Abstract
Shaggy aorta is severe luminal surface degeneration of the aorta leading to an increased risk of plaque destabilization and embolization to the peripheral or visceral vessel beds. It represents a challenging clinical entity for both endovascular and open repair owing to potential atheroembolization, increased early morbidity and mortality, and poor long-term survival. Patients may be denied repair owing to its high risks. Herein, we present a novel approach to open repair of a juxtarenal abdominal aortic aneurysm with shaggy aorta using moderate hypothermic circulatory arrest with antegrade cerebral perfusion and concurrent flow modification to mitigate the risk of atheroma embolism.
Shaggy aorta (SA) refers to severe luminal surface degeneration of the aorta with a characteristic irregularly shaped and spiculated aorta on computed tomography angiography (CTA). It may be associated with atheromatous embolization, termed SA syndrome (SAS).1 Not surprisingly, SA is more prone to embolism-related early adverse events and poor long-term survival after both open and endovascular aortic repairs (EVARs).2, 3, 4, 5, 6, 7, 8 It has been identified as an independent predictor for spinal cord injury, cerebral and peripheral embolization, and death after aortic interventions. As such, patients with SA are often denied a repair. Herein, we present a novel approach to open abdominal aortic aneurysm (AAA) repair for a patient with SA using moderate hypothermic circulatory arrest and antegrade cerebral perfusion. Consent for publication was obtained from the patient.
Case presentation
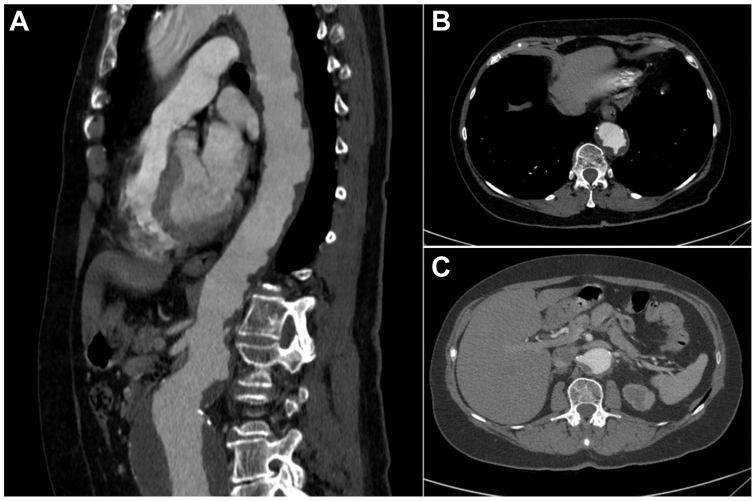
A 72-year-old woman presented with an incidentally discovered asymptomatic 7.5-cm juxtarenal AAA on CTA (Fig 1, A). The descending thoracic aorta was involved with diffuse atherosclerosis and circumferential mural thrombosis, consistent with SA (with a very high shaggy score9 of 22) along with severe stenosis of the left renal artery (Fig 1, B and C). Her past medical history was notable for chronic obstructive pulmonary disease secondary to her extensive tobacco use (>50 pack-years), hypertension, coronary artery disease, and hypothyroidism. Although her activities were limited owing to chronic obstructive pulmonary disease, she was otherwise functional and able to perform her daily activities of living.
Fig 1.
Preoperative computed tomography reconstructions of descending and abdominal aorta with extensive shaggy morphology and abdominal aneurysm in (A) sagittal view, (B) axial view of descending thoracic aorta, and (C) axial view of infrarenal aorta with severe stenosis of left renal artery.
Owing to the extensive atheromatous changes of her aorta with shaggy score of 22, she was deemed a poor candidate for either EVAR or open surgical repair (OSR). The atheroma burden in the paravisceral and descending thoracic aorta prevented any aortic clamping. After discussion at a multidisciplinary aortic center conference with our cardiac surgery colleagues, the joint decision was made to proceed with OSR using total cardiopulmonary bypass (CPB) with moderate hypothermic circulatory arrest (MHCA) and antegrade cerebral perfusion (ACP) to mitigate the risk of atheroembolization and neuroischemic complications. Right axillary artery-common femoral vein circuit was chosen for CPB because it also allows ACP during circulatory arrest. Common femoral artery cannulation was to be avoided to prevent retrograde flow and the attendant risk of cerebral embolism, especially in this patient with SA. Back-bleeding into the aorta during ACP would be controlled by clamping the innominate and the left common carotid artery via suprasternal exposure of these vessels.
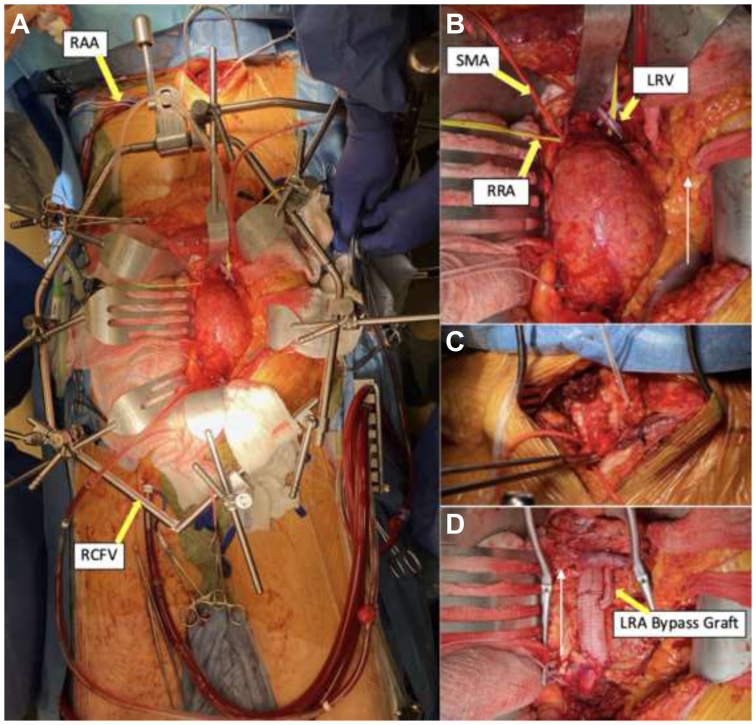
Through a transperitoneal midline abdominal incision, the supra-superior mesenteric artery (SMA) aorta, SMA, and bilateral renal and bilateral common iliac arteries were isolated and dissected free (Fig 2, A and B). Due precautions were taken during dissection of the pararenal and suprarenal aorta to minimize the risk of atheroembolism. The left renal vein was preserved and mobilized fully by ligating the left adrenal and lumbar veins. The right common femoral vein was accessed percutaneously and a 22F cannula placed at the atriocaval junction under echocardiographic guidance (Fig 2, A).
Fig 2.
(A) Complete operative exposure with bypass circuit (RCFV access and RAA), suprasternal, and midline transperitoneal incisions. (B) Abdominal aortic exposure through transperitoneal approach (white arrow notates cephalad direction). (C) Close up of suprasternal incision used to obtain control of the great vessels to prevent backbleeding. (D) Completion of aortic repair with left renal bypass (white arrow notates cephalad direction). RAA, Right axillary artery; RCFV, right common femoral vein; RRA, right renal artery; LRV, left renal vein; SMA, superior mesenteric artery.
Under full heparinization, the right axillary artery was cannulated with an 18F cannula. The innominate and left common carotid arteries were exposed through a suprasternal incision (Fig 2, C). The patient was placed on CPB and systemically cooled down to bladder temperature of 22°C. After 35 minutes of cooling, MHCA with ACP was initiated by clamping the takeoff of the innominate artery and the left common carotid artery. The flow was decreased to approximately 1 L/min. No changes were noted with cerebral oximetry.
Control was obtained of the SMA and bilateral renal arteries to prevent atheroembolism during removal of the atheroma. The aorta was opened and transected between the right and left renal arteries (the right was more proximal of the two). Endarterectomy of the pararenal aorta was effected and the friable atheromatous debris from the paravisceral aorta removed manually and with suction evacuation. A 20-mm woven polyester graft was sewn onto the aorta incorporating the right renal artery into the anastomosis. After de-airing, the graft was clamped and CPB flow rate returned to normal with full rewarming instituted after an appropriate period of cold reperfusion. The remainder of aortic reconstruction was effected in the standard fashion, including the left renal artery bypass grafting with a 6-mm graft (Fig 2, D).
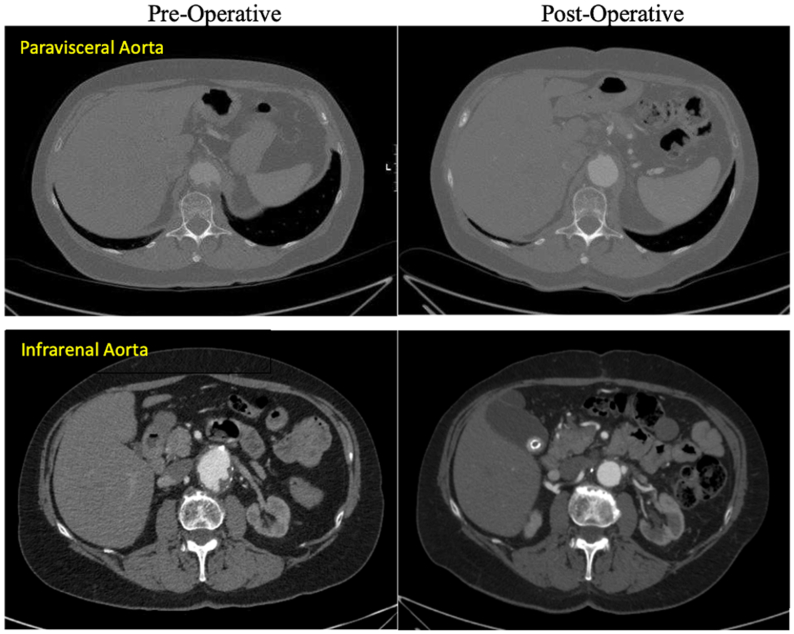
The total circulatory arrest time was 25 minutes and pump time was 162 minutes. Her postoperative course was uneventful with no evidence of atheroembolism or neurological complications. She was discharged to home on postoperative day 7. At the 9-month follow-up, she was clinically well and abstaining from cigarette use since the operation. CTA showed intact repair with notably atheroma-free paravisceral aorta and patent renal bypass graft (Fig 3).
Fig 3.
Comparison of preoperative (left) and 6 months postoperative (right) computed tomography angiography (CTA) (axial views) showing atheroma-free paravisceral abdominal aorta with patent renal bypass graft.
Discussion
This case report, to the authors' knowledge, is the first to describe the use of MHCA with ACP during OSR of juxtarenal AAA with SA. MHCA with ACP obviates the need for aortic cross-clamping, thereby mitigating the risk of atheroma destabilization and embolization while maintaining brain perfusion.
Kazmier first coined the term SA in 19891,2 to describe the peculiar aorta with soft, mobile, and grumous atheroma that may be complicated by spontaneous peripheral and visceral atheromatous embolization, known as SAS. SAS needs to be differentiated from other embolic disease processes that can be readily managed, including (1) embolic lesion from the atrium, or aneurysms that can be managed by thrombectomy and anticoagulation or aneurysm repair and (2) blue toe syndrome, which often results from isolated atherosclerotic ulcerative lesion often found in the superficial femoral artery that can also be treated surgically. In contrast, there is no effective treatment for SAS.8 Anticoagulation therapy is thought to be contraindicated for SAS.2,10, 11, 12, 13, 14 Statins have been shown to be effective in atheroma regression in AAA.15 However, the rapidity and extent of regression to allow aortic cross clamping remains to be seen.
Furthermore, surgical outcomes for patients with SA are inferior to those without.2, 3, 4, 5, 6, 7 SA has been identified as an independent risk factor for stroke, spinal cord ischemia (SCI), renal and mesenteric embolization, lower extremity embolization, skin infarction, and death after aortic interventions, either open or endovascular.3, 4, 5, 6, 7, 8,16,17 Aortic manipulation, aortic cross-clamping and unclamping during OSR, and instrumentation with wires, catheters, sheaths, and devices during EVAR can cause destabilization and embolization of the atheroma. It also seems that higher the atheroma burden, higher the risk of postoperative adverse events.3,7,9,18 Maeda et al9 reported a statistically higher shaggy score (7.9 vs 2.0) in patients who suffered embolic complications after TEVAR. Similarly, Huynh et al7 observed a higher shaggy score (9.3 vs 4.1; P = .06) in patients who experienced SCI after branched EVAR.
Although the risks of embolization are significant with both EVAR and OSR, open repair is thought to be preferable to endovascular if a repair was needed.2,17,19 To mitigate the risk of embolic complications, several intraoperative adjunctive maneuvers or techniques have been used successfully for thoracic aortic pathologies with SA, including MHCA with ACP, intermittent visceral and/or carotid artery clamping, and extracorporeal circulation circuit to filter the atheromatous debris.6,20,21 However, no adjunctive maneuvers or techniques have been reported for abdominal aortic pathologies with SA and patients are often denied a repair.22,23
MHCA and ACP proved to be an innovative approach to mitigate the risk of atheroembolic event during OSR of AAA for our patient who was deemed to have prohibitive risk for EVAR with shaggy score of 22 and not a candidate for a standard OSR. CPB with MHCA and ACP offers several advantages: (1) obviate the need to cross clamp the aorta; (2) allow a bloodless field to inspect and remove atheroma; and (3) protect the central nervous system by maintaining cerebral perfusion and hypothermic spinal cord protection. The protective effects of MHCA against SCI during TAAA repair have been well demonstrated by Kulik et al.24
Conclusions
This report demonstrates that MHCA with ACP may provide an effective means of mitigating the risk of atheroembolization during open repair of AAA with SA in select patients in whom traditional OSR or EVAR carries prohibitive risk of embolic complications.
Disclosures
None.
From the Eastern Vascular Society
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kazmier F.J. In: Aortic surgery. Bergan J.J.Y.J.S.T., editor. W. B. Saunders; 1989. Shaggy aorta sydrome and disseminated atheromatous embolization. [Google Scholar]
- 2.Hollier L.H., Kazmier F.J., Ochsner J., Bowen J.C., Procter C.D. "Shaggy" aorta syndrome with atheromatous embolization to visceral vessels. Ann Vasc Surg. 1991;5:439–444. doi: 10.1007/BF02133048. [DOI] [PubMed] [Google Scholar]
- 3.Patel S.D., Constantinou J., Hamilton H., Davis M., Ivancev K. Editor's choice - A shaggy aorta is associated with mesenteric embolisation in patients undergoing fenestrated endografts to treat paravisceral aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47:374–379. doi: 10.1016/j.ejvs.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Kwon H., Han Y., Noh M., Gwon J.G., Cho Y.P., Kwon T.W. Impact of shaggy aorta in patients with abdominal aortic aneurysm following open or endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2016;52:613–619. doi: 10.1016/j.ejvs.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Yokawa K., Ikeno Y., Henmi S., Yamanaka K., Okada K., Okita Y. Impact of shaggy aorta on outcomes of open thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg. 2020;160:889–897.e1. doi: 10.1016/j.jtcvs.2019.07.112. [DOI] [PubMed] [Google Scholar]
- 6.Serra R., Bracale U.M., Jiritano F., et al. The shaggy aorta syndrome: an updated review. Ann Vasc Surg. 2021;70:528–541. doi: 10.1016/j.avsg.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Huynh C., Liu I., Sommer A., et al. Descending thoracic aortic mural ulceration is associated with postoperative spinal cord ischemia after branched endovascular aortic aneurysm repair. J Vasc Surg. 2024;79:732–739. doi: 10.1016/j.jvs.2023.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Shintani T., Mitsuoka H., Hasegawa Y., et al. Effect of atheromatous aorta on thromboembolic complications after endovascular aortic aneurysm. Ann Vasc Dis. 2020;13:273–280. doi: 10.3400/avd.oa.20-00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda K., Ohki T., Kanaoka Y., Shukuzawa K., Baba T., Momose M. A novel shaggy aorta scoring system to predict embolic complications following thoracic endovascular aneurysm repair. Eur J Vasc Endovasc Surg. 2020;60:57–66. doi: 10.1016/j.ejvs.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Kazmier F.J., Sheps S.G., Bernatz P.E., Sayre G.P. Livedo reticularis and digital infarcts: a syndrome due to cholesterol emboli arising from atheromatous abdominal aortic aneurysms. Vasc Dis. 1966;3:12–24. [PubMed] [Google Scholar]
- 11.Moldveen-Geronimus M., Merriam J.C., Jr. Cholesterol embolization. From pathological curiosity to clinical entity. Circulation. 1967;35:946–953. doi: 10.1161/01.cir.35.5.946. [DOI] [PubMed] [Google Scholar]
- 12.Bruns F.J., Segel D.P., Adler S. Control of cholesterol embolization by discontinuation of anticoagulant therapy. Am J Med Sci. 1978;275:105–108. doi: 10.1097/00000441-197801000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Kazmier F.J. Current practice with vitamin K antagonist therapy. Mayo Clin Proc. 1974;49:918–922. [PubMed] [Google Scholar]
- 14.Hyman B.T., Landas S.K., Ashman R.F., Schelper R.L., Robinson R.A. Warfarin-related purple toes syndrome and cholesterol microembolization. Am J Med. 1987;82:1233–1237. doi: 10.1016/0002-9343(87)90231-2. [DOI] [PubMed] [Google Scholar]
- 15.Nemoto M., Hoshina K., Takayama T., et al. Statins reduce extensive aortic atheromas in patients with abdominal aortic aneurysms. Ann Vasc Dis. 2013;6:711–717. doi: 10.3400/avd.oa.13-00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinaldi E., Loschi D., Santoro A., et al. A comparison of thoracoabdominal aortic aneurysms open repair in patients with or without "shaggy aorta". J Vasc Surg. 2023;77:347–356.e2. doi: 10.1016/j.jvs.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Hoshina K., Hosaka A., Takayama T., et al. Outcomes after open surgery and endovascular aneurysm repair for abdominal aortic aneurysm in patients with massive neck atheroma. Eur J Vasc Endovasc Surg. 2012;43:257–261. doi: 10.1016/j.ejvs.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Katsanos A.H., Giannopoulos S., Kosmidou M., et al. Complex atheromatous plaques in the descending aorta and the risk of stroke: a systematic review and meta-analysis. Stroke. 2014;45:1764–1770. doi: 10.1161/STROKEAHA.114.005190. [DOI] [PubMed] [Google Scholar]
- 19.Zempo N., Sakano H., Ikenaga S., et al. Fatal diffuse atheromatous embolization following endovascular grafting for an abdominal aortic aneurysm: report of a case. Surg Today. 2001;31:269–273. doi: 10.1007/s005950170185. [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T., Takase S., Satokawa H., Misawa Y., Wakamatsu H., Yokoyama H. Thoracic endovascular aortic repair with visceral arteries intermittent clamp technique for descending thoracic aortic aneurysm with shaggy aorta. Ann Vasc Surg. 2013;27:974.e11–974.e14. doi: 10.1016/j.avsg.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Takano T., Takase S., Kagoshima A., Yokoyama H. Prevention of embolization in concomitant endovascular therapy for thoracic and abdominal aneurysms with severely atheromatous aorta. Ann Vasc Dis. 2016;9:345–348. doi: 10.3400/avd.cr.16-00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wada H., Nishimura M., Matsumura H., Yamamoto S., Sekine Y., Hosoda Y. Risk factors in the treatment of abdominal aortic aneurysms in the endovascular ERA. Ann Thorac Cardiovasc Surg. 2014;20:299–303. doi: 10.5761/atcs.oa.12.02241. [DOI] [PubMed] [Google Scholar]
- 23.Coscas R., Maumias T., Capdevila C., Javerliat I., Goeau-Brissonniere O., Coggia M. Mini-invasive treatment of abdominal aortic aneurysms: current roles of endovascular, laparoscopic, and open techniques. Ann Vasc Surg. 2014;28:123–131. doi: 10.1016/j.avsg.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Kulik A., Castner C.F., Kouchoukos N.T. Outcomes after thoracoabdominal aortic aneurysm repair with hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141:953–960. doi: 10.1016/j.jtcvs.2010.06.010. [DOI] [PubMed] [Google Scholar]