodistribution of Adeno-Associated Virus Gene Therapy Following Cerebrospinal Fluid-Directed Administration. Hum. Gene Ther. 2023;34:94–111. doi: 10.1089/hum.2022.163. [DOI] [PubMed] [Google Scholar]
- 10.Riyad J.M., Weber T. Intracellular trafficking of adeno-associated virus (AAV) vectors: challenges and future directions. Gene Ther. 2021;28:683–696. doi: 10.1038/s41434-021-00243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]