Abstract
Study Design
Retrospective Matched Cohort Study.
Objectives
Optimization of medical comorbidities is an essential part of preoperative management. However, the isolated effects of individual comorbidities have not been evaluated within a homogenous spine surgery population. This exact matching study aims to assess the independent effects of cancer on outcomes following single-level lumbar fusions for non-cancer surgery.
Methods
4680 consecutive patients undergoing single-level posterior-only lumbar fusion were retrospectively enrolled. Univariate statistics and coarsened exact matching (CEM) were computed to evaluate outcomes between cancer patients and those without comorbidities.
Results
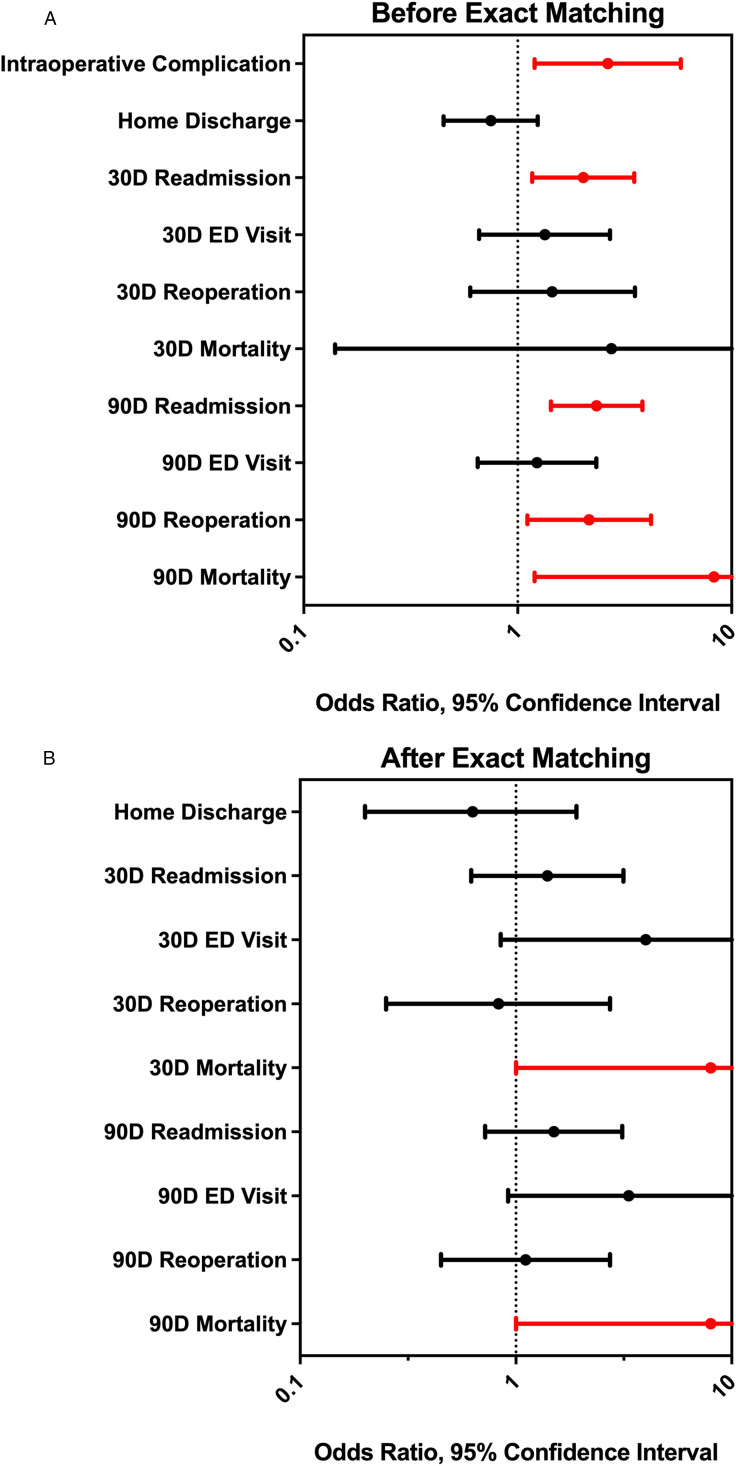
By logistic regression, malignancy conferred a higher risk of surgical complication (P = 0.016, OR = 2.64, CI = [1.200,5.790]), 30- and 90- day readmission (P = 0.012, OR = 2.025, CI = [1.170-3.510]; P < 0.001, OR = 2.34, CI = [1.430, 3.830], respectively), 90-day reoperation (P < 0.001, OR = 2.16, [1.110, 4.200]), and death at 90-days (P = 0.032, OR = 8.27, CI = [1.200, 56.850]). After matching, malignancy was associated with increased odds of incidental durotomy (6 vs 0 cases, P = 0.048) and death at both 30 and 90 days (both: OR = 8.0, P = 0.020, CI = [1.00, 63.960]). No cases of durotomy occurred in cases with mortality in the matched sample, suggesting independent relationships. There were no differences in length of stay, non-home discharge, ED evaluation, readmission, or reoperations.
Conclusion
Among otherwise exact-matched patients undergoing single level lumbar fusion, history of malignancy conferred a higher risk of short-term mortality, but not other outcomes suggestive of surgical failure. Increased mortality after lumbar fusion should be studied further and may play a role in surgical decision-making and patient discussions.
Keywords: lumbar fusion, cancer, malignancy, spine surgery, coarsened exact matching
Introduction
Lumbar fusion is a common and increasingly performed procedure worldwide. 1 Baseline complication and mortality rates are low2-4 and studies suggest that the cost/quality-adjusted life years ratio of lumbar fusion may be preferable to that of conservative care for degenerative spine conditions.5,6 However, given the overall increase in cost burden to health systems from lumbar fusions, it remains necessary to optimize the safety and the cost-to-value ratio of this procedure.
Many patient-specific risk factors for markers of poor surgical outcomes are being studied to guide the selection for surgery and focus targets for perioperative management.7-12 Medical comorbidity is a common risk factor examined by such studies. The Charlson Comorbidity Index (CCI) and American Society of Anesthesiologists (ASA) Classification are composite risk scores of comorbidity and have been shown to be associated with adverse short-term outcomes from lumbar surgery.7,8,12-14 In addition to composite measures like the CCI or global assessments of disease severity like the ASA classification, individual comorbidities have also been studied, showing that malignancy, rheumatoid disease, diabetes, and asthma may independently relate to patient outcomes following spinal surgery.12,15,16
Malignancy of any kind has been repeatedly shown in nationwide databases to associate with increased reoperations and mortality after lumbar fusion surgeries.17,18 Furthermore, patients with cancer have several related conditions, such as poor functional status, electrolyte abnormalities, and hypercoagulability, that impact surgical outcomes. 19 Despite this growing literature, databases are susceptible to errors 20 and well-controlled single institution studies can provide external validity. As perioperative patient care pathways are becoming increasingly standardized, it is imperative to have a thorough understanding of patient-specific risk factors to refine perioperative medical management to meet each patient’s unique needs. Additionally, the trend towards value-based care places strong incentives on hospital management to develop risk mitigation pathways on a health systems-level. We aim to examine how a specific medical comorbidity is associated with outcomes and rigorously control for the complex interactions between demographic, medical, and surgery specific risk factors by matching patients undergoing a precisely defined surgical procedure. Given malignancy as a leading cause of mortality in western societies, we focus herein on history of malignancy as it directly relates to the odds of adverse surgical outcomes following single-level lumbar fusion.
Methods
Patient Selection
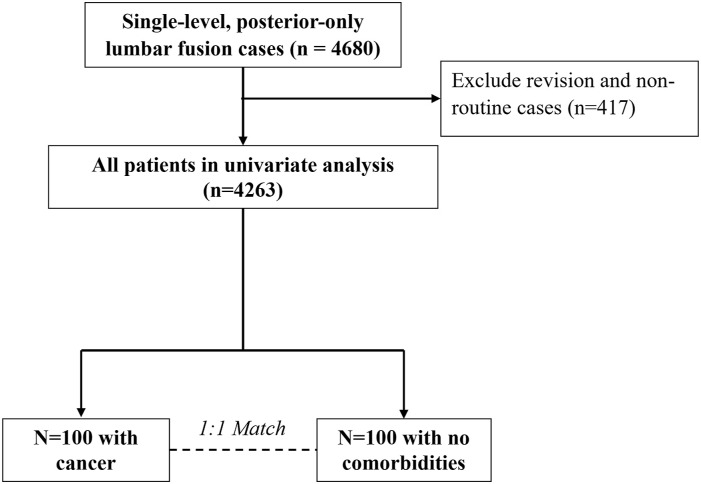
A total of 4680 consecutive cases of adult patients undergoing single-level posterior-only lumbar fusion at a single multihospital academic medical center from 2013 to 2021 were prospectively enrolled and retrospectively studied. The indications for spinal fusion in our cohort included symptomatic spondylolisthesis, radiculopathy, and degenerative scoliosis. 21 Inclusion criteria included cases that were non-emergent, inpatient admissions, used general anesthesia, had clean wounds, and had complete follow-up information. A total of 4263 cases analyzed further (Figure 1).
Figure 1.
Study selection and sample size.
Data Extraction
Patient characteristics and surgical outcome data were extracted from the electronic medical record (EMR) using EpiLog, a non-proprietary system integrated with the EMR to streamline data collection and quality improvement initiatives. 22 Patient variables controlled for included median household income (MHI) cross-referenced to zip-code (adjusted to 2016 US dollars), body mass index (BMI), age, sex, race, ASA score, smoking status, prior surgical history, insurance type (public vs private), duration of surgery, and presence of each comorbidity measured within the CCI score. Outcomes measured included surgical complication, length of stay, discharge home vs non-home, and 30- and 90- day emergency department (ED) evaluation, readmissions, reoperation, and all-cause mortality.
Statistical Analysis
Patients with any malignancy (n = 121) were compared to patients with no medical comorbidities (n = 2329), as measured by all CCI variables excluding age. First, univariate logistic regression was performed to determine if malignancy correlated with short-term surgical outcomes. Next, coarsened exact matching (CEM) was employed to seek ideal matches on patient characteristic known to impact outcome, except malignancy. CEM was performed by binning the following 10 controlling variables into categorical levels: age (categorized by decade), ASA grade (exact matched), health insurance type (private vs public insurer), gender (male vs female), smoking history (prior smoker vs never user), prior surgical procedures (binary), prior surgery within 30 days of index operation (binary), BMI (<18.5, 18.5-30, or >30), MHI (above or below median), and duration of surgery (above or below median). Patients with malignancy were then matched 1:1 to those without any medical comorbidities (n = 200 matched patients). Patient demographics were compared using chi-squared and non-parametric tests. Outcomes in the CEM cohort were analyzed using the McNemar’s and non-parametric tests for categorical and continuous variables, respectively.
The SAS version 9.4 (SAS Institute Inc) program was used to bin covariates and remove missing values for CEM and subsequent matching done via the MatchIt programming package in R Statistics (R Core Team, 2017). All other statistical analyses were performed with SAS version 9.4. Significance for all analyses was set as P-value <0.05.
Results
Patient Demographics
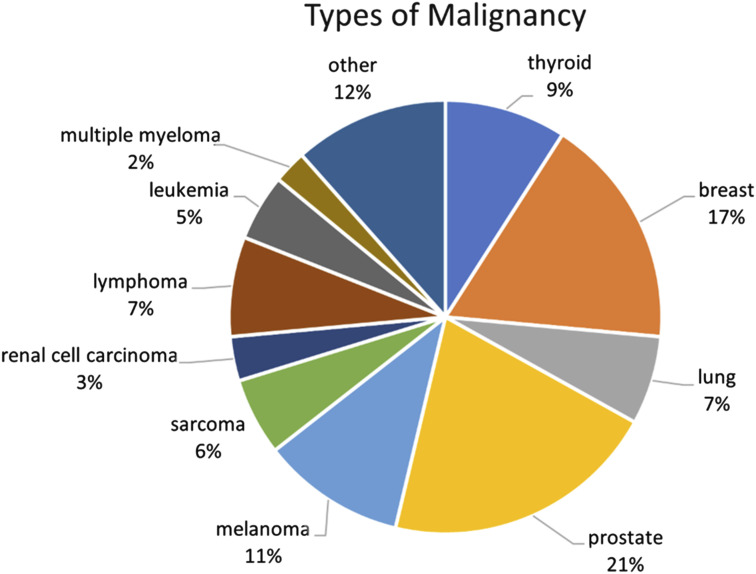
Before matching, patients with malignancy (n = 121) tended to be older (66.50 vs 58.75, P < 0.001), smoke less (4.13% vs 14.30%, P = 0.0005), have higher ASA scores (2.48 vs 2.24, P < 0.0001), be publicly insured (64.5% vs 42.9%, P < 0.0001), and have more prior surgeries (0.92 vs 0.52, P = 0.0004) than patients without comorbidity (n = 2329). After matching, there were no statistically significant differences in age, tobacco use, insurance type, or surgical history between matched groups. Patient demographic information before and after matching is presented in Table 1 and breakdown of cancer types in the malignancy cohort is displayed in Figure 2.
Table 1.
Demographics in the Cohort of Cancer Analysis.
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| No Comorbidities (n = 2329) | Cancer History (n = 121) | P-value | No Comorbidities (n = 100) | Cancer History (n = 100) | P-value | |
| Gender (n, %) | ||||||
| Male | 1050 (45.08%) | 403 (47.93%) | 0.5745 | 48 (48.00%) | 48 (48.00%) | 1 |
| Female | 1279 (54.92%) | 231 (50.55%) | 52 (52.00%) | 52 (52.00%) | ||
| Age (mean, range) | ||||||
| 58.75 [15.0,90.0] | 66.50 [27.0,84.0] | <0.0001 | 65.98 [31.0,85.0] | 66.42 [27.0,83.0] | 0.5829 | |
| Race (n, %) | ||||||
| White | 1959 (84.11%) | 105 (86.78%) | 0.0849 | 91 (91.00%) | 87 (87.00%) | 0.1189 |
| Non-White | 370 (15.89%) | 16 (13.22%) | 9 (9.00%) | 13 (13.00%) | ||
| BMI (mean, range) | ||||||
| 29.26 [15.0, 54.4] | 30.50 [13.64, 56.58] | 0.2957 | 28.92 [19.75, 54.4] | 28.58 [18.6,41.75] | 0.93 | |
| Tobacco use (n, %) | ||||||
| 333 (14.30%) | 5 (4.13%) | 0.0005 | 2 (2.00%) | 2 (2.00%) | 1 | |
| Insurance type (n, %) | ||||||
| Private | 1329 (57.1%) | 43 (35.5%) | <0.0001 | 35 (35.0%) | 35 (35.0%) | 1 |
| Public | 1000 (42.9%) | 78 (64.5%) | 65 (65.0%) | 65 (65.0%) | ||
| Prior surgery (mean, range) | ||||||
| 0.52 [0,11] | 0.92 [0,17] | 0.0004 | 0.65 [0,7] | 0.87 [0,17] | 0.90 | |
| Prior surgery 30D (mean, range) | ||||||
| 0.03 [0,3] | 0.02 [0,1] | 0.668 | 0.03 [0,3] | 0.02 [0,1] | 1 | |
| ASA score (mean, range) | ||||||
| 2.24 [1.0,4.0] | 2.48 [2.0,3.0] | <0.0001 | 2.45 [2.0,3.0] | 2.45 [2.0,3.0] | 1 | |
Figure 2.
Representation of cancer type in malignancy cohort (n = 121).
Statistical Analysis
In the univariate regression model (Figure 3A), malignancy was associated with higher odds of incidental durotomy (P = 0.016, OR = 2.64, CI = [1.200,5.790]), 30- and 90- day readmission (P = 0.012, OR = 2.025, CI = [1.170-3.510]; P < 0.001, OR = 2.34, CI = [1.430, 3.830], respectively), 90-day reoperation (P < 0.001, OR = 2.16, CI = [1.110, 4.200]), and death at 90-days (P = 0.032, OR = 8.27, CI = [1.200, 56.850]). There were no differences in rates of non-home discharge, 30- or 90- day ED visits, 30-day reoperation rates, or 30-day mortality. After CEM, malignancy was found to be associated with significantly greater incidental durotomy (6 vs 0 cases, P = 0.048) and increased odds of death at both 30 and 90 days (both: OR = 8.0, P = 0.020, CI = [1.00, 63.960]) but no different length of stay, or odds of discharge home vs not home, ED evaluation, readmission, or reoperations (Figure 3B). No deaths occurred among the patients with incidental durotomy in the matched cohort.
Figure 3.
(A) Forest plot for univariate regression analysis showing odds ratios and 95% confidence intervals for postoperative outcomes. Significant values (P-value <0.05) are denoted in red. (B) Forest plot for coarsened exact matching analysis showing odds ratios and 95% confidence intervals for postoperative outcomes. Significant values (P-value <0.05) are denoted in red.
Discussion
This study examines how the presence or absence of any cancer history affects the short-term outcomes of non-cancer-related lumbar fusion surgery in a single-center cohort study. Univariate analysis showed that history of malignancy was related to increased odds of surgical complications, readmissions, reoperations, and death. After controlling for confounders by matching patients with malignancy to those without any medical comorbidities on 10 factors associated with outcomes, malignancy was associated with higher odds of incidental durotomy, and, unrelatedly, death, but not length of stay, discharge home vs non-home, or short-term ED visits, readmissions or reoperations. It has been shown previously that metastatic cancer and lymphoma are associated with complications, readmissions and mortality in multivariate modeling, and our finding is a novel addition to this literature providing an analysis exactly matching cancer patients to patients without medical comorbidities.3,15,23
It is likely expected that there are higher odds of death after surgery in patients with a history of malignancy compared to those without. Despite higher mortality rates, patients with cancer did not demonstrate any differences in length of stay, ED visits, readmissions, or repeat operations compared to otherwise exactly matched peers without malignancy. These findings may suggest that single-level lumbar fusion in well-selected patients, even with malignancy, can remain a safe and effective procedure, despite a higher baseline mortality rate from malignancy alone.
Cancer is the second leading cause of mortality in America and worldwide.24,25 While this study was conducted in a United States medical institution, our results provide relevant insights into the interaction between a common medical comorbidity and outcomes of a spine procedure that is widely conducted in other countries.26-28 Regardless of the specific healthcare system, it is important to understand how patient characteristics may impact surgical management and our study may provide groundwork for further analyses at international institutions.
The risks of major surgery such as lumbar fusion may be magnified in patients with malignancy due to the systemic effects of cancer on the body, irrespective of the cancer subtype. Patients with cancer share several of the same underlying risk factors, including greater rates of anemia, frailty, and are predisposed to medical complications such as deep vein thrombosis and pulmonary embolism.29-32 Blood loss is associated with greater morbidity and mortality during spine surgery, surgical/hospital practices on blood transfusions are variable and contribute to outcomes, and malignancy history may influence surgical decisions regarding transfusion.33,34 Furthermore, as a product of both the underlying disease process and the effects of treatment, cancer patients are subject to increased levels of immunosuppression relative to healthy peers. 35 There are also several psychosocial factors and significant psychiatric comorbidities that are associated with having a life-threatening and chronic disease such as cancer.36,37 A combination of these baseline patient factors with the immunosuppressive effects of surgical trauma, 38 the effect of blood loss on pre-existing anemia and frailty, and the increased risk of DVT and PE seen following spine surgery may all contribute to the observed increased mortality rate seen in these individuals in addition to the increased baseline risk. 39
As a result of these risk factors, the perioperative management of cancer patients must be carefully tailored. 19 After a potential patient is identified to have a history of malignancy, the surgeon should first assess whether they would still be a good surgical candidate given their comorbidity and its implications on life expectancy. The patient should also be informed of the additional risk, so that they can weigh it with the potential benefit of the intervention. Additionally, because cancer is associated with various acute and chronic pain syndromes, 40 there should be consideration of the efficacy of surgery and whether the patient is willing to tolerate the postoperative pain. Once the decision to proceed with surgery is made, the patient should be engaged with other specialists to optimize their medical management prior to surgery, including comprehensive assessments of functional status, baseline cancer-related pain, and nutritional status. Furthermore, chemotherapy and/or radiation timelines should be considered when scheduling an elective surgery given their effects on wound healing. 41 Patients should be optimized with regards to anticoagulation due to their increased hypercoagulability 39 and perioperative antibiotics may need to be tailored given that many cancer patients are immunocompromised. 42 Additionally, many cancer patients have adrenal insufficiency due to chronic steroid treatment 43 and may therefore require increased dosing of glucocorticoids. Postoperatively, close follow-up should be coordinated with the patient’s oncologist and primary care provider to manage pain, wound healing complications, and psychosocial issues.36,37
In addition to the increased odds of mortality, we note that patients with cancer had increased incidental durotomy than those without cancer, although between matched patients there was no co-occurrence of durotomy and mortality. Notably, this study was performed on a cohort of patients undergoing lumbar fusion surgery for reasons other than spinal tumors. This is an important area for future investigation as understanding the role of a patient’s cancer status on dural integrity and surgical complications could facilitate adjustments to surgical technique or perioperative management to mitigate these occurrences.
Limitations
This study comes with important limitations. The patients analyzed in this study were from a single multihospital academic medical center, potentially introducing bias. By design of the retrospective analysis, this study matched and compared within a cohort of patients selected for and undergoing surgery. Lacking a control group of similar patients with malignancy who did not undergo surgery, we cannot determine whether the observed mortality rate in this surgical cohort differs from the baseline mortality rate for malignancy alone. We are limited to observing that, for well-selected patients, higher odds of mortality did not coincide with any relationship to the length of stay, ED visits, readmissions, or reoperations. Additionally, we were unable to assess the specific reasons for increased mortality and further studies are warranted to understand the underlying causes. Another limitation is that the outcome variables, although selected for interpretability and importance to patients, providers, and payers, do not capture subjective patient outcomes. Finally, separating all patients with a history of malignancy together in our matching cohort from those with no comorbidities, while enabling a larger cohort by our matching method, likely oversimplifies the complex heterogeneity among all cancers. Informed by our results and the prior literature, ongoing and future studies should explore these nuances of cancer history, types, and staging may influence patient experiences of degenerative spine disease and functional surgical outcomes.
Conclusion
Patients with a history of malignancy undergoing single level lumbar fusion have a higher short-term mortality risk compared to exact-matched patients without cancer. However, there is no significant difference in other measures of healthcare utilization indicating surgical differences. In this cohort, surgeons appear to deliver equally safe short-term immediate surgical outcomes to patients with or without malignancy; however, increased early death in patients with malignancy should lead to additional research and play a role in strategies to optimize care for cancer patients.
Acknowledgments
The EpiLog Project and The Bernadette and Kevin McKenna Family Research Fund.
Appendix.
Abbreviations
- MHI
Median Household Income
- ED
Emergency Department
- OR
Odds Ratio
- CEM
Coarsened exact matching
- BMI
Body mass index
- ASA
American Society of Anesthesiologists
- CCI
Charlson Comorbidity Index
Footnotes
Author Contributions: RSG and NRM were involved in the design and conception of this manuscript. RSG, RK, EX, CAW, AJB, and NRM performed the literature review and compiled the primary manuscript. RSG, AJB, JN, TC, and NRM collected and analyzed data. RSG and RK compiled the figures and tables. RSG, RK, EX, CAW, AJB, JN, SM, and NRM critically revised the manuscript. All authors approved the manuscript as it is written.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the NRM received support from the Bernadette and Kevin McKenna Family Research Fund.
Ethical Statement
Ethical Approval
This study was approved by the IRB at the Hospital of the University of Pennsylvania. The IRB number for this study is 832794. All ethical guidelines and rules were followed to protect patient privacy.
ORCID iDs
Ryan S. Gallagher https://orcid.org/0000-0002-1789-9142
Ritesh Karsalia https://orcid.org/0000-0001-6002-3296
Neil R. Malhotra https://orcid.org/0000-0002-0754-6423
References
- 1.Martin BI, Mirza SK, Spina N, Spiker WR, Lawrence B, Brodke DS. Trends in lumbar fusion procedure rates and associated hospital costs for degenerative spinal diseases in the United States, 2004 to 2015. Spine. 2019;44(5):369-376. [DOI] [PubMed] [Google Scholar]
- 2.Pumberger M, Chiu YL, Ma Y, Girardi FP, Vougioukas V, Memtsoudis SG. Perioperative mortality after lumbar spinal fusion surgery: an analysis of epidemiology and risk factors. Eur Spine J. 2012;21(8):1633-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goz V, Weinreb JH, McCarthy I, Schwab F, Lafage V, Errico TJ. Perioperative complications and mortality after spinal fusions: analysis of trends and risk factors. Spine. 2013;38(22):1970-1976. [DOI] [PubMed] [Google Scholar]
- 4.Fjeld OR, Grøvle L, Helgeland J, et al. Complications, reoperations, readmissions, and length of hospital stay in 34 639 surgical cases of lumbar disc herniation. Bone Joint Lett J. 2019;101-b(4):470-477. [DOI] [PubMed] [Google Scholar]
- 5.Tosteson AN, Skinner JS, Tosteson TD, et al. The cost effectiveness of surgical versus nonoperative treatment for lumbar disc herniation over two years: evidence from the Spine Patient Outcomes Research Trial (SPORT). Spine. 2008;33(19):2108-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glassman SD, Polly DW, Dimar JR, Carreon LY. The cost effectiveness of single-level instrumented posterolateral lumbar fusion at 5 years after surgery. Spine. 2012;37(9):769-774. [DOI] [PubMed] [Google Scholar]
- 7.Borja AJ, Connolly J, Kvint S, et al. Charlson Comorbidity Index score predicts adverse post-operative outcomes after far lateral lumbar discectomy. Clin Neurol Neurosurg. 2021;206:106697. [DOI] [PubMed] [Google Scholar]
- 8.Winter E, Detchou DK, Glauser G, et al. Predicting patient outcomes after far lateral lumbar discectomy. Clin Neurol Neurosurg. 2021;203:106583. [DOI] [PubMed] [Google Scholar]
- 9.Phan K, Lee NJ, Kothari P, Kim JS, Cho SK. Risk factors for readmissions following anterior lumbar interbody fusion. Spine. 2018;43(5):364-369. [DOI] [PubMed] [Google Scholar]
- 10.Lim S, Edelstein AI, Patel AA, Kim BD, Kim JYS. Risk factors for postoperative infections after single-level lumbar fusion surgery. Spine. 2018;43(3):215-222. [DOI] [PubMed] [Google Scholar]
- 11.Shin JI, Kothari P, Phan K, et al. Frailty index as a predictor of adverse postoperative outcomes in patients undergoing cervical spinal fusion. Spine. 2017;42(5):304-310. [DOI] [PubMed] [Google Scholar]
- 12.Khor S, Lavallee D, Cizik AM, et al. Development and validation of a prediction model for pain and functional outcomes after lumbar spine surgery. JAMA Surg. 2018;153(7):634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lubelski D, Feghali J, Nowacki AS, et al. Patient-specific prediction model for clinical and quality-of-life outcomes after lumbar spine surgery. J Neurosurg Spine. 2021;34(4):580-588. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 15.Akins PT, Harris J, Alvarez JL, et al. Risk factors associated with 30-day readmissions after instrumented spine surgery in 14,939 patients: 30-day readmissions after instrumented spine surgery. Spine. 2015;40(13):1022-1032. [DOI] [PubMed] [Google Scholar]
- 16.Lubelski D, Ehresman J, Feghali J, et al. Prediction calculator for nonroutine discharge and length of stay after spine surgery. Spine J. 2020;20(7):1154-1158. [DOI] [PubMed] [Google Scholar]
- 17.Durand WM, Eltorai AEM, Depasse JM, Yang J, Daniels AH. Risk factors for unplanned reoperation within 30 Days following elective posterior lumbar spinal fusion. Global Spine J. 2018;8(4):388-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song J, Katz AD, Perfetti D, et al. Impact of discharge to rehabilitation on postdischarge morbidity following multilevel posterior lumbar fusion. Clin Spine Surg. 2022;35(1):24-30. [DOI] [PubMed] [Google Scholar]
- 19.Lefor AT. Perioperative management of the patient with cancer. Chest. 1999;115(5 Suppl):165S-171S. [DOI] [PubMed] [Google Scholar]
- 20.Rolston JD, Han SJ, Chang EF. Systemic inaccuracies in the National Surgical Quality Improvement Program database: implications for accuracy and validity for neurosurgery outcomes research. J Clin Neurosci. 2017;37:44-47. [DOI] [PubMed] [Google Scholar]
- 21.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1(1):2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farooqi AS, Borja AJ, Detchou DKE, et al. Postoperative outcomes and the association with overlap before or after the critical step of lumbar fusion. J Neurosurg Spine. 2022;36(3):366-375. [DOI] [PubMed] [Google Scholar]
- 23.Shah AA, Devana SK, Lee C, et al. Prediction of major complications and readmission after lumbar spinal fusion: a machine learning–driven approach. World Neurosurg. 2021;152:e227-e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Global Burden of Disease 2019 Cancer Collaboration. Kocarnik JM, Compton K, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the global burden of disease study 2019. JAMA Oncol. 2022;8(3):420-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran KB, Lang JJ, Compton K, et al. The global burden of cancer attributable to risk factors, 2010–19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2022;400(10352):563-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vertuani S, Nilsson J, Borgman B, et al. A cost-effectiveness analysis of minimally invasive versus open surgery techniques for lumbar spinal fusion in Italy and the United Kingdom. Value Health. 2015;18(6):810-816. [DOI] [PubMed] [Google Scholar]
- 27.Pereira P, Park Y, Arzoglou V, et al. Anterolateral versus posterior minimally invasive lumbar interbody fusion surgery for spondylolisthesis: comparison of outcomes from a global, multicenter study at 12-months follow-up. Spine J. 2023;23(10):1494-1505. [DOI] [PubMed] [Google Scholar]
- 28.Kim HC, Jeong YH, Oh SH, et al. Single-position oblique lumbar interbody fusion and percutaneous pedicle screw fixation under O-arm navigation: a retrospective comparative study. J Clin Med. 2022;12(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21(6):345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Prim. 2022;8(1):11. [DOI] [PubMed] [Google Scholar]
- 31.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091-1101. [DOI] [PubMed] [Google Scholar]
- 32.Kroemer G, McQuade JL, Merad M, André F, Zitvogel L. Bodywide ecological interventions on cancer. Nat Med. 2023;29(1):59-74. [DOI] [PubMed] [Google Scholar]
- 33.Pennington Z, Ehresman J, Westbroek EM, Lubelski D, Cottrill E, Sciubba DM. Interventions to minimize blood loss and transfusion risk in spine surgery: a narrative review. Clin Neurol Neurosurg. 2020;196:106004. [DOI] [PubMed] [Google Scholar]
- 34.Wu W-C, Trivedi A, Friedmann PD, et al. Association between hospital intraoperative blood transfusion practices for surgical blood loss and hospital surgical mortality rates. Ann Surg. 2012;255(4):708-714. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Razeq H, Hashem H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol. 2020;145:102837. [DOI] [PubMed] [Google Scholar]
- 36.Rasic DT, Belik SL, Bolton JM, Chochinov HM, Sareen J. Cancer, mental disorders, suicidal ideation and attempts in a large community sample. Psycho Oncol. 2008;17(7):660-667. [DOI] [PubMed] [Google Scholar]
- 37.Mehnert A, Brähler E, Faller H, et al. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol. 2014;32(31):3540-3546. [DOI] [PubMed] [Google Scholar]
- 38.Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao C-M, Zhang Y, Yang S-D, et al. Risk factors for lower limb deep vein thrombosis in patients with single-level lumbar fusion: a prospective study of 710 cases. Clin Appl Thromb Hemost. 2018;24(9_suppl):157S-162S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Portenoy RK, Ahmed E. Cancer pain syndromes. Hematol Oncol Clin N Am. 2018;32(3):371-386. [DOI] [PubMed] [Google Scholar]
- 41.Słonimska P, Sachadyn P, Zieliński J, Skrzypski M, Pikuła M. Chemotherapy-mediated complications of wound healing: an understudied side effect. Adv Wound Care. 2024;13(4):187-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nanayakkara AK, Boucher HW, Fowler VG, Jr, Jezek A, Outterson K, Greenberg DE. Antibiotic resistance in the patient with cancer: escalating challenges and paths forward. CA - Cancer J Clin. 2021;71(6):488-504. [DOI] [PubMed] [Google Scholar]
- 43.Hayes AG, Rushworth RL, Torpy DJ. Risk assessment, diagnosis, and treatment of cancer treatment-related adrenal insufficiency. Expet Rev Endocrinol Metabol. 2022;17(1):21-33. [DOI] [PubMed] [Google Scholar]