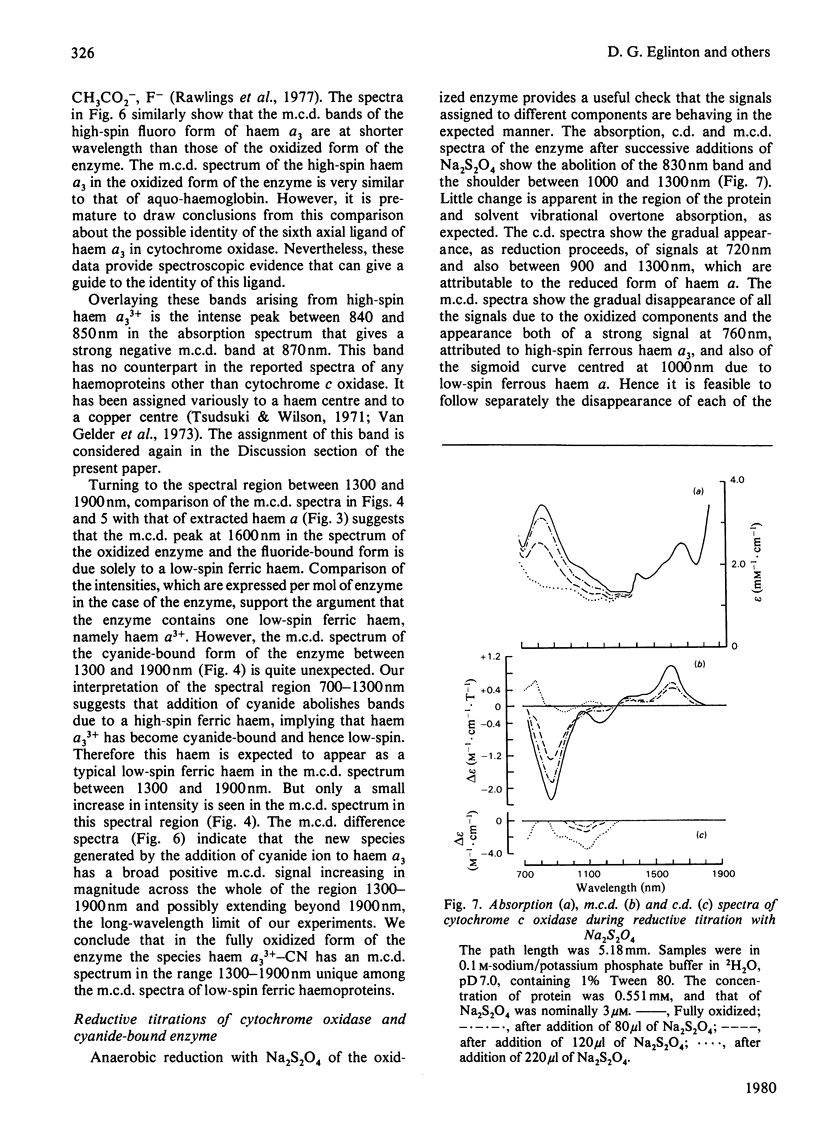
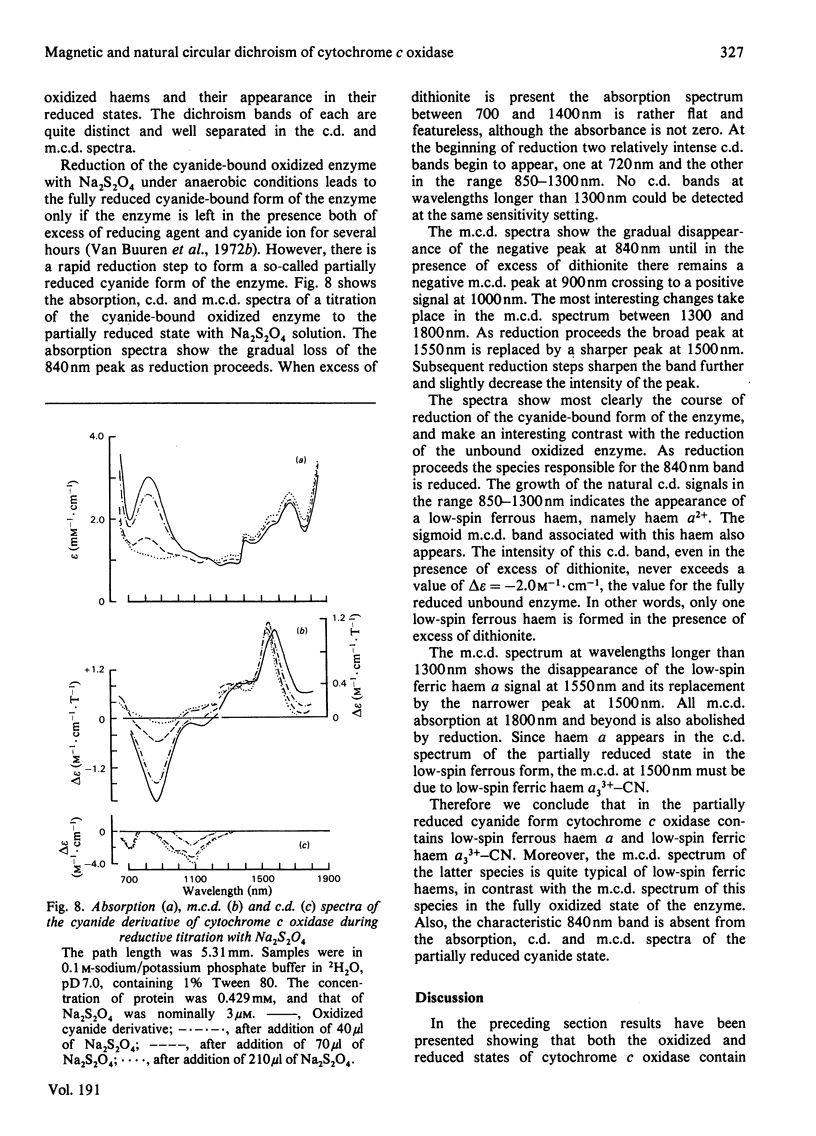
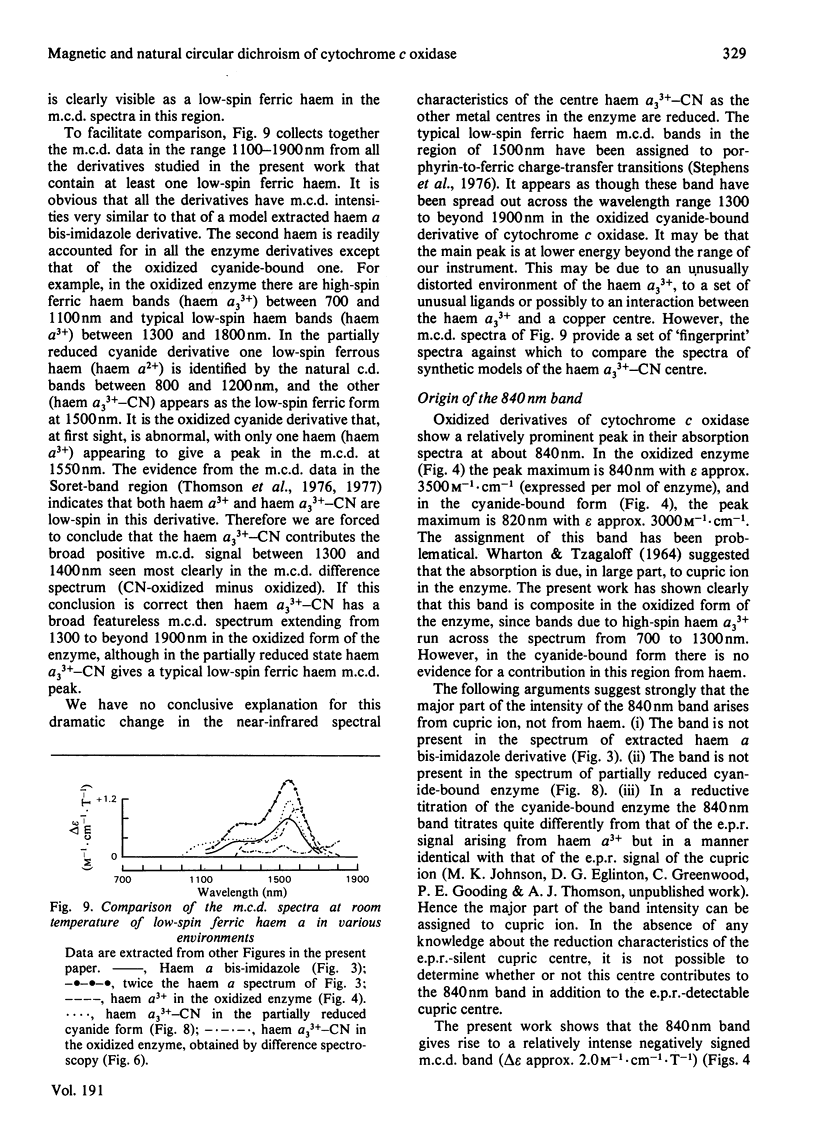
Abstract
A detailed study is presented of the room-temperature absorption, natural and magnetic circulation-dichroism (c.d. and m.c.d.) spectra of cytochrome c oxidase and a number of its derivatives in the wavelength range 700-1900 nm. The spectra of the reduced enzyme show a strong negative c.d. band peaking at 1100nm arising from low-spin ferrous haem a and a positive m.c.d. peak at 780nm assigned to high-spin ferrous haem a3. Addition of cyanide ion doubles the intensity of the low-spin ferrous haem c.d. band and abolishes reduced carbonmonoxy derivative the haem a32+-CO group shows no c.d. or m.c.d. bands at wavelengths longer than 700nm. A comparison of the m.c.d. spectra of the oxidized and cyanide-bound oxidized forms enables bands characteristic of the high-spin ferric form of haem a33+ to be identified between 700 and 1300nm. At wavelengths longer than 1300nm a broad positive m.c.d. spectrum, peaking at 1600nm, is observed. By comparison with the m.c.d. spectrum of an extracted haem a-bis-imidazole complex this m.c.d. peak is assigned to one low-spin ferric haem, namely haem a3+. On binding of cyanide to the oxidized form of the enzyme a new, weak, m.c.d. signal appears, which is assigned to the low-spin ferric haem a33+-CN species. A reductive titration, with sodium dithionite, of the cyanide-bound form of the enzyme leads to a partially reduced state in which low-spin haem a2+ is detected by means of an intense negative c.d. peak at 1100 nm and low-spin ferric haem a33+-CN gives a sharp positive m.c.d. peak at 1550nm. The c.d. and m.c.d. characteristics of the 830nm absorption band in oxidized cytochrome c oxidase are not typical of type 1 blue cupric centres.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Vickery L. E., Palmer G. Electronic state of heme in cytochrome oxidase. I. Magnetic circular dichroism of the isolated enzyme and its derivatives. J Biol Chem. 1976 Dec 25;251(24):7907–7919. [PubMed] [Google Scholar]
- Brittain T., Greenwood C., Springall J. P., Thomson A. J. Magnetic-circular-dichroism studies of haem a and its derivatives. Biochem J. 1978 Aug 1;173(2):411–417. doi: 10.1042/bj1730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day P., Smith D. W., Williams R. J. Crystal spectra of some ferric hemoproteins. Biochemistry. 1967 Dec;6(12):3747–3750. doi: 10.1021/bi00864a018. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Charney E. Near-infrared absorption and circular dichroism spectra of ferrocytochrome c: d-d transitions. J Chem Phys. 1969 Nov 15;51(10):4502–4505. doi: 10.1063/1.1671818. [DOI] [PubMed] [Google Scholar]
- Hill H. A., Smith B. E. Characteristics of azurin from Pseudomonas aeruginosa via 270-MHz 1H nuclear magnetic resonance spectroscopy. J Inorg Biochem. 1979 Oct;11(2):79–93. doi: 10.1016/s0162-0134(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Lemberg M. R. Cytochrome oxidase. Physiol Rev. 1969 Jan;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- Nicholls P. A new carbon monoxide-induced complex of cytochrome c oxidase. Biochem J. 1978 Dec 1;175(3):1147–1150. doi: 10.1042/bj1751147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa T., Yamamoto T., Hatano M. Infrared magnetic circular dichroism of myoglobin derivatives. Biochim Biophys Acta. 1976 Mar 18;427(1):28–37. doi: 10.1016/0005-2795(76)90282-8. [DOI] [PubMed] [Google Scholar]
- Powers L., Blumberg W. E., Chance B., Barlow C. H., Leigh J. S., Jr, Smith J., Yonetani T., Vik S., Peisach J. The nature of the copper atoms of cytochrome c oxidase as studied by optical and x-ray absorption edge spectroscopy. Biochim Biophys Acta. 1979 Jun 5;546(3):520–538. doi: 10.1016/0005-2728(79)90085-9. [DOI] [PubMed] [Google Scholar]
- Rawlings J., Stephens P. J., Nafie L. A., Kamen M. D. Near-infrared magnetic circular dichroism of cytochrome c'. Biochemistry. 1977 Apr 19;16(8):1725–1729. doi: 10.1021/bi00627a032. [DOI] [PubMed] [Google Scholar]
- Rotillio G., Calabrese L., Coleman J. E. Magnetic circular dichroism of cobalt-copper and zinc-copper bovine superoxide dismutase. J Biol Chem. 1973 Jun 10;248(11):3855–3859. [PubMed] [Google Scholar]
- Solomon E. I., Hare J. W., Gray H. B. Spectroscopic studies and a structural model for blue copper centers in proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1389–1393. doi: 10.1073/pnas.73.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. H., Bocian D. F., Chan S. I. EPR studies of 15NO-ferrocytochrome alpha3 in cytochrome c oxidase. FEBS Lett. 1979 Jan 15;97(2):314–316. doi: 10.1016/0014-5793(79)80110-6. [DOI] [PubMed] [Google Scholar]
- TAKEMORI S., KING T. E. EFFECT OF ALKALI AND BOROHYDRIDE ON CARDIAC CYTOCHROME OXIDASE. FORMATION OF SCHIFF BASE. J Biol Chem. 1965 Jan;240:504–513. [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. P. Variable-temperature magnetic-circular-dichroism spectra of cytochrome c oxidase and its derivatives. Biochem J. 1977 Aug 1;165(2):327–336. doi: 10.1042/bj1650327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. Determination of the heme spin states in cytochrome c oxidase using magnetic circular dichroism. FEBS Lett. 1976 Aug 1;67(1):94–98. doi: 10.1016/0014-5793(76)80877-0. [DOI] [PubMed] [Google Scholar]
- Tsudzuki T., Wilson D. F. The oxidation-reduction potentials of the hemes and copper of cytochrome oxidase from beef heart. Arch Biochem Biophys. 1971 Jul;145(1):149–154. doi: 10.1016/0003-9861(71)90021-x. [DOI] [PubMed] [Google Scholar]
- Tweedle M. F., Wilson L. J. Electronic state of heme in cytochrome oxidase III. The magnetic susceptibility of beef heart cytochrome oxidase and some of its derivatives from 7-200 K. Direct evidence for an antiferromagnetically coupled Fe (III)/Cu (II) pair. J Biol Chem. 1978 Nov 25;253(22):8065–8071. [PubMed] [Google Scholar]
- WHARTON D. C., TZAGOLOFF A. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LVII. THE NEAR INFRARED ABSORPTION BAND OF CYTOCHROME OXIDASE. J Biol Chem. 1964 Jun;239:2036–2041. [PubMed] [Google Scholar]
- Wikström K. F., Harmon H. J., Ingledew W. J., Chance B. A re-evaluation of the spectral, potentiometric and energy-linked properties of cytochrome c oxidase in mitochondria. FEBS Lett. 1976 Jun 15;65(3):259–277. doi: 10.1016/0014-5793(76)80127-5. [DOI] [PubMed] [Google Scholar]
- Wilson M. T., Greenwood C., Brunori M., Antonini E. Kinetic studies on the reaction between cytochrome c oxidase and ferrocytochrome c. Biochem J. 1975 Apr;147(1):145–153. doi: 10.1042/bj1470145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]
- van Buuren K. J., Nicholis P., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972 Feb 28;256(2):258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]
- van Gelder B. F., Tiesjema R. H., Muijsers A. O., van Buuren K. J., Wever R. Mechanism of action of cytochrome c oxidase and its implications for energy conservation. Fed Proc. 1973 Sep;32(9):1977–1980. [PubMed] [Google Scholar]


