Abstract
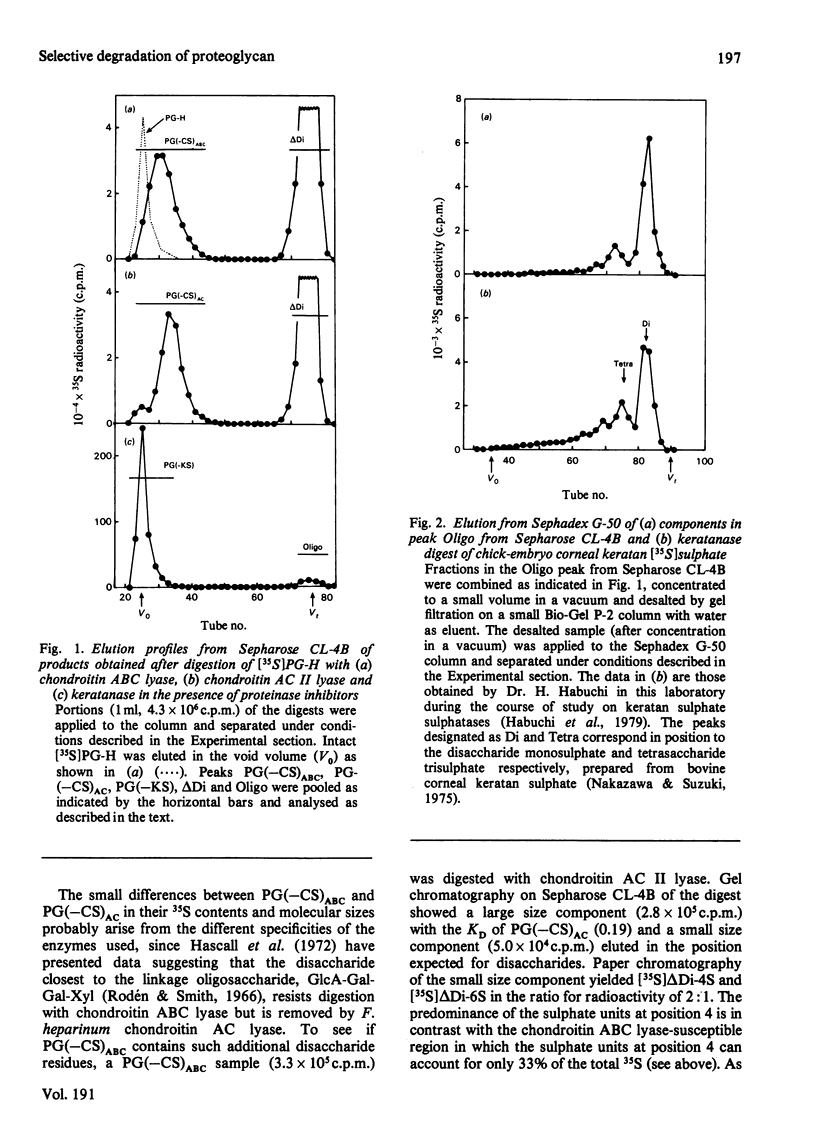
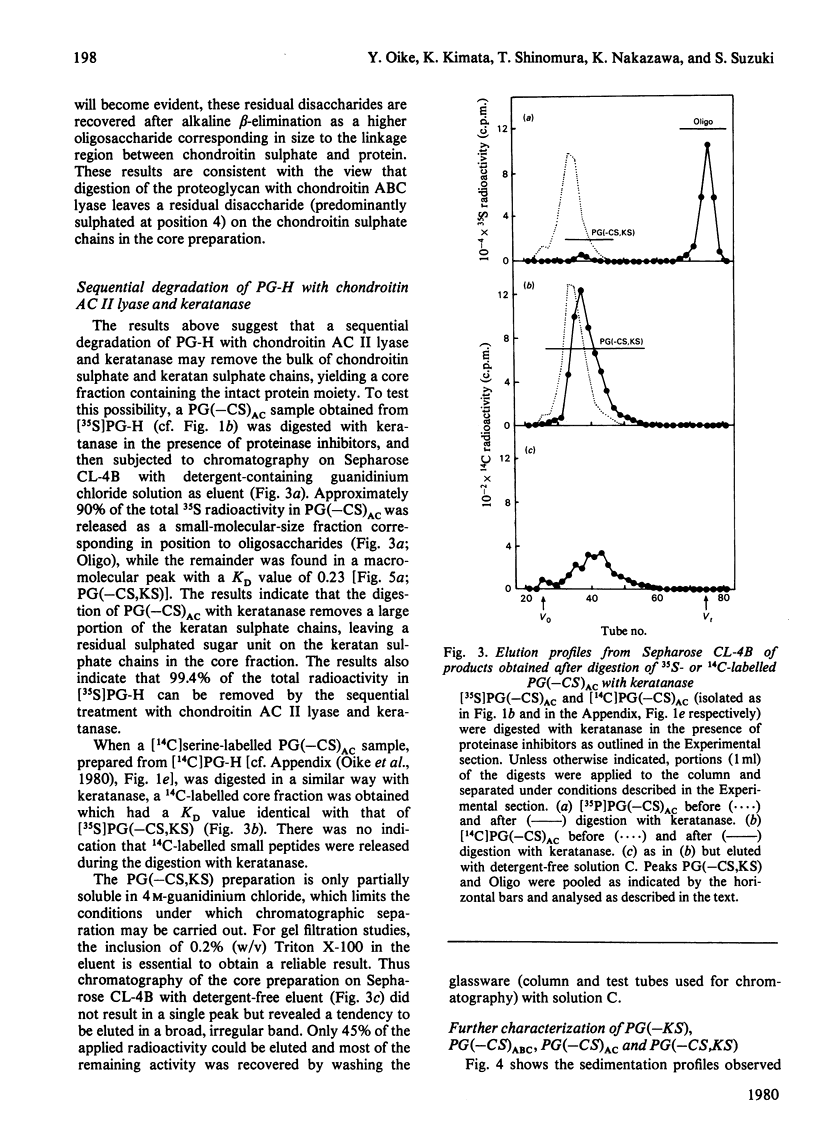
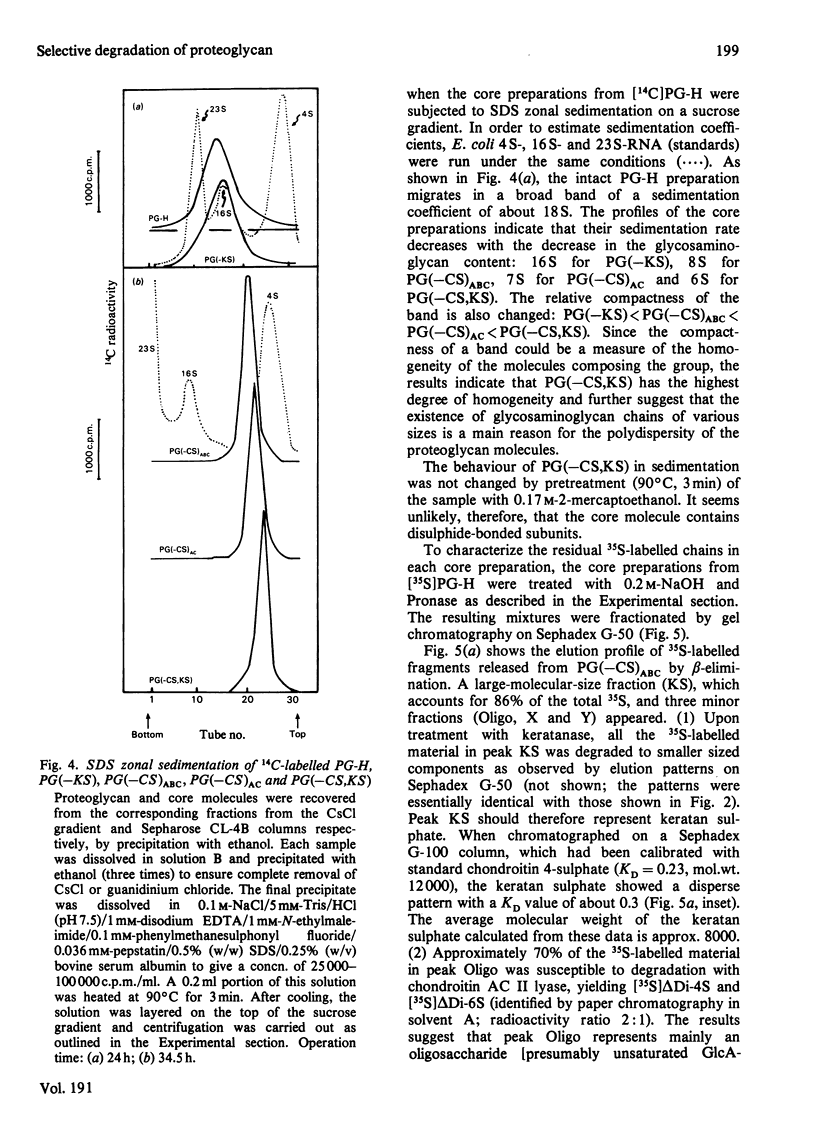
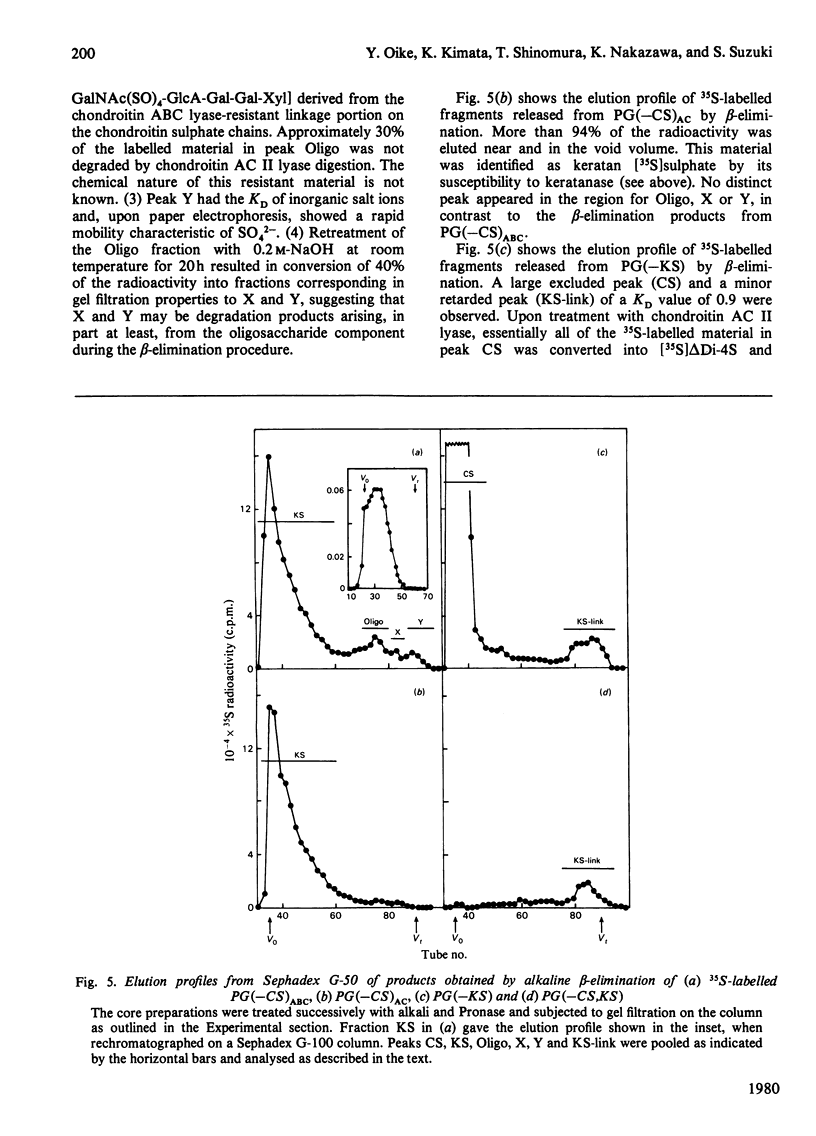
Digestion of chick-embryo cartilage proteoglycan (type H) with chondroitin AC II lyase or keratanase, in the presence of EDTA, N-ethylmaleimide, phenylmethanesulphonyl fluoride and pepstatin, resulted in the removal of the bulk of the chondroitin sulphate or keratan sulphate chains respectively, without altering the protein portion of the macromolecule. An exhaustive treatment of the proteoglycan with chondroitin AC II lyase followed by digestion with keratanase yielded a core fraction having the enzymically modified linkage oligosaccharides. Zonal sedimentation of this core preparation on a sucrose gradient in 0.5% SDS resulted in a single narrow band with a sedimentation coefficient of 6S. In 4 M-guanidinium chloride, the core preparation showed a tendency to aggregate to multiple-molecular-weight forms which could dissociate in the presence of Triton X-100. The results indicate that the preponderance of glycosaminoglycans in the proteoglycan molecule is a main reason for both polydispersity and hydrophilicity of the proteoglycan preparation, and further suggest that the enzymic procedures could prove useful as a method to obtain new information about the structure and properties of proteoglycan core molecules.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Baxter E., Muir H. The nature of the protein moieties of cartilage proteoglycans of pig and ox. Biochem J. 1975 Sep;149(3):657–668. doi: 10.1042/bj1490657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca S., Heinegård D., Hascall V. C., Kimura J. H., Caplan A. I. Chemical and physical changes in proteoglycans during development of chick limb bud chondrocytes grown in vitro. J Biol Chem. 1977 Oct 10;252(19):6600–6608. [PubMed] [Google Scholar]
- Goetinck P. F., Pennypacker J. P., Royal P. D. Proteochondroitin sulfate synthesis and chondrogenic expression. Exp Cell Res. 1974 Aug;87(2):241–248. doi: 10.1016/0014-4827(74)90476-5. [DOI] [PubMed] [Google Scholar]
- Habuchi H., Tsuji M., Nakanishi Y., Suzuki S. Separation and properties of five glycosaminoglycan sulfatases from rat skin. J Biol Chem. 1979 Aug 25;254(16):7570–7578. [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L. Characteristics of the protein-keratan sulfate core and of keratan sulfate prepared from bovine nasal cartilage proteoglycan. J Biol Chem. 1972 Jul 25;247(14):4529–4538. [PubMed] [Google Scholar]
- Hascall V. C., Riolo R. L., Hayward J., Jr, Reynolds C. C. Treatment of bovine nasal cartilage proteoglycan with chondroitinases from Flavobacterium heparinum and Proteus vulgaris. J Biol Chem. 1972 Jul 25;247(14):4521–4528. [PubMed] [Google Scholar]
- Heinegård D. Hyaluronidase digestion and alkaline treatment of bovine tracheal cartilage proteoglycans. Isolation and characterisation of different keratan sulfate proteins. Biochim Biophys Acta. 1972 Nov 28;285(1):193–207. doi: 10.1016/0005-2795(72)90191-2. [DOI] [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. Studies on the polydispersity and heterogeneity of cartilage proteoglycans. Identification of 3 proteoglycan structures in bovine nasal cartilage. Biochem J. 1975 Dec;151(3):581–594. doi: 10.1042/bj1510581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Robinson H. C. The alkali-labile linkage between keratan sulphate and protein. Biochem J. 1974 Jul;141(1):57–69. doi: 10.1042/bj1410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa K., Kimata K., Ito K., Kato Y., Suzuki S. Morphological and biochemical differentiation of limb bud cells cultured in chemically defined medium. Dev Biol. 1979 Jun;70(2):287–305. doi: 10.1016/0012-1606(79)90029-0. [DOI] [PubMed] [Google Scholar]
- Kato Y., Kimata K., Ito K., Karasawa K., Suzuki S. Effect of beta-D-xyloside and cycloheximide on the synthesis of two types of proteochondroitin sulfate in chick embryo cartilage. J Biol Chem. 1978 Apr 25;253(8):2784–2789. [PubMed] [Google Scholar]
- Kimata K., Oike Y., Ito K., Karasawa K., Suzuki S. The occurrence of low buoyant density proteoglycans in embryonic chick cartilage. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1431–1439. doi: 10.1016/0006-291x(78)91163-4. [DOI] [PubMed] [Google Scholar]
- Kimura J. H., Osdoby P., Caplan A. I., Hascall V. C. Electron microscopic and biochemical studies of proteoglycan polydispersity in chick limb bud chondrocyte cultures. J Biol Chem. 1978 Jul 10;253(13):4721–4729. [PubMed] [Google Scholar]
- Kitamura K., Yamagata T. The occurrence of a new type of proteochondroitin sulfate in the developing chick embryo. FEBS Lett. 1976 Dec 1;71(2):337–340. doi: 10.1016/0014-5793(76)80965-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lohmander L. S., Hascall V. C., Caplan A. I. Effects of 4-methyl umbelliferyl-beta-D-xylopyranoside on chondrogenesis and proteoglycan synthesis in chick limb bud mesenchymal cell cultures. J Biol Chem. 1979 Oct 25;254(20):10551–10561. [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Suzuki S. Proteinase activity in chondroitin lyase (chondroitinase) and endo-beta-D-galactosidase (keratanase) preparations and a method to abolish their proteolytic effect on proteoglycan. Biochem J. 1980 Oct 1;191(1):203–207. doi: 10.1042/bj1910203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama M., Pacifici M., Holtzer H. Differences among sulfated proteoglycans synthesized in nonchondrogenic cells, presumptive chondroblasts, and chondroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3224–3228. doi: 10.1073/pnas.73.9.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M. Immunoglobulin M biosynthesis. Production of intermediates and excess of light-chain in mouse myeloma MOPC 104E. Biochem J. 1971 Jul;123(4):635–641. doi: 10.1042/bj1230635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. The stability of bovine nasal cartilage proteoglycans during isolation and storage. Biochim Biophys Acta. 1977 Jun 23;498(1):176–188. doi: 10.1016/0304-4165(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Wolfenstein-Todel C., Margolis R., Pal S., Strider W. Proteoglycans from bovine proximal humeral articular cartilage. Structural basis for the polydispersity of proteoglycan subunit. J Biol Chem. 1976 Oct 25;251(20):6439–6444. [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Upholt W. B., Vertel B. M., Dorfman A. Translation and characterization of messenger RNAs in differentiating chicken cartilage. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4847–4851. doi: 10.1073/pnas.76.10.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]


