Abstract
Background and Aims
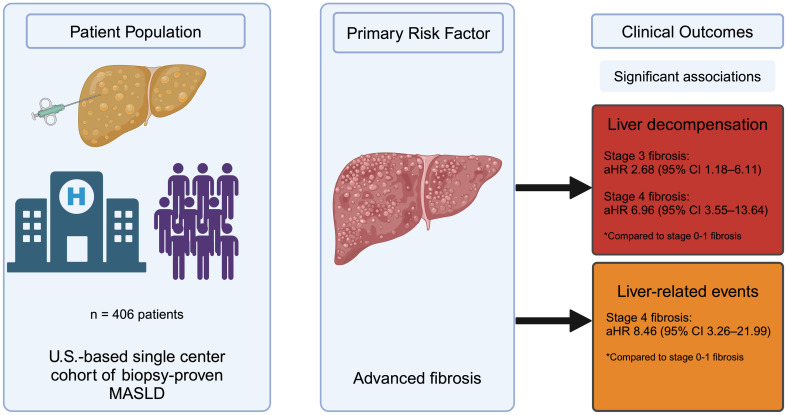
Data regarding risk factors and long-term outcomes of U.S. patients with biopsy-proven metabolic dysfunction-associated steatotic liver disease (MASLD) are limited. This study aimed to investigate the role of clinical and histologic risk factors on long-term outcomes in patients with MASLD.
Methods
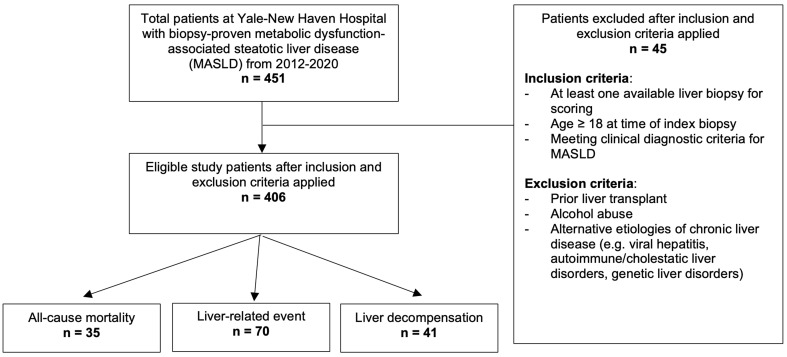
A retrospective cohort study of 451 adults with biopsy-proven MASLD was conducted at a U.S. academic hospital from 2012 to 2020. An experienced pathologist evaluated the index liver biopsy. Patients with a prior liver transplant or alternative etiologies of chronic liver disease were excluded. The duration of the risk exposure was determined from the date of the index liver biopsy to an outcome event or the last follow-up examination. Outcome events of interest included incident liver-related events, liver decompensation, and all-cause mortality.
Results
In the final cohort of 406 patients followed for a median of 3.7 years (interquartile range: 4.8 years), 35 patients died, 41 developed hepatic decompensation, and 70 experienced a liver-related event. Among histologic risk factors, stage 3 (adjusted Hazard ratio (aHR) 2.68, 95% confidence interval (CI) 1.18–6.11) and stage 4 (aHR 6.96, 95% CI 3.55–13.64) fibrosis were associated with incident liver-related events compared to stage 0–1 fibrosis. Stage 4 (aHR 8.46, 95% CI 3.26–21.99) fibrosis alone was associated with incident liver decompensation events compared to stage 0–1 fibrosis. Among clinical risk factors, hypertension (aHR 2.58, 95% CI 1.05–6.34) was associated with incident liver decompensation.
Conclusions
In a U.S. single-center cohort of patients with biopsy-proven MASLD, advanced fibrosis was the primary risk factor for incident liver decompensation and liver-related events.
Keywords: Liver fibrosis, Metabolic dysfunction-associated steatotic liver disease, Liver decompensation, Liver event, Long-term outcomes, Risk factor, Histology
Graphical abstract
Introduction
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is the most common cause of chronic liver disease and a leading cause of liver transplantation in the U.S.1–3 The prevalence of MASLD is projected to increase to 100.9 million affected individuals in the U.S. by 2030, growing in parallel with the epidemic of metabolic syndrome, including obesity and type 2 diabetes.4 MASLD also contributes to a substantial national economic burden, with $103 billion spent annually on direct medical costs in the U.S.5
Liver biopsy is considered the gold standard for diagnosing and grading the severity of MASLD, which ranges from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), with or without fibrosis.6,7 The fibrosis stage is one of the most important prognostic factors in patients with MASLD—an increasing fibrosis stage is associated with a higher risk of overall mortality and liver-related outcomes such as hepatocellular carcinoma and hepatic decompensation.8–11
Understanding risk factors with prognostic implications can help stratify patients who may benefit from early specialty referral and MASH-directed therapy. Most previous studies investigating clinical outcomes in patients with biopsy-confirmed MASLD have been based on international populations, either through pooled data from multiple countries or single-country studies outside the U.S.9,12–15 Data on long-term outcomes in U.S.-based populations with biopsy-confirmed MASLD remain limited. MASLD is a complex and heterogeneous condition that can vary geographically due to differences in ethnicity, diet, and metabolic comorbidities.16,17 Therefore, the goal of this study was to investigate the role of clinical and histologic risk factors on the long-term prognosis of U.S. patients with biopsy-proven MASLD.
Methods
Study design and data source
We conducted a retrospective cohort study of patients with biopsy-confirmed MASLD at Yale-New Haven Hospital (YNHH), a large U.S. academic medical center. YNHH uses an EPIC electronic medical record system which was initiated in early 2010, with full adoption across hospital services by 2012. EPIC contains comprehensive data on demographics, medical diagnoses, social and family history, medications, and laboratory results. Our study utilized the YNHH pathology database, which contains documentation of all liver biopsy specimens, pathology reports, and pathology slides. The study was approved by the Institutional Review Board of Yale University. The reporting of this study followed the STROBE guidelines.
Study population and selection criteria
The source cohort was identified by reviewing and retrieving original biopsy slides from the YNHH pathology database for patients with a biopsy diagnosis keyword of “steatosis” or “steatohepatitis” between January 2012 and December 2020. An experienced gastrointestinal pathologist (D.J.), who was unaware of the patient’s clinical and laboratory data, reviewed the obtained biopsy slides to assess if the original report findings were consistent with a histologic diagnosis of MASLD. Each patient’s clinical chart was then thoroughly reviewed for imaging, serologic workup, and clinical documentation to verify a diagnosis of clinical MASLD without alternative etiology. Patients with reported weekly alcohol consumption exceeding 140 g for women and 210 grams for men at the time of the index biopsy and during follow-up were categorized as having alcohol-associated fatty liver disease and were excluded. Additional exclusion criteria included a prior history of liver transplant or alternative etiologies of chronic liver disease, such as viral hepatitis, autoimmune or cholestatic liver disorders, and genetic or metabolic liver disorders. All included patients were aged ≥18 years at the time of the index biopsy and had at least one adequate liver biopsy for scoring.
Histopathology evaluation
All index liver biopsy slides for eligible study patients were reviewed and scored again by our gastrointestinal pathologist (D.J.). The index liver biopsy was defined as the patient’s first liver biopsy if they had undergone multiple biopsies. A minimum of 5% hepatocyte steatosis was required on the index biopsy for a MASLD diagnosis. Liver biopsies were scored for various histologic features based on NASH Clinical Research Network criteria, including fibrosis stage (0–4), steatosis (0–3), lobular inflammation (0–3), portal inflammation (0–3), ballooning (0–2), and Mallory bodies (0–2).18 The NAFLD activity score was the sum of scores for steatosis, lobular inflammation, and ballooning.
Baseline characteristics and laboratory data
Baseline demographics, anthropometrics, medical comorbidities, and medication data were extracted from patient charts at the time of the index liver biopsy. Diagnoses of medical comorbidities were identified using ICD-9 and ICD-10 codes. A former smoker was defined as an adult who had smoked at least 100 cigarettes in their lifetime but had quit smoking at least 28 days prior to the index biopsy. BMI was calculated as weight (in kg) divided by height (in meters2). Medications were grouped into classes, specifically statins, metformin, thiazolidinediones, GLP-1 agonists, DPP-4 inhibitors, and vitamin E. Laboratory results within a one-month period closest to the index liver biopsy were considered baseline values. These included routine liver function tests (alanine transaminase, aspartate transaminase, alkaline phosphatase, total bilirubin, direct bilirubin, and albumin), complete blood count, fasting lipids, and hemoglobin A1C.
Outcome events
The primary outcome events of interest were any liver-related event, defined as liver decompensation, hepatopulmonary syndrome, development of varices, hepatocellular carcinoma, or liver-related death; liver decompensation, defined as ascites, variceal hemorrhage, hepatic encephalopathy, spontaneous bacterial peritonitis, or hepatorenal syndrome; and all-cause mortality. Individual outcome events and their dates were obtained from the patient charts based on ICD-9 and ICD-10 codes. Events occurring within 90 days of the index biopsy were excluded.
Exposure and follow-up
Risk exposure was determined from the date of the index liver biopsy to the earliest occurrence of an outcome event or the last follow-up examination. All-cause mortality was determined using the date of death recorded in the patient chart.
Statistical analysis
Analyses were performed using SAS software (version 9.4, SAS Institute, Cary, NC). For descriptive analyses, we reported medians and interquartile ranges for continuous variables and frequencies and percentages for categorical variables unless otherwise specified. Kaplan-Meier analysis was used to estimate cumulative survival probabilities for patients in each fibrosis stage, with comparisons among groups performed using the Log-rank test. Given that all-cause mortality is a competing risk for liver-related events and liver decompensation, cumulative incidence functions were plotted for liver-related events and decompensation, and comparisons among fibrosis groups were made using Fine and Grey’s test. We performed cause-specific multivariable Cox regression analysis to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for risk factors associated with time-to-event outcomes. Variables were chosen for inclusion in the multivariable model based on univariable analysis findings, frontline clinical experience, and review of published data. p-values less than 0.05 were considered statistically significant.
Results
Demographics and baseline laboratory studies
A flow diagram of the study selection is shown in Figure 1. A total of 451 patients with biopsy-proven MASLD at YNHH were identified during the study period. After applying inclusion and exclusion criteria, the final study cohort consisted of 406 patients (Table 1). The cohort had a median age of 54 years (interquartile range [IQR] 20.4) and a median BMI of 33 (IQR 11). Patients were predominantly female (58.2%), White (76.3%), and never smokers (57.4%). The most common medical comorbidities included obesity (69%), hypertension (60.3%), and type 2 diabetes mellitus (49.8%). Laboratory results showed median serum ALT levels (48 mg/dL, IQR 51 mg/dL) greater than AST (42 mg/dL, IQR 34 mg/dL), with elevated hemoglobin A1C (6.3%, IQR 2.2%). Median platelet count (221.5 × 103 platelets/µL, IQR 97.5 × 103 platelets/µL), international normalized ratio (1.0, IQR 0.2), and albumin levels (4.3 g/dL, IQR 0.6 g/dL) were within normal ranges.
Fig. 1. The flow of patients through the study.
Table 1. Summary of baseline characteristics of the patient cohort with biopsy-proven metabolic dysfunction-associated steatotic liver disease (MASLD).
| Study cohort (n = 406) | |
|---|---|
| Demographics | |
| Age, median (IQR) | 54 (20.4) |
| Male sex, n (%) | 170 (41.8%) |
| Race, n (%) | |
| White | 310 (76.3%) |
| Black | 20 (4.9%) |
| Asian | 9 (2.2%) |
| Unknown | 68 (16.7%) |
| Ethnicity, n (%) | |
| Hispanic | 101 (24.8%) |
| Non-Hispanic | 298 (73.3%) |
| Unknown | 8 (2%) |
| Smoking | |
| Current | 40 (9.8%) |
| Former | 131 (32.3%) |
| Never | 233 (57.4%) |
| Unknown | 3 (0.7%) |
| BMI, median (IQR) | 33 (11) |
| Medical comorbidities | |
| Hypertension, n (%) | 245 (60.3%) |
| Overweight, n (%) | 95 (23.4%) |
| Obese, n (%) | 280 (69%) |
| Type 2 diabetes mellitus, n (%) | 202 (49.8%) |
| Coronary artery disease, n (%) | 36 (8.8%) |
| Chronic kidney disease, n (%) | 30 (7.4%) |
| Congestive heart failure, n (%) | 15 (3.69%) |
| Obstructive sleep apnea, n (%) | 94 (23.1%) |
| Medications | |
| Statin, n (%) | 168 (41.4%) |
| Metformin, n (%) | 39 (9.6%) |
| Thiazolidinedione, n (%) | 5 (1.2%) |
| GLP-1 agonist, n (%) | 21 (5.2%) |
| DPP-4 inhibitor, n (%) | 8 (2%) |
| Vitamin E, n (%) | 8 (2%) |
| Laboratory data | |
| Alanine transaminase (ALT), median (IQR) | 48 mg/dL (51 mg/dL) |
| Aspartate transaminase (AST), median (IQR) | 42 mg/dL (34 mg/dL) |
| Alkaline phosphatase, median (IQR) | 84 mg/dL (43 mg/dL) |
| Albumin, median (IQR) | 4.3 g/dL (0.6g/dL) |
| Total bilirubin, median (IQR) | 0.5 mg/dL (0.4 mg/dL) |
| Direct bilirubin, median (IQR) | 0.2 mg/dL (0.1 mg/dL) |
| Hemoglobin A1C, median (IQR) | 6.3% (2.2%) |
| International normalized ratio (INR), median (IQR) | 1.0 (0.2) |
| Platelets, median (IQR) | 221.5 × 103/ µL (97.5 × 103/ µL) |
| Triglycerides, median (IQR) | 138 mg/dL (99 mg/dL) |
| Low-density lipoprotein (LDL) cholesterol, median (IQR) | 95 mg/dL (44 mg/dL) |
| High-density lipoprotein (HDL) cholesterol, median (IQR) | 43mg/dL (20 mg/dL) |
| Triglycerides, median (IQR) | 138 mg/dL (99mg/dL) |
| Total cholesterol, median (IQR) | 171 mg/dL (51mg/dL) |
IQR, interquartile range; BMI, body mass index.
Index liver biopsy characteristics
The largest proportion of the study cohort had an index liver biopsy suggestive of stage 0–1 fibrosis (44.3%), with the remaining distribution including stage 2 (19.2%), stage 3 (14.8%), and stage 4 (21.7%) fibrosis. The median NAFLD activity score was 4 (IQR 2). A detailed description of the index liver biopsy characteristics is provided in Table 2.
Table 2. Index liver biopsy characteristics.
| Study cohort (n = 406) | |
|---|---|
| Fibrosis, n (%) | |
| 0–1 | 180 (44.3%) |
| 2 | 78 (19.2%) |
| 3 | 60 (14.8%) |
| 4 | 88 (21.7%) |
| Steatosis, n (%) | |
| 0–1 | 146 (35.9%) |
| 2 | 162 (40.0%) |
| 3 | 98 (24.1%) |
| Lobular inflammation, n (%) | |
| 0 | 70 (17.2%) |
| 1 | 176 (43.4%) |
| 2 | 134 (33.0%) |
| 3 | 26 (6.4%) |
| Portal inflammation, n (%) | |
| 0 | 269 (66.3%) |
| 1 | 115 (28.3%) |
| 2 | 22 (5.4%) |
| Ballooning, n (%) | |
| 0 | 203 (50%) |
| 1 | 177 (43.6%) |
| 2 | 26 (6.4%) |
| Mallory bodies, n (%) | |
| 0 | 331 (81.5%) |
| 1 | 68 (16.7%) |
| 2 | 7 (1.7%) |
| Acidophils, n (%) | |
| 0 | 386 (95.1%) |
| 1 | 20 (4.9%) |
| NAFLD activity score, median (IQR) | 4 (2) |
| Microvesicular fat, median (IQR) | 0 (0) |
| Iron, median (IQR) | 0 (0) |
IQR, Interquartile range; NAFLD, non-alcoholic fatty liver disease.
Follow-up and outcomes
The median follow-up duration for the 406 patients was 3.7 years (IQR 4.8 years), as shown in Table 3. During this period, 35 patients died (8.6%), 70 developed a liver-related event (17.2%), and 41 experienced liver decompensation (10%). We sought to understand the clinical and histologic risk factors associated with the development of any liver-related event, liver decompensation, and all-cause mortality. Univariable-unadjusted hazard ratios for clinical and histologic risk factors associated with clinical outcome events are reported in Supplementary Tables 1–3.
Table 3. Summary of follow-up and outcomes for patient cohort.
| Study cohort (n = 406) | |
|---|---|
| Follow-up time, median (IQR) | 3.7 years (4.8 years) |
| All-cause mortality, n (%) | 35 (8.6%) |
| Liver decompensation, n (%) | 41 (10%) |
| Hepatic encephalopathy | 35 |
| Spontaneous bacterial peritonitis | 6 |
| Variceal bleeding | 7 |
| Ascites | 1 |
| Liver-related event, n (%) | 70 (17.2%) |
| Liver decompensation | 41 |
| Hepatocellular carcinoma | 16 |
| Variceal development | 45 |
| Hepatopulmonary syndrome | 2 |
IQR, Interquartile range.
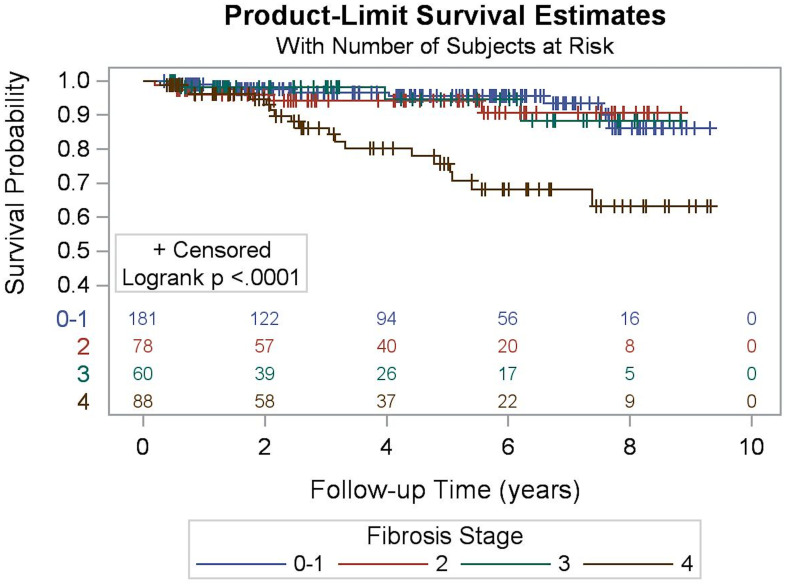
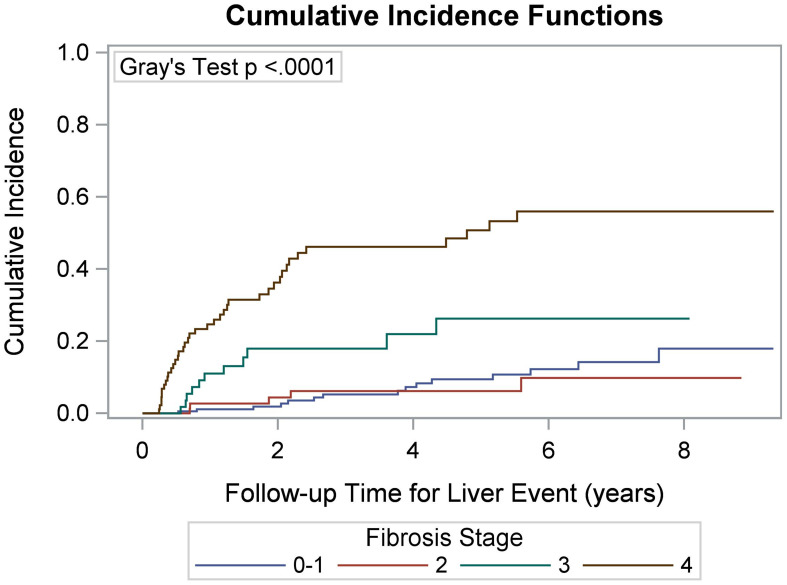
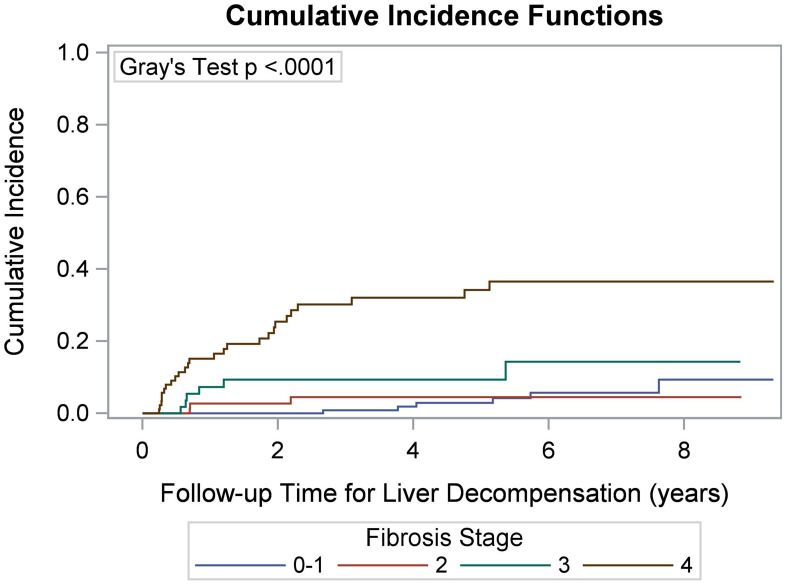
A Kaplan-Meier survival curve for all-cause mortality was generated (Fig. 2) and compared among fibrosis stage groups, showing that patients with stage 0–1 fibrosis had significantly better survival than those in the other three stages (p <0.0001). Cumulative incidence curves of any liver-related event (Fig. 3) and liver decompensation (Fig. 4) also demonstrated that patients with stage 3 and 4 fibrosis had higher cumulative event rates over time compared to those with stages 0–2 (p < 0.0001).
Fig. 2. Overall survival according to index liver fibrosis staging.
Fig. 3. Cumulative incidence of a liver-related event according to initial index liver fibrosis staging.
Fig. 4. Cumulative incidence of liver decompensation according to initial index liver fibrosis staging.
In multivariable analysis (Table 4), patients with stage 3 (aHR 2.68, 95% CI 1.18–6.11) and stage 4 (aHR 6.96, 95% CI 3.55–13.64) liver fibrosis had significantly higher rates of liver-related events compared to those with stage 0–1 fibrosis, in a stepwise fashion. Stage 4 fibrosis alone (aHR 8.46, 95% CI 3.26–21.99) was associated with an increased rate of liver decompensation compared to stage 0–1 fibrosis. Among clinical risk factors, hypertension (aHR 2.58, 95% CI 1.05–6.34) was associated with a higher risk of liver decompensation. Former smoking (aHR 2.60, 95% CI 1.18–5.70) was significantly associated with higher all-cause mortality compared to non-smokers. No other clinical risk factors or histologic features were significantly associated with the outcome events.
Table 4. Multivariate-adjusted hazard ratios and 95% confidence intervals (CI) of selected covariates and outcome events.
| Adjusted hazard ratio (aHR) | 95% Confidence interval (CI) of aHR | p-value | |
|---|---|---|---|
| All-cause mortality | |||
| Fibrosis stage | |||
| 0–1 | Reference | Reference | |
| 2 | 1.06 | 0.35–3.23 | 0.92 |
| 3 | 0.75 | 0.19–2.93 | 0.68 |
| 4 | 2.35 | 0.93–5.94 | 0.07 |
| Age | 1.02 | 0.99–1.06 | 0.15 |
| Body mass index (BMI) | |||
| Underweight/Normal | Reference | Reference | |
| Overweight | 0.76 | 0.21–2.75 | 0.68 |
| Obese | 0.55 | 0.17–1.77 | 0.32 |
| Race | |||
| White | Reference | Reference | |
| Asian | 1.52 | 0.18–12.61 | 0.70 |
| Black | 1.05 | 0.14–8.10 | 0.97 |
| Gender | |||
| Male | Reference | Reference | |
| Female | 0.98 | 0.48–2.01 | 0.95 |
| Type 2 diabetes mellitus | 1.89 | 0.84–4.27 | 0.12 |
| Hypertension | 1.34 | 0.56–3.23 | 0.51 |
| Smoker | |||
| Never smoker | Reference | Reference | |
| Former smoker | 2.60 | 1.18–5.70 | 0.02 |
| Current smoker | 1.18 | 0.26–5.40 | 0.83 |
| Liver-related event | |||
| Fibrosis stage | |||
| 0–1 | Reference | Reference | |
| 2 | 0.78 | 0.28–2.20 | 0.65 |
| 3 | 2.68 | 1.18–6.11 | 0.02 |
| 4 | 6.96 | 3.55–13.64 | <0.01 |
| Age | 1.02 | 1.00–1.04 | 0.06 |
| BMI | |||
| Underweight/Normal | Reference | Reference | |
| Overweight | 2.44 | 0.79–7.51 | 0.12 |
| Obese | 1.06 | 0.36–3.10 | 0.92 |
| Race | |||
| White | Reference | Reference | |
| Asian | 0.56 | 0.07–4.32 | 0.58 |
| Black | 1.76 | 0.52–5.96 | 0.36 |
| Gender | |||
| Male | Reference | Reference | |
| Female | 0.66 | 0.40–1.08 | 0.10 |
| Type 2 diabetes mellitus | 1.18 | 0.68–2.04 | 0.56 |
| Hypertension | 1.18 | 0.64–2.16 | 0.59 |
| Smoker | |||
| Never smoker | Reference | Reference | |
| Former smoker | 1.26 | 0.75–2.15 | 0.74 |
| Current smoker | 0.61 | 0.21–1.76 | 0.36 |
| Liver decompensation | |||
| Fibrosis stage | |||
| 0–1 | Reference | Reference | |
| 2 | 1.03 | 0.25–4.16 | 0.97 |
| 3 | 2.90 | 0.90–9.28 | 0.07 |
| 4 | 8.46 | 3.26–21.99 | <0.01 |
| Age | 1.02 | 0.99–1.05 | 0.12 |
| BMI | |||
| Underweight/Normal | Reference | Reference | |
| Overweight | 7.24 | 0.90–58.45 | 0.06 |
| Obese | 2.10 | 0.27–16.40 | 0.48 |
| Race | |||
| White | Reference | Reference | |
| Asian | 0.73 | 0.09–5.94 | 0.77 |
| Black | 2.27 | 0.50–10.36 | 0.29 |
| Gender | |||
| Male | Reference | Reference | |
| Female | 0.92 | 0.48–1.76 | 0.80 |
| Type 2 diabetes mellitus | 1.15 | 0.56–2.35 | 0.71 |
| Hypertension | 2.58 | 1.05–6.34 | 0.04 |
| Smoker | |||
| Never smoker | Reference | Reference | |
| Former smoker | 1.62 | 0.82–3.22 | 0.17 |
| Current smoker | 0.62 | 0.14–2.77 | 0.53 |
Discussion
In a large observational cohort of patients with biopsy-proven MASLD, advanced fibrosis was the primary histologic risk factor associated with the development of liver-related and liver decompensation events, demonstrating a stepwise increase in risk for liver-related events in stage 3 (aHR 2.68) and stage 4 (aHR 6.96) fibrosis. Furthermore, patients with stage 4 fibrosis experienced higher rates of liver decompensation events (aHR 8.46). Hypertension was predictive of liver decompensation.
Given the heterogeneous burden of MASLD across geographical regions, this study of a U.S. cohort provides further evidence confirming the primary importance of the histologic fibrosis stage as a predictor of clinical outcomes.16,19 Our findings are consistent with those from prior studies involving cohorts of patients with biopsy-proven MASLD. A multicenter retrospective study of patients with biopsy-proven MASLD from the U.S., Europe, and Thailand found that patients with stage 3 and 4 fibrosis had 14.2 times and 51.5 times the risk of liver-related events, respectively, compared to stage 0 fibrosis.9 Similarly, a prospective U.S. multicenter registry study with biopsy-proven MASLD found that the incidence of liver-related decompensation for stage 3 and 4 fibrosis was 18.7 times and 46.1 times that of patients with stages 0 to 2 fibrosis, respectively.20 Unlike prior studies, we did not find a statistically significant association between advanced fibrosis and mortality, which may be attributable to the limited number of events and statistical power.
The association between advanced fibrosis and both liver decompensation and liver-related events is mechanistically related to portal hypertension.21,22 Extensive liver fibrosis disrupts liver architecture and increases resistance to portal blood flow, ultimately leading to portal hypertension.23 Patients with a hepatic venous pressure gradient above 5 mmHg are directly at risk for clinical events such as ascites, variceal bleeding, and hepatic encephalopathy.24,25
While current management of MASLD is based on weight loss (through diet, exercise, and/or medications such as GLP-1 receptor agonists) and strict control of metabolic comorbidities, current tools to prevent and treat MASH fibrosis remain limited.26 Novel investigational medications addressing MASH and MASH fibrosis are currently under evaluation in clinical trials, offering hope for improving clinical outcomes.27,28 Due to the strong association between advanced fibrosis and liver-related outcomes, patients with liver stage 3 fibrosis or greater should be prioritized for MASH-directed therapy. Our study further supports clinical care pathways that prioritize risk stratification to identify patients with MASH fibrosis who may represent priority candidates for weight loss interventions and MASH pharmacotherapy.29,30
A key strength of our study was the restriction of the study population to patients with biopsy-proven MASLD, rather than those diagnosed based on noninvasive tests alone. Additionally, a single experienced pathologist reviewed and staged all liver biopsies using a validated scoring system (NASH Clinical Research Network system). To our knowledge, this study represents the largest U.S. single-center cohort study evaluating clinical outcomes in patients with biopsy-confirmed MASLD to date. While there is growing interest in non-invasive testing to assess liver fibrosis, these tests are limited by variability, inadequate accuracy, and potential error factors.31 Our study provides further justification for using liver biopsy as the gold-standard diagnostic modality to grade fibrosis, given its prognostic value for liver outcomes. Our study had several important limitations. With only 21.7% of the cohort having cirrhosis at baseline, our median follow-up time of 3.7 years may have been insufficient to observe an adequate number of clinical events to determine differences between individual fibrosis stages, particularly regarding liver decompensation and hepatocellular carcinoma. Due to the limited number of outcome events, we were restricted in the number of covariates that could be included in the multivariable model to control for confounders. Most of our patients were White, so these results may not be generalizable to non-White patients, although 24.8% were identified as Hispanic. These demographics are similar to other U.S. studies, including a large U.S.-based multicenter study20 and an MASLD cohort within the U.S. National Health and Nutrition Examination Survey.32 Lastly, while our study carefully excluded patients with alcohol use disorder at the time of the index liver biopsy, the retrospective nature of our study could not account for interval increases in alcohol intake over time or alternative sources of hepatic injury during the follow-up period, which could contribute to the development of outcome events.
Conclusions
Our study showed that advanced fibrosis in a large U.S. single-center cohort of patients with biopsy-proven MASLD is associated with hepatic decompensation and liver-related events. Patients with advanced fibrosis should be identified using risk stratification and staging tools and prioritized for weight loss interventions and future MASH-directed pharmacotherapy.
Supporting information
Ethical statement
The study was approved by the Institutional Review Board of Yale University (IRB # 2000029192). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The individual consent for this retrospective analysis was waived.
Data sharing statement
Data, analytic methods, and study materials may be made available to researchers upon request.
References
- 1.Arshad T, Golabi P, Henry L, Younossi ZM. Epidemiology of Non-alcoholic Fatty Liver Disease in North America. Curr Pharm Des. 2020;26(10):993–997. doi: 10.2174/1381612826666200303114934. [DOI] [PubMed] [Google Scholar]
- 2.Burra P, Becchetti C, Germani G. NAFLD and liver transplantation: Disease burden, current management and future challenges. JHEP Rep. 2020;2(6):100192. doi: 10.1016/j.jhepr.2020.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terrault NA, Francoz C, Berenguer M, Charlton M, Heimbach J. Liver Transplantation 2023: Status Report, Current and Future Challenges. Clin Gastroenterol Hepatol. 2023;21(8):2150–2166. doi: 10.1016/j.cgh.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64(5):1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- 6.Tsai E, Lee TP. Diagnosis and Evaluation of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Including Noninvasive Biomarkers and Transient Elastography. Clin Liver Dis. 2018;22(1):73–92. doi: 10.1016/j.cld.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 8.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology. 2018;155(2):443–457.e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149(2):389–97.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng CH, Lim WH, Hui Lim GE, Hao Tan DJ, Syn N, Muthiah MD, et al. Mortality Outcomes by Fibrosis Stage in Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2023;21(4):931–939.e5. doi: 10.1016/j.cgh.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Simon TG, Roelstraete B, Khalili H, Hagström H, Ludvigsson JF. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70(7):1375–1382. doi: 10.1136/gutjnl-2020-322786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kogiso T, Sagawa T, Kodama K, Taniai M, Hashimoto E, Tokushige K. Outcomes of Japanese patients with non-alcoholic fatty liver disease according to genetic background and lifestyle-related diseases. Ann Hepatol. 2021;21:100260. doi: 10.1016/j.aohep.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Hirose S, Matsumoto K, Tatemichi M, Tsuruya K, Anzai K, Arase Y, et al. Nineteen-year prognosis in Japanese patients with biopsy-proven nonalcoholic fatty liver disease: Lean versus overweight patients. PLoS One. 2020;15(11):e0241770. doi: 10.1371/journal.pone.0241770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017;65(1):54–64. doi: 10.1002/hep.28697. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Xu Y, Zhu Z, Li Y, Li X, Li Y, et al. Changes in the global, regional, and national burdens of NAFLD from 1990 to 2019: A systematic analysis of the global burden of disease study 2019. Front Nutr. 2022;9:1047129. doi: 10.3389/fnut.2022.1047129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pal P, Palui R, Ray S. Heterogeneity of non-alcoholic fatty liver disease: Implications for clinical practice and research activity. World J Hepatol. 2021;13(11):1584–1610. doi: 10.4254/wjh.v13.i11.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 19.Wong VW, Ekstedt M, Wong GL, Hagström H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J Hepatol. 2023;79(3):842–852. doi: 10.1016/j.jhep.2023.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N Engl J Med. 2021;385(17):1559–1569. doi: 10.1056/NEJMoa2029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryou M, Stylopoulos N, Baffy G. Nonalcoholic fatty liver disease and portal hypertension. Explor Med. 2020;1:149–169. doi: 10.37349/emed.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suk KT, Kim DJ. Staging of liver fibrosis or cirrhosis: The role of hepatic venous pressure gradient measurement. World J Hepatol. 2015;7(3):607–615. doi: 10.4254/wjh.v7.i3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwakiri Y. Pathophysiology of portal hypertension. Clin Liver Dis. 2014;18(2):281–291. doi: 10.1016/j.cld.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simonetto DA, Liu M, Kamath PS. Portal Hypertension and Related Complications: Diagnosis and Management. Mayo Clin Proc. 2019;94(4):714–726. doi: 10.1016/j.mayocp.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134(6):1715–1728. doi: 10.1053/j.gastro.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, Barb D, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023;77(5):1797–1835. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison SA, Loomba R, Dubourg J, Ratziu V, Noureddin M. Clinical Trial Landscape in NASH. Clin Gastroenterol Hepatol. 2023;21(8):2001–2014. doi: 10.1016/j.cgh.2023.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, Wang L. Current, emerging, and potential therapies for non-alcoholic steatohepatitis. Front Pharmacol. 2023;14:1152042. doi: 10.3389/fphar.2023.1152042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko CW, Siddique SM, Patel A, Harris A, Sultan S, Altayar O, et al. AGA Clinical Practice Guidelines on the Gastrointestinal Evaluation of Iron Deficiency Anemia. Gastroenterology. 2020;159(3):1085–1094. doi: 10.1053/j.gastro.2020.06.046. [DOI] [PubMed] [Google Scholar]
- 30.Kanwal F, Shubrook JH, Adams LA, Pfotenhauer K, Wai-Sun Wong V, Wright E, et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2021;161(5):1657–1669. doi: 10.1053/j.gastro.2021.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel K, Sebastiani G. Limitations of non-invasive tests for assessment of liver fibrosis. JHEP Rep. 2020;2(2):100067. doi: 10.1016/j.jhepr.2020.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le MH, Devaki P, Ha NB, Jun DW, Te HS, Cheung RC, et al. Prevalence of non-alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017;12(3):e0173499. doi: 10.1371/journal.pone.0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.