Abstract
Background and purpose
Cognitive impairment (CI) in multiple sclerosis (MS) is associated with bidirectional changes in resting‐state centrality measures. However, practicable functional magnetic resonance imaging (fMRI) biomarkers of CI are still lacking. The aim of this study was to assess the graph‐theory‐based degree rank order disruption index (k D) and its association with cognitive processing speed as a marker of CI in patients with MS (PwMS) in a secondary cross‐sectional fMRI analysis.
Methods
Differentiation between PwMS and healthy controls (HCs) using k D and its correlation with CI (Symbol Digit Modalities Test) was compared to established imaging biomarkers (regional degree, volumetry, diffusion‐weighted imaging, lesion mapping). Additional associations were assessed for fatigue (Fatigue Scale for Motor and Cognitive Functions), gait and global disability.
Results
Analysis in 56 PwMS and 58 HCs (35/27 women, median age 45.1/40.5 years) showed lower k D in PwMS than in HCs (median −0.30/−0.06, interquartile range 0.55/0.54; p = 0.009, Mann–Whitney U test), yielding acceptable yet non‐superior differentiation (area under curve 0.64). k D and degree in medial prefrontal cortex (MPFC) correlated with CI (k D/MPFC Spearman's ρ = 0.32/−0.45, p = 0.019/0.001, n = 55). k D also explained fatigue (ρ = −0.34, p = 0.010, n = 56) but neither gait nor disability.
Conclusions
k D is a potential biomarker of CI and fatigue warranting further validation.
Keywords: biomarkers, cognitive processing speed, fatigue, fMRI, multiple sclerosis
Multiple sclerosis was associated with global disruption of degree rank ordering (k D), a graph‐theoretical measure of resting‐state functional connectivity. Degree rank order disruption explained deficits in cognitive processing speed and fatigue score, but was not correlated with gait or global disability assessments. Whilst slowing of cognitive processing was associated with increased degree centrality in the medial prefrontal cortex, fatigue was accompanied by a higher degree in the cerebellum and caudate nuclei and a lower degree in the left fusiform gyrus.
INTRODUCTION
Multiple sclerosis (MS) is frequently associated with cognitive impairment (CI) [1]. Being often neglected in routine neurological examination [1, 2], CI and its imaging biomarkers, such as grey matter volume, white matter integrity or resting‐state functional connectivity (rsFC), are attracting increasing attention [1, 3]. Recent resting‐state functional magnetic resonance imaging (fMRI) studies utilizing graph‐theoretical approaches have identified a relationship between CI and decreased overall mean degree [4] or increased centrality in the default mode network (DMN) accompanied by decreased centrality outside the DMN [5, 6, 7, 8, 9, 10]. Moreover, changes in centrality also seem to precede the actual cognitive decline, indicating its potential utility as a prognostic biomarker [6]. However, the ultimate goal, that is, providing individual predictions applicable in day‐to‐day practice, remains far from achieved, thus urging further refinement of imaging and analytical methods [3].
Whereas a voxel‐wise centrality assessment would require considerable time and personal resources, a recently introduced centrality‐derived global scalar metric, the degree rank order disruption index (k D) [11], could serve as a biomarker reflecting simultaneous focal increases and decreases in degree centrality throughout the brain, requiring less demanding interpretation. The k D has previously been demonstrated to be associated with brain‐wide degree centrality changes in impaired consciousness [12] and chronic pain [11], but so far it was evaluated neither as a biomarker of the MS‐related brain damage nor as a predictor of CI in MS.
Hence, the aim of this study was to investigate the relationship between k D and the presence of MS and its clinical presentation (reduced cognitive processing speed as a marker of global CI) in comparison to established multimodal MRI biomarkers (resting‐state fMRI with regional degree assessment, diffusion‐weighted imaging [DWI], volumetry and lesion mapping) in patients with MS and matched healthy controls. The hypotheses were as follows: (1) k D differs between patients with MS (PwMS) and matched healthy controls (HCs); (2a) k D is superior to the regional degree centrality in the pre‐selected regions of interest (ROIs) in differentiating between PwMS and HCs and (2b) improves such differentiation when used in conjunction with established structural imaging diagnostic biomarkers of MS (lesion load, global atrophy, global white matter integrity); (3) k D correlates with deficits in cognitive processing speed in PwMS; (4a) k D provides superior correlation with cognitive processing speed in comparison to the regional degree centrality in pre‐selected ROIs and (4b) improves the regression model of cognitive processing speed when added on top of established structural imaging diagnostic biomarkers of MS.
In addition, the following exploratory hypotheses were tested to assess associations between k D and potential confounding factors: (5) k D correlates with global disability, fatigue and motor performance (gait) and (6) improves regression models for these clinical outcomes when used jointly with the established structural imaging diagnostic biomarkers of MS (lesion load, global atrophy, global white matter integrity); (7) k D correlates with these structural imaging biomarkers; and (8) the structural imaging diagnostic biomarkers of MS differ between PwMS and matched HCs.
METHOD
Study design and participant selection
The secondary analysis was performed on cross‐sectional imaging and behavioural data of 65 PwMS and 65 HCs matched for age and sex, with participants recruited from MS centres across Czechia. The same cohort has been partially analysed and published using other methods [13, 14]. Original inclusion criteria were the diagnosis of MS [15]; spastic paraparesis as a prominent clinical feature; stable clinical status for at least 3 months preceding the study (determined by a neurologist); physical ability to undergo clinical testing—consistent with an Expanded Disability Status Scale (EDSS) score ≤7.5. Subjects were excluded in the case of missing imaging data or imaging artefacts and conditionally excluded if exceeding the motion outlier criteria (see the section ‘Pre‐processing’ in the Supplementary Methods). To address potential attrition bias, a sensitivity analysis was performed in a sample including motion outlier subjects.
Clinical assessment and questionnaires
Clinical parameters comprised an objective assessment of cognitive performance using a Symbol Digit Modalities Test (SDMT) [16] and possible confounding factors such as fatigue (Fatigue Scale for Motor and Cognitive Functions, FSMC) [17], global disability (EDSS) [18] and motor performance (Timed Up and Go Test, TUG) [19].
Standard protocol approvals, registrations and patient consents
The secondary data analysis was pre‐registered at osf.io (https://osf.io/v8ejw). The original study was approved by the Ethics Committee of the Faculty Hospital Královské Vinohrady, approval no. EK‐VP/22/0/2014. All patients gave their written informed consent to participate in the study.
MRI data acquisition
Imaging was performed using a 3 T magnetic resonance scanner (Siemens Trio Tim, Erlangen, Germany) equipped with a 12‐channel phased‐array head coil. The MRI protocol included blood oxygenation level dependent resting‐state fMRI, as well as high‐resolution 1‐mm T1‐weighted and T2‐weighted imaging and 2‐mm DWI. Detailed acquisition parameters have been published elsewhere [13, 14].
Imaging data analysis
Following the pre‐processing, subject‐specific functional connectivity matrices containing Fisher‐transformed Pearson's r coefficients were computed in CONN toolbox v. 21a [20] for 4632 large voxels created using 6‐mm resampling of a common grey matter mask (see Supplementary Methods for more details). Degree centrality was then computed for each subject using a brain connectivity toolbox (https://sites.google.com/a/brain‐connectivity‐toolbox.net/bct/) with 10% link density [11]. Regional degree centrality was extracted by averaging nodal degree from the DMN (four ROIs) [5, 6, 8], basal ganglia (six ROIs) [6, 8], thalamus, hippocampus and cerebellum (five ROIs) [8] and from the multimodal ROIs explicitly participating in the SDMT: superior parietal lobule (two ROIs), dorsolateral prefrontal cortex (two ROIs) and anterior cingulate cortex (ACC) (1 ROI) [21] (see Figure S1 and Table S1). Finally, k D was calculated using custom MATLAB scripts implementing a modified approach according to Mansour et al. [11], as described in Supplementary Methods.
The pre‐processing of T1‐weighted and DWI data, the calculation of grey matter volume (GMV) as a measure of cortical atrophy and extraction of fractional anisotropy (FA) as a measure of white matter integrity, as well as the ROI definition for GMV and FA are described elsewhere [14]. Finally, the lesion load (LL) was calculated using the lesion segmentation tool (https://www.statistical‐modelling.de/lst.html) with the lesion prediction algorithm [22].
Statistical analysis
Initially, normality was assessed for all continuous variables using the Kolmogorov–Smirnov test. Non‐parametric tests were applied in case normality was violated. Additionally, regressors considerably deviating from the normal distribution (LL) were log‐transformed prior to any subsequent analysis to meet regression model assumptions. Demographic variables were compared between groups using Fisher's exact test and the Mann–Whitney U test. Pairwise deletion was applied in the case of missing clinical data. A summary of all variables, outcome measures and statistical tests for each hypothesis is provided in Table S2. All tests were performed using SPSS v29.0.1.1 (IBM, Armonk, NY, USA). p < 0.05 was considered significant. For correlations of regional degree centrality (4a), Bonferroni–Holm correction for multiple comparisons across 18 ROIs was applied (α = 0.0028). One‐tailed tests were used where superiority was assumed by the hypotheses or statistics with one‐tailed distribution were employed (2a, 2b, 4b and 6), with two‐tailed tests applied otherwise. Details on figure preparation, power analysis as well as additional post hoc analyses are provided in Supplementary Methods.
RESULTS
Study sample
Out of the original sample of 65 PwMS and 65 HCs, one PwMS and one HC were excluded due to missing data (incomplete field of view and susceptibility artefact) and another PwMS was excluded due to a suspected vascular lesion. In the remaining sample, 13 subjects with excessive motion levels were identified (seven PwMS and six HCs) (see Figure S2 for the inclusion/exclusion diagram). Here, only results in 56 PwMS and 58 HCs after excluding motion outliers are reported (‘final’ sample), whereas results in the sample with outliers are provided in Supplementary Results. Whilst both analyses yielded mostly similar results, two differences are explicitly stated below. Demographic details of the ‘final’ sample and summary statistics for clinical parameters are provided in Table 1.
TABLE 1.
Demographic and clinical data.
| Variable | Statistic | PwMS | HC | p | |
|---|---|---|---|---|---|
| Number | Count | 56 | 58 | ||
| Sex [women/men] | 35/21 | 27/31 | 0.095 a | ||
| Age [years] | Median ± IQR | 45.1 ± 17 | 40.5 ± 17 | 0.090 b | |
| Diagnosis | RRMS | Count, % | 35, 62.5% | ||
| SPMS | 15, 26.8% | ||||
| PPMS | 5, 8.9% | ||||
| no data | 1, 1.8% | ||||
| Time since diagnosis [years] | Mean ± SD | 12.6 ± 6.2 | |||
| EDSS | Median ± IQR | 4.5 ± 2.5 | |||
| SDMT | 45 ± 29 | ||||
| FSMC | 57 ± 23 | ||||
| TUG [s] | 10.3 ± 9 | ||||
Abbreviations: EDSS, Expanded Disability Status Scale; FSMC, Fatigue Scale for Motor and Cognitive Functions; HCs, healthy controls; IQR, interquartile range; MS, multiple sclerosis; PPMS, primary progressive MS; PwMS, patients with MS; RRMS, relapsing–remitting MS; SD, standard deviation; SDMT, Symbol Digit Modalities Test; SPMS, secondary progressive MS; TUG, Timed Up and Go Test.
Fisher's exact test.
Mann–Whitney U test.
Group differences and differentiation between PwMS and HCs
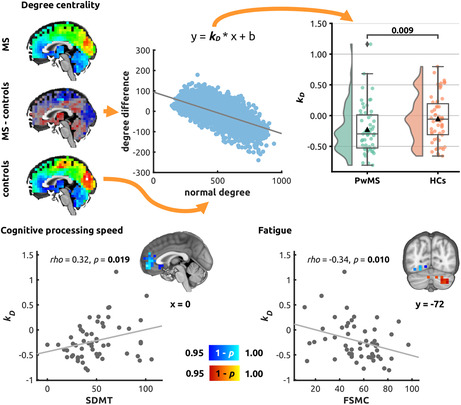
Patients with MS showed significantly lower k D compared to HCs (PwMS, median −0.298, interquartile range [IQR] 0.549; HCs, median −0.058, IQR = 0.542; p = 0.009; Mann–Whitney U test) (Figure 1). Underlying raw degree centrality data are summarized in Figure S3.
FIGURE 1.
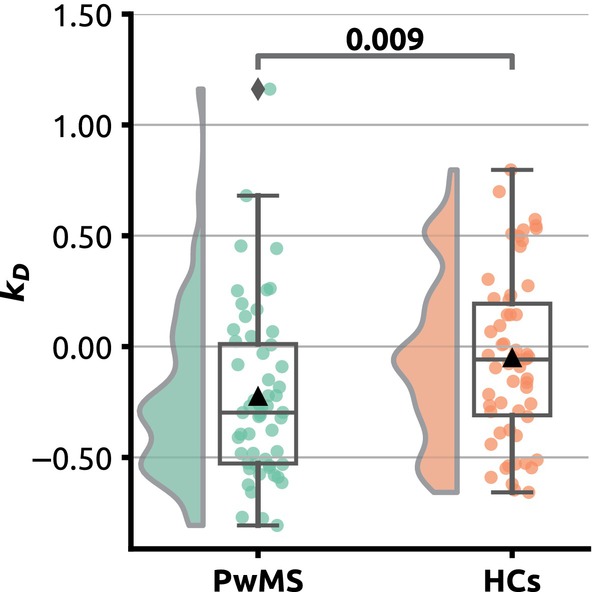
Group differences in degree rank order disruption index (k D). Raincloud plots illustrating individual k D values and distribution. Patients with multiple sclerosis are shown in green, healthy controls in red. Mann–Whitney U test p value is provided in annotation.
The ROC analysis for differentiation between PwMS and HCs yielded significant above‐chance area under curve (AUC) for k D (AUC = 0.642, p = 0.007; two‐tailed asymptotic significance for null hypothesis AUC = 0.5), the left lateral parietal portion of the DMN (AUC = 0.671, p = 0.001) and the ACC (AUC = 0.619; p = 0.026) (Figure 2 and Table S3). In pairwise comparisons, AUC for k D was significantly higher than AUC for six ROIs and did not significantly differ from the remaining ROIs (Table S3).
FIGURE 2.
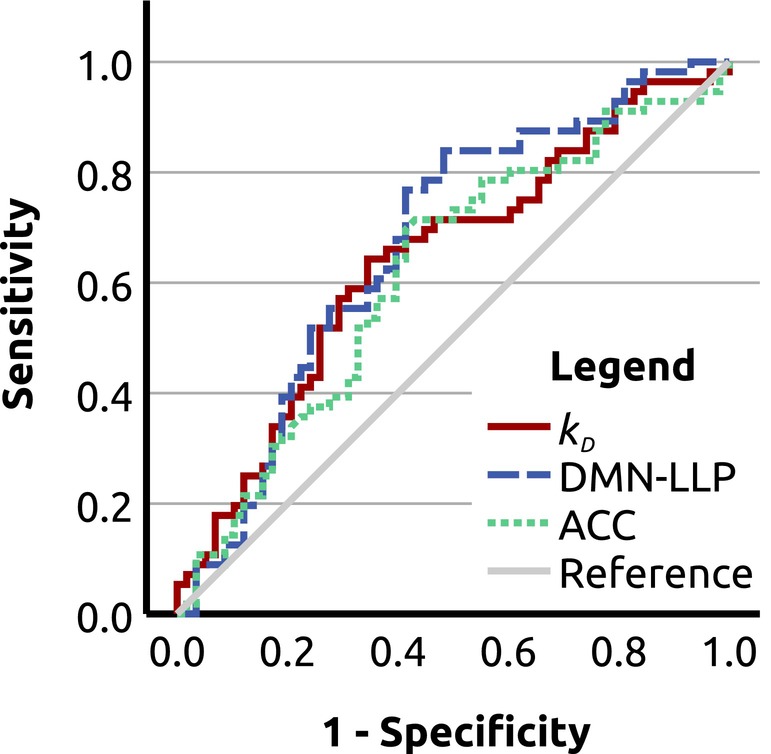
Receiver operating characteristic (ROC) analysis. ROC curves for differentiation between patients with multiple sclerosis and healthy controls using degree rank order disruption index (k D) (area under the curve [AUC] = 0.642; p = 0.007; two‐tailed asymptotic significance for null hypothesis AUC = 0.5, uncorrected); the left lateral parietal portion of the default mode network (DMN‐LLP; AUC = 0.671; p = 0.001) and the anterior cingulate cortex (ACC; AUC = 0.619; p = 0.026).
No significant improvement was observed in a multiple logistic regression model differentiating between PwMS and HCs after adding k D as an additional regressor on top of GMV, FA, log(LL) (χ 2 step 0.007, p = 0.934).
Correlation with cognitive processing speed
A significant correlation was detected between k D and SDMT (Spearman's ρ = 0.32, p = 0.019, n = 55, Figure 3). For the regional degree centrality, significant correlation with SDMT was observed in the medial prefrontal part of the DMN, yielding slightly higher effect size than the k D (Table 2). An ordinal regression model including GMV, FA, log(LL), age, sex and years since diagnosis as regressors of the SDMT score was significantly improved after adding k D (χ 2 step 4.49, p = 0.034, likelihood ratio test; see Table S4). In contrast, neither significant correlation with SDMT nor improvement of the regression model for SDMT were observed in the analysis with motion outliers (see Supplementary Results).
FIGURE 3.
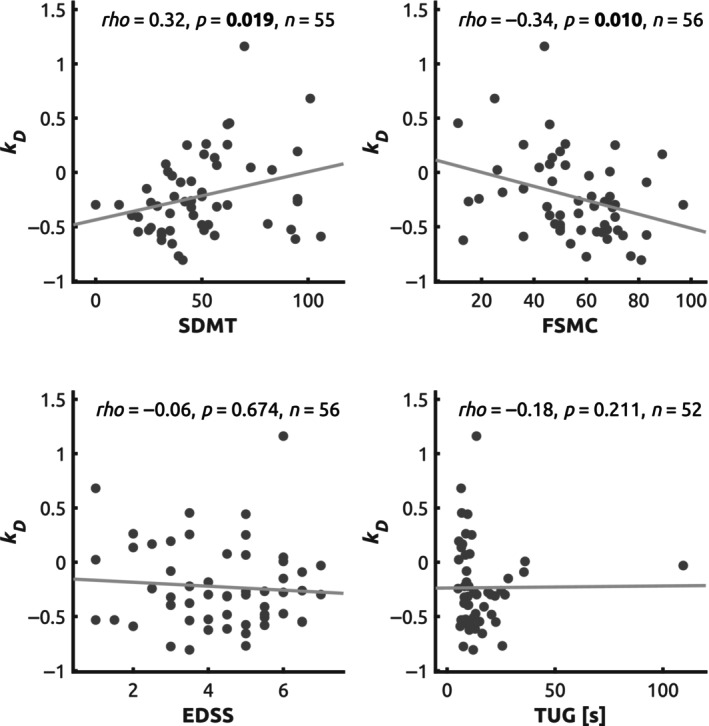
Correlation between k D and clinical scores. Scatter plots illustrating relationship between the degree rank order disruption index (k D) and cognitive processing speed (Symbol Digit Modalities Test, SDMT), global disability (Expanded Disability Status Scale, EDSS), fatigue (Fatigue Scale for Motor and Cognitive Functions, FSMC) and motor performance (Timed Up and Go Test, TUG). Spearman's rank correlation coefficient (rho), two‐tailed uncorrected significance and number of valid observations are provided.
TABLE 2.
Correlation between regional degree and clinical scores.
| ROI | SDMT n = 55 | FSMC n = 56 | ||
|---|---|---|---|---|
| ρ a | p a | ρ a | p a | |
| DMN‐MPFC | −0.45 | 0.001 | 0.23 | 0.081 |
| DMN‐LP | ||||
| L | −0.03 | 0.847 | 0.12 | 0.365 |
| R | −0.28 | 0.036 | 0.27 | 0.041 |
| DMN‐PCC | −0.18 | 0.177 | 0.13 | 0.346 |
| Putamen | ||||
| L | −0.35 | 0.009 | 0.38 | 0.004 |
| R | −0.27 | 0.044 | 0.30 | 0.024 |
| Caudate nucleus | ||||
| L | −0.35 | 0.008 | 0.41 | 0.002 |
| R | −0.38 | 0.004 | 0.40 | 0.002 |
| Thalamus | ||||
| L | −0.27 | 0.043 | 0.30 | 0.025 |
| R | −0.27 | 0.046 | 0.25 | 0.059 |
| Hippocampus | ||||
| L | −0.24 | 0.084 | 0.23 | 0.085 |
| R | −0.33 | 0.015 | 0.26 | 0.053 |
| Cerebellum | −0.25 | 0.067 | 0.34 | 0.010 |
| Superior parietal lobule | ||||
| L | −0.04 | 0.781 | −0.21 | 0.118 |
| R | −0.12 | 0.396 | −0.12 | 0.369 |
| DLPFC | ||||
| L | −0.05 | 0.728 | −0.24 | 0.069 |
| R | −0.11 | 0.424 | 0.01 | 0.913 |
| ACC | −0.21 | 0.120 | 0.06 | 0.657 |
Abbreviations: ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; DMN, default mode network; DMN‐LP, lateral parietal part of the DMN; DMN‐MPFC, DMN ‐ medial prefrontal cortex; DMN‐PCC, DMN ‐ posterior cingulate cortex; FSMC, Fatigue Scale for Motor and Cognitive Functions; L, left; R, right; ROI, region of interest; SDMT, Symbol Digit Modalities Test; SPL, superior parietal lobule.
Spearman's rank correlation coefficient ρ, significant correlations at Bonferroni–Holm‐corrected α = 0.0028 are in bold type.
Correlation with fatigue, global disability and motor performance
A significant correlation was detected between k D and FSMC (Spearman's ρ = −0.34, p = 0.010, n = 56) but not for EDSS (Spearman's ρ = −0.06, p = 0.674, n = 56) or TUG (Spearman's ρ = −0.18, p = 0.211, n = 52) (Figure 4). In ordinal regression, k D significantly improved the model fit for fatigue (FSMC) when added on top of GMV, FA, log(LL), age, sex and years since diagnosis, but not for EDSS or TUG (Table S4).
FIGURE 4.
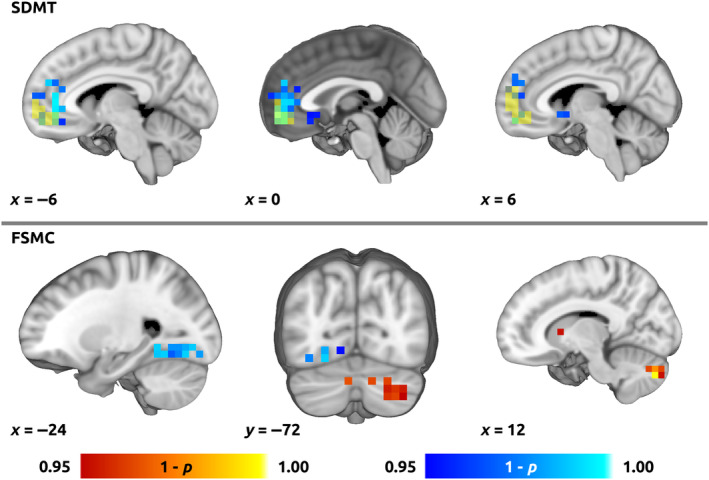
Voxel‐wise correlation between degree and clinical scores. Colour overlays illustrating spatial distribution of correlation of the voxel‐wise degree centrality with (top) cognitive processing speed (Symbol Digit Modalities Test, SDMT) and (bottom) fatigue (Fatigue Scale for Motor and Cognitive Functions, FSMC). Red–yellow and blue–light blue overlays indicate positive and negative correlation, respectively. Thresholded using non‐parametric threshold‐free cluster enhancement (10,000 permutations, family‐wise error‐corrected p = 0.05). In the top panel, yellow overlay shows overlap with the medial prefrontal cortex default mode network (MPFC‐DMN) region of interest.
Relationship between k D and structural imaging biomarkers
No significant correlation was observed between k D and structural imaging parameters, that is, GMV, LL and global FA (see Figure S4). In analysis with motion outliers, however, k D was significantly correlated with LL (see Supplementary Results). All structural imaging parameters significantly differed between PwMS and HCs (see Table S5).
Analysis with motion outliers
For complete results of the sensitivity analysis with motion outliers, see Supplementary Results and Tables S6–S10.
Post hoc analyses
Voxel‐wise group differences are illustrated in Figure S3. Voxel‐wise regression analysis for degree centrality as response variable and SDMT as explanatory variable yielded significant clusters with negative effect mainly in medial prefrontal cortex (MPFC) and ACC, and to a lesser degree in the subcallosal cortex and the right nucleus accumbens, overlapping in part with the DMN‐MPFC ROI (Figure 4, Figure S3 for unthresholded data). The regression for FSMC yielded a positive effect in predominantly the right cerebellum, right caudate nucleus, right inferior frontal gyrus and a negative effect in the left temporo‐occipital fusiform and lateral occipital cortex (Figure 4, Figure S3 for unthresholded data).
The ROI analysis for FSMC yielded significant correlation in the left and right caudate nuclei (left, Spearman's ρ = 0.41, p = 0.002; right, Spearman's ρ = 0.40, p = 0.002; n = 56; Bonferroni–Holm‐corrected across the 18 ROIs, i.e., α = 0.0028). See Table 2 for complete results.
DISCUSSION
The present study aimed to investigate the potential utility of k D as a new functional imaging biomarker of cognitive processing speed (and hence of CI) in MS in comparison to regional degree centrality. Whilst k D in PwMS significantly differed from HCs and was a significant explanatory variable for cognitive processing speed (SDMT) in PwMS, it yielded weaker correlation than the mean degree centrality in the frontal hub (MPFC) of the DMN. In an exploratory analysis, k D turned out to be a significant explanatory variable for self‐reported fatigue. A post hoc analysis indicated that the correlation with fatigue might be driven by degree centrality changes in the cerebellum, basal ganglia (caudate nuclei) and left fusiform gyrus. These results were shown to be largely independent of structural imaging parameters, which were not significantly correlated with k D.
Differentiation between PwMS and HCs
Our primary observation of decreased k D in PwMS captures the global character of changes in nodal centrality (both degree and eigenvector) that have recently been reported on the local and network‐wide level in MS [5, 7, 8, 23, 24]. Lower k D in PwMS suggests less centralized and more diffusely distributed rsFC, which is analogous to a previously described disruption of the rich‐club topology of the brain network in MS [25]. However, our multiple regression analysis indicated that k D did not improve differentiation between PwMS and HCs when added on top of structural imaging parameters. Taken together with the considerable overlap between k D distributions in PwMS and HCs (Figure 1), it can be inferred that degree reordering is not primarily driven by the mere presence of MS and is more probably related to the resulting neurological deficits.
Correlation with cognitive processing speed
Our next main analysis demonstrated that k D was a significant explanatory variable for cognitive processing speed compared to global structural imaging parameters. Whilst the negative correlation between k D and SDMT is a novel finding, it is in line with previous evidence for a weak positive association between SDMT score and rsFC of the peripheral nodes outside the rich club [25]. The correlation between k D and SDMT yielded an effect size similar to some previously reported structural imaging biomarkers of cognitive processing speed, including fractional anisotropy in the superior longitudinal fascicle [26] or grey matter atrophy‐based brain age gap [27], but was lower than overall effect size in a recent meta‐analysis of multimodal structural MRI data [28].
In our dataset, a superior correlation with SDMT was achieved using regional degree centrality in the MPFC hub of the DMN, yielding an effect size comparable with structural imaging biomarkers [28, 29]. Correspondingly, cognitive impairment in MS has been shown to be associated with increased degree or eigenvector centrality in the DMN [6, 7, 8, 9, 10] and decreased centrality in the visual [7, 9, 10] and sensorimotor network [7], whilst eigenvector and degree centrality show high agreement even within the same group [8]. Nevertheless, our results add novel evidence for the strength of the relationship between degree centrality in the DMN and cognitive processing speed since no such correlation in MS has been reported before.
Role of the DMN in pathophysiology of cognitive deterioration in MS
The ROI and post hoc voxel‐wise analyses indicated that the MPFC and ACC (i.e., anterior DMN) provided the highest correlation between degree and cognitive processing speed. The role of abnormalities in DMN in CI remains controversial, possibly being non‐specifically linked to the dysfunction of the entire brain network [3]. However, an excessively central and less dynamic DMN has also been proposed to directly hinder externally oriented cognitive processing by superfluous introspective thoughts [7]. Additionally, rsFC studies using the Paced Auditory Serial Addition Test also point to dysfunction of DMN and subcallosal cortex [24, 29]. Hence, graph‐theoretical measures extracted from the anterior portion of the DMN are potential future candidates for even more accurate biomarkers of cognitive processing speed than k D.
Association with fatigue
Our results indicate that, cognitive processing speed aside, k D was also a significant explanatory variable for the self‐reported global fatigue score (FSMC). Whilst cognitive performance and fatigue have been shown to be associated with similar rsFC dysfunctions, such as increased rsFC in posterior DMN and reduced rsFC in the anterior DMN [30], our post hoc analyses on ROI and voxel‐wise level suggested potential differentiation between mechanisms underlying cognitive decline and global fatigue. Whereas SDMT correlated with average degree in DMN‐MPFC, fatigue scores were more strongly associated with degree in the caudate nuclei, cerebellum and fusiform cortex (Table 2 and Figure 4).
From the network perspective, fatigue has been associated with damage to cortico‐subcortical pathways and with particular involvement of the prefrontal cortex [31]. On the computational (metacognitive) level, it has been proposed to result from mismatch between predicted and measured output from cognitive and sensorimotor networks [3, 32]. Our results fit in by emphasizing the role of basal ganglia [33] and cerebellum, which is involved in maintaining internal forward models and error monitoring [34]. Future dedicated studies should evaluate the specificity and stability of the here identified biomarkers and their accuracy with respect to the motor and cognitive sub‐domains of FSMC.
Limitations and future directions
Whilst the main strength of the study was rigorous pre‐registration of all main analyses, there are also several limitations related to the fact that this study was carried out as a secondary analysis: SDMT reflects mainly cognitive processing speed and involves visual processing. Further assessments across multiple cognitive domains and sensory modalities as well as consideration of depression as a potential confounding factor are warranted. Furthermore, normative data, assessment of test–retest reliability, consideration of compound predictive models (involving regional degree centrality) and longitudinal evaluation are also necessary. Finally, our data cannot be currently generalized to all individuals with MS (see our inclusion criteria); hence, a cross‐validation of our results in an independent dataset is necessary before translating the results into clinical practice.
CONCLUSION
Although our results require further cross‐validation, they suggest that obtaining a single scalar functional imaging biomarker of cognitive processing speed and CI in general is feasible and may provide an important diagnostic tool to assess performance decline due to CI and fatigue.
AUTHOR CONTRIBUTIONS
Pavel Hok: Writing – original draft; writing – review and editing; conceptualization; methodology; software; formal analysis; visualization. Quang Thong Thai: Formal analysis; writing – review and editing. Barbora Rehák Bučková: Investigation; conceptualization; methodology; data curation; writing – review and editing. Martin Domin: Software; formal analysis; writing – review and editing; writing – original draft. Kamila Řasová: Investigation; writing – review and editing. Jaroslav Tintěra: Investigation; writing – review and editing. Martin Lotze: Conceptualization; writing – review and editing; supervision; resources. Matthias Grothe: Writing – review and editing; conceptualization; supervision. Jaroslav Hlinka: Conceptualization; methodology; investigation; supervision; writing – review and editing; writing – original draft; validation.
FUNDING INFORMATION
P. Hok was awarded a Gerhard‐Domagk fellowship by University Medicine Greifswald for undertaking this study. K. Řasová was supported by Charles University, programme Cooperatio (Neuroscience) and 260648/SVV/2024. J. Hlinka was supported by the ERDF‐Project Brain dynamics, No. CZ.02.01.01/00/22_008/0004643.
CONFLICT OF INTEREST STATEMENT
None.
Supporting information
Data S1.
ACKNOWLEDGEMENT
Open Access funding enabled and organized by Projekt DEAL.
Hok P, Thai QT, Bučková BR, et al. Global functional connectivity reorganization reflects cognitive processing speed deficits and fatigue in multiple sclerosis. Eur J Neurol. 2025;32:e16421. doi: 10.1111/ene.16421
Matthias Grothe and Jaroslav Hlinka contributed equally to this work.
DATA AVAILABILITY STATEMENT
Part of the dataset (imaging data for 60 PwMS with the respective global disability and motor scales) is publicly available in an on‐line repository at https://osf.io/p2kj7/. Data not provided in the article may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results, upon signing a data sharing agreement. The custom MATLAB script for k D calculation is available at https://github.com/pavelhok/calculate_kd/tree/MS‐project.
REFERENCES
- 1. Benedict RHB, Amato MP, DeLuca J, Geurts JJG. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19(10):860‐871. doi: 10.1016/S1474-4422(20)30277-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sumowski JF, Benedict R, Enzinger C, et al. Cognition in multiple sclerosis: state of the field and priorities for the future. Neurology. 2018;90(6):278‐288. doi: 10.1212/WNL.0000000000004977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chard DT, Alahmadi AAS, Audoin B, et al. Mind the gap: from neurons to networks to outcomes in multiple sclerosis. Nat Rev Neurol. 2021;17(3):173‐184. doi: 10.1038/s41582-020-00439-8 [DOI] [PubMed] [Google Scholar]
- 4. Rocca MA, Valsasina P, Meani A, Falini A, Comi G, Filippi M. Impaired functional integration in multiple sclerosis: a graph theory study. Brain Struct Funct. 2016;221(1):115‐131. doi: 10.1007/s00429-014-0896-4 [DOI] [PubMed] [Google Scholar]
- 5. Carotenuto A, Valsasina P, Schoonheim MM, et al. Investigating functional network abnormalities and associations with disability in multiple sclerosis. Neurology. 2022;99(22):e2517‐e2530. doi: 10.1212/WNL.0000000000201264 [DOI] [PubMed] [Google Scholar]
- 6. Dekker I, Schoonheim MM, Venkatraghavan V, et al. The sequence of structural, functional and cognitive changes in multiple sclerosis. Neuroimage Clin. 2021;29:102550. doi: 10.1016/j.nicl.2020.102550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eijlers AJC, Wink AM, Meijer KA, Douw L, Geurts JJG, Schoonheim MM. Reduced network dynamics on functional MRI signals cognitive impairment in multiple sclerosis. Radiology. 2019;292(2):449‐457. doi: 10.1148/radiol.2019182623 [DOI] [PubMed] [Google Scholar]
- 8. Eijlers AJC, Meijer KA, Wassenaar TM, et al. Increased default‐mode network centrality in cognitively impaired multiple sclerosis patients. Neurology. 2017;88(10):952‐960. doi: 10.1212/WNL.0000000000003689 [DOI] [PubMed] [Google Scholar]
- 9. Huiskamp M, Eijlers AJC, Broeders TAA, et al. Longitudinal network changes and conversion to cognitive impairment in multiple sclerosis. Neurology. 2021;97(8):e794‐e802. doi: 10.1212/WNL.0000000000012341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eijlers AJC, Meijer KA, van Geest Q, Geurts JJG, Schoonheim MM. Determinants of cognitive impairment in patients with multiple sclerosis with and without atrophy. Radiology. 2018;288(2):544‐551. doi: 10.1148/radiol.2018172808 [DOI] [PubMed] [Google Scholar]
- 11. Mansour A, Baria AT, Tetreault P, et al. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep. 2016;6(1):34853. doi: 10.1038/srep34853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Achard S, Delon‐Martin C, Vértes PE, et al. Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci USA. 2012;109(50):20608‐20613. doi: 10.1073/pnas.1208933109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bučková B, Kopal J, Řasová K, Tintěra J, Hlinka J. Open access: the effect of neurorehabilitation on multiple sclerosis—unlocking the resting‐state fMRI data. Front Neurosci. 2021;15:662784. doi: 10.3389/fnins.2021.662784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rehák Bučková B, Mareš J, Škoch A, et al. Multimodal‐neuroimaging machine‐learning analysis of motor disability in multiple sclerosis. Brain Imaging Behav. 2023;17(1):18‐34. doi: 10.1007/s11682-022-00737-3 [DOI] [PubMed] [Google Scholar]
- 15. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292‐302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith A. Symbol Digit Modalities Test. Western Psychological Services; 2017. [Google Scholar]
- 17. Penner IK, Raselli C, Stöcklin M, Opwis K, Kappos L, Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis‐related fatigue. Mult Scler. 2009;15(12):1509‐1517. doi: 10.1177/1352458509348519 [DOI] [PubMed] [Google Scholar]
- 18. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444‐1452. doi: 10.1212/wnl.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 19. Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142‐148. doi: 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 20. Whitfield‐Gabrieli S, Nieto‐Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125‐141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 21. Grothe M, Domin M, Hoffeld K, Nagels G, Lotze M. Functional representation of the symbol digit modalities test in relapsing remitting multiple sclerosis. Mult Scler Relat Disord. 2020;43:102159. doi: 10.1016/j.msard.2020.102159 [DOI] [PubMed] [Google Scholar]
- 22. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR‐hyperintense white‐matter lesions in multiple sclerosis. NeuroImage. 2012;59(4):3774‐3783. doi: 10.1016/j.neuroimage.2011.11.032 [DOI] [PubMed] [Google Scholar]
- 23. Schoonheim MM, Geurts J, Wiebenga OT, et al. Changes in functional network centrality underlie cognitive dysfunction and physical disability in multiple sclerosis. Mult Scler. 2014;20(8):1058‐1065. doi: 10.1177/1352458513516892 [DOI] [PubMed] [Google Scholar]
- 24. Tommasin S, De Giglio L, Ruggieri S, et al. Multi‐scale resting state functional reorganization in response to multiple sclerosis damage. Neuroradiology. 2020;62(6):693‐704. doi: 10.1007/s00234-020-02393-0 [DOI] [PubMed] [Google Scholar]
- 25. Stellmann JP, Hodecker S, Cheng B, et al. Reduced rich‐club connectivity is related to disability in primary progressive MS. Neurol Neuroimmunol Neuroinflam. 2017;4(5):e375. doi: 10.1212/NXI.0000000000000375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grothe M, Jochem K, Strauss S, et al. Performance in information processing speed is associated with parietal white matter tract integrity in multiple sclerosis. Front Neurol. 2022;13:982964. doi: 10.3389/fneur.2022.982964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Denissen S, Engemann DA, De Cock A, et al. Brain age as a surrogate marker for cognitive performance in multiple sclerosis. Eur J Neurol. 2022;29(10):3039‐3049. doi: 10.1111/ene.15473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pike AR, James GA, Drew PD, Archer RL. Neuroimaging predictors of longitudinal disability and cognition outcomes in multiple sclerosis patients: a systematic review and meta‐analysis. Mult Scler Relat Disord. 2022;57:103452. doi: 10.1016/j.msard.2021.103452 [DOI] [PubMed] [Google Scholar]
- 29. Has Silemek AC, Fischer L, Pöttgen J, et al. Functional and structural connectivity substrates of cognitive performance in relapsing remitting multiple sclerosis with mild disability. Neuroimage Clin. 2020;25:102177. doi: 10.1016/j.nicl.2020.102177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bisecco A, Nardo FD, Docimo R, et al. Fatigue in multiple sclerosis: the contribution of resting‐state functional connectivity reorganization. Mult Scler. 2018;24(13):1696‐1705. doi: 10.1177/1352458517730932 [DOI] [PubMed] [Google Scholar]
- 31. Penner IK, Paul F. Fatigue as a symptom or comorbidity of neurological diseases. Nature Rev Neurol. 2017;13(11):662‐675. doi: 10.1038/nrneurol.2017.117 [DOI] [PubMed] [Google Scholar]
- 32. Manjaly ZM, Harrison NA, Critchley HD, et al. Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2019;90(6):642‐651. doi: 10.1136/jnnp-2018-320050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fleischer V, Ciolac D, Gonzalez‐Escamilla G, et al. Subcortical volumes as early predictors of fatigue in multiple sclerosis. Ann Neurol. 2022;91(2):192‐202. doi: 10.1002/ana.26290 [DOI] [PubMed] [Google Scholar]
- 34. Diedrichsen J, King M, Hernandez‐Castillo C, Sereno M, Ivry RB. Universal transform or multiple functionality? Understanding the contribution of the human cerebellum across task domains. Neuron. 2019;102(5):918‐928. doi: 10.1016/j.neuron.2019.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
Part of the dataset (imaging data for 60 PwMS with the respective global disability and motor scales) is publicly available in an on‐line repository at https://osf.io/p2kj7/. Data not provided in the article may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results, upon signing a data sharing agreement. The custom MATLAB script for k D calculation is available at https://github.com/pavelhok/calculate_kd/tree/MS‐project.


