Abstract
Objectives
In this study, the impacts of various processing parameters on the quality and consumer satisfaction of dragon fruit beverages were investigated in order to establish an informative framework for the manufacturing of commercial dragon fruit beverages, focusing on health benefits and safety for consumers, and sensory appeal of the products.
Methods
This study employed various analytical methods to quantify some important phytochemical compounds in dragon fruit juice such as total and reducing sugars, vitamin C, betacyanins, anthocyanins, polyphenol, and so forth. This study also employed the Box–Behnken response surface experimental design to optimize various processing parameters for quality and consumer satisfaction, which include enzymatic pectin hydrolysis parameters and formulation for dragon fruit energy drink.
Results
The results from this study indicated a causal relationship between geological origin and some important characteristics of dragon fruit juice, emphasizing the need to track the source of fruits to ensure consistent quality of juice products. This study also systematically determined the impacts of various enzymatic hydrolysis, thermal sterilization, and formulation parameters on both nutrition content and sensory aspects of dragon fruit beverages, while also successfully optimized these parameters for the best outcomes.
Conclusion
This study had successfully achieved the objective of establishing a valuable framework for the manufacturing of commercial dragon fruit beverages, focusing on health benefits and safety for consumers, and sensory appeal of the products.
Keywords: Selenicereus costaricensis, pectinase hydrolysis, Box–Behnken optimization, healthy drink, natural extract
Introduction
In recent years, consumers are increasingly opting for healthy food choices that will help maintain their body weight, meet their nutritional requirements, and prevent chronic diseases. One of these choices is fruit juices — a rich source of natural vitamins, minerals, and antioxidants. The consumption of fruit juices has been evidently linked to numerous health benefits, which include reducing inflammation, protecting cardiovascular health, aiding in muscle recovery, boosting immune function, and promoting digestive health. 1 Due to such demand, the global fruit juice market size reached a value of about 150.52 billion US dollars in 2023, and was assessed to grow at an annual rate of 5.2% in the next 10 years, reaching the value of around 237.54 billion US dollars by 2032. 2
Known for its vivid color and being rich in betacyanins — a group of potent antioxidants, dragon fruit (Selenicereus costaricensis) have become increasingly popular worldwide both for its aesthetic appeal and for its health-promoting properties.3,4 In particular, betacyanins were shown to exhibit antioxidative, anti-inflammatory, and even anti-carcinogenic effects, making them highly desirable in diet and health-conscious consumer circles. 5 However, the incorporation of dragon fruit into beverages presents significant technical challenges, particularly in terms of maintaining the stability of these sensitive compounds during processing and ensuring the microbial safety of the final product without compromising its sensory and nutritional quality.
Processing techniques play a pivotal role in determining the quality and safety of fruit-based beverages. For example, thermal processes commonly employed to ensure microbial safety were known to cause the degradation of heat-sensitive nutrients such as betacyanins and vitamin C — both of which are abundant in dragon fruit and its juice.6,7 Such degradation not only diminishes the health benefits of the drink, but also affects its color and overall sensory attributes, which are crucial for consumer acceptance. Moreover, the application of enzymatic treatments to enhance the yield and clarity of fruit juices through breaking down complex polysaccharides like pectin has been well documented. 8 Nevertheless, optimizing these processes to balance enzyme activity, temperature control, and incubating duration to maximize both yield and nutrient preservation poses significant challenges that require thorough investigation.
This study aims to address these challenges by optimizing the enzymatic hydrolysis and thermal processing parameters specifically for the production of red-fleshed dragon fruit beverages. By systematically evaluating the impact of various processing conditions on the stability of betacyanins and the microbial safety of the juice, this research seeks to develop a scientifically validated approach that can be implemented commercially to produce dragon fruit beverages that are both high in nutrition content, safe to consume, and sensory appealing. The outcomes are expected to provide valuable insights for beverage manufacturers, contributing to the growing market for functional drinks and supporting consumer demand for healthy, natural, and safe beverage options.
Materials and methods
Materials
The red-fleshed dragon fruit used in this study was sourced from local farms in Vietnam, particularly in the provinces of Binh Thuan, Long An, Tien Giang, and Tra Vinh, which are known for their good quality and high yield of dragon fruit cultivation. Fresh fruits were harvested at the commercial ripening stage, then selected and prepared in accordance with CODEX Standard 237-2003 for pitahayas. 9
Pectinase enzyme (with an enzyme activity of 5000 PGU/g) was obtained from Sigma-Aldrich, USA. Food-grade refined sugar was acquired from Bien Hoa Sugar Co. (Vietnam). Food-grade citric acid was purchased from Shandong Ensign Industry Co. (China). Food-grade energy drink flavor was procured from MQFlavor (Vietnam).
Other chemical reagents were of analytical grade, and used in accordance with the instructions in relevant analytical standards without further processing, unless stated otherwise.
Experimental methods
The analysis on geological origin in this study was mostly conducted at the Faculty of Food Science and Technology and the Faculty of Tourism and Culinary (Ho Chi Minh City University of Industry and Trade) during the period from April 2023 to September 2023. Other experiments were both performed at aforementioned faculties, and also at the Center for High Technology Research and Development (Vietnam Academy of Science and Technology) during the period from October 2023 to April 2024.
For the analysis on geological origin and the impacts of sterilization parameters on microbial control and betacyanin stability, each experiment was performed in at least triplicate, with the results expressed as an average value. Statistical processing was performed using the JMP Pro 17 statistical software, utilized the analysis of variance (ANOVA) method and the Tukey's range test to evaluate the differences between samples (significance level = 0.05).
For the optimization of enzymatic pectin hydrolysis parameters and the optimizing formulation for dragon fruit energy drink, Box–Behnken experimental design was used, with specific experimental design matrix, results processing, and statistical analysis performed automatically utilizing the Design Expert 12 software. Details on Box–Behnken experimental designs were clarified below in the respective section, and in the attached Supplementary Materials 01.
Fruit juice extraction and enzymatic treatment procedure
In a general procedure, fresh dragon fruits were firstly peeled and diced into chunks of appropriate dimensions. The fruit chunks were then subjected to mechanical pressing using a hydraulic press in order to extract a bright red juice rich with pulp. Pectinase enzyme solutions were prepared by diluting the stock pectinase enzyme solution in distilled water, resulting in solutions with concentrations as presented in Table S1 (see Supplementary Materials 01).
The enzymatic treatment of the freshly pressed juice was conducted by firstly mixing the juice with a pectinase enzyme solution of specific concentration (volumetric ratio between the enzyme solution and the juice was 1:10). The mixture was then incubated at a particular temperature, allowing the pectinase-catalyzed hydrolysis reaction of pectin in the mixture to process. After some particular duration, the hydrolysis reaction was halted through enzyme deactivation by increasing the temperature of the mixture. Finally, the mixture was filtered to clear out undissolved solid contents, producing a clarified juice which was more eye-appealing and not prone to layer separation. 10
Specific parameters for each experiment were presented in Table S2 (see Supplementary Materials 01).
Fruit juice analysis
Freshly pressed juices and enzymatic treated juices were analyzed for key quality parameters and important bioactive contents, including vitamin C, polyphenols, and betacyanins. In particular, pH value and total dissolved solid content of the samples were determined directly using portable analysis devices, while total acidity of the samples were quantified through potentiometric titration method. 11 Vitamin C content (including dehydroascorbic acid and ascorbic acid), betacyanin content, and relative clarity of the samples were measured using spectrophotometric methods, respectively at the wavelength of 521 nm (after 2,4-dinitrophenylhydrazine coupling), 12 538 nm, 13 and 660 nm (expressed as the percentage of light transmittance). 14 Anthocyanin assessment of the samples were conducted through pH differential method, 15 while total polyphenol content of the samples were quantified using the Folin-Ciocalteu method. 16 Both reducing sugar content and total sugar content of the samples were determined through colorimetric method with 3,5-dinitrosalicylic acid (DNS) assay. 17
In addition to these parameters, antioxidant capability of the samples were also determined using the DPPH radical scavenging assay method. 18 For sterilized samples, the existent of microorganisms, yeasts, and molds were assessed through enumeration methods in accordance with international standards ISO 4833-1:2013 (colony count at 30°C by the pour plate technique) and ISO 21527-1:2008 (colony count technique in products with water activity greater than 0.95).
Sensory evaluation
Aside from subjective analysis, sensory evaluation was also conducted to assess the effects of some food additives and the sterilization process on consumer acceptability of the functional drink produced from red-fleshed dragon fruit juice. In particular, a panel of 20 trained assessors were instructed to evaluate prepared samples for their color, taste, aroma, and overall acceptability on a 7-point hedonic scale, from 1 (dislike extremely) to 7 (like extremely). 19
Box–Behnken design
In this study, 3-level Box–Behnken experimental design was applied to investigate and validate both enzymatic pectin hydrolysis process parameters influencing the clarity of red-fleshed dragon fruit juice, and amount of supplemental food additives affecting the sensory quality of dragon fruit energy drink. Particularly, there were four variable input parameters in enzymatic pectin hydrolysis experimental design, which included: pH (X1: 4–6), temperature (X2: 40–60°C), duration (X3: 60–180 min), and initial enzyme concentration (X4: 0.1–0.3%). Conversely, there were three variable input parameters in food additive supplementation experimental design, which included: amount of added sugar (X1: 60–140 g/L), amount of added citric acid (X2: 1–3 g/L), and amount of added energy drink flavor (X3: 1–3 g/L). The factor levels were coded as −1 (low), 0 (middle), and 1 (high). 20 Definitions of statistical terms and their significance were provided in detail by Montgomery. 21
Results and discussion
Regional variations in physicochemical properties and bioactive contents of dragon fruit
An comprehensive assessment of physicochemical properties and bioactive compounds of red-fleshed, pink-skinned dragon fruit collected from four provinces in Vietnam (namely: Binh Thuan, Long An, Tien Giang, and Tra Vinh) were conducted in order to investigate the possible influence of regional environmental conditions on fruit characteristics. The analytical results were presented in Table 1 and Supplementary Materials 02.
Table 1.
Physicochemical properties, chemical composition, and biological activity of red-fleshed, pink-skinned dragon fruit from four main cultivation provinces in Vietnam.
| Parameters | Binh Thuan | Long An | Tien Giang | Tra Vinh |
|---|---|---|---|---|
| Total dissolved solids (°Bx) | 12.50 ± 0.50 | 14.00 ± 0.50 | 13.17 ± 0.29 | 12.67 ± 0.29 |
| pH value | 4.90 ± 0.14 | 4.52 ± 0.09 | 4.56 ± 0.10 | 4.71 ± 0.06 |
| Reducing sugars (g/L) | 99.23 ± 6.50 | 115.65 ± 4.80 | 106.57 ± 4.79 | 101.67 ± 4.07 |
| Total sugars (g/L) | 109.94 ± 6.38 | 127.76 ± 7.34 | 116.80 ± 7.35 | 112.94 ± 2.25 |
| Vitamin C (ppm) | 229.34 ± 17.78 | 273.86 ± 16.81 | 257.56 ± 13.83 | 251.38 ± 12.10 |
| Total betacyanins (mg/100g) | 12.05 ± 0.49 | 10.52 ± 0.51 | 10.95 ± 0.68 | 11.44 ± 0.46 |
| Anthocyanins (mg/100g) | 212.77 ± 12.95 | 176.31 ± 7.35 | 197.66 ± 10.64 | 200.53 ± 8.97 |
| Polyphenols (ppm) | 625.69 ± 14.88 | 572.88 ± 9.93 | 588.11 ± 11.07 | 617.45 ± 11.54 |
| DPPH radical scavenging activity (%) | 42.78 ± 1.21 | 46.52 ± 1.75 | 46.28 ± 1.22 | 44.21 ± 1.40 |
| Total acidity (g/L) | 4.76 ± 0.32 | 5.89 ± 0.29 | 5.69 ± 0.11 | 5.35 ± 0.34 |
Data in Table 1 highlighted the significant variability in physicochemical properties, chemical composition, and biological activity of red-fleshed dragon fruit cultivated in different geological regions of Vietnam.
In particular, the analyzed value of reducing sugars and total sugars showed significant variation among the provinces, with fruits from Long An possessed the highest values (115.65 ± 4.80 and 127.76 ± 7.34 ppm, respectively), while fruits from Binh Thuan possessed the lowest values (99.23 ± 6.50 and 109.94 ± 6.38 ppm, respectively). These results indicated that fruits from Long An were generally sweeter, possibly due to more intense sunlight or other specific agricultural practices promoting the sugar accumulation of the cultivated fruits. On the other hand, the environmental conditions in Binh Thuan appeared to be less favorable for the natural sugar synthesis process of red-fleshed dragon fruit.22,23 High sugar content also suggested that the juices from red-fleshed dragon fruit cultivated in Vietnam are suitable as components in functional drinks, since fruit sugars are widely considered the more healthy alternative to refined sugars in providing both sweetness and energy. 24
The analyzed value of total dissolved solids content also showed significant variation among red-fleshed dragon fruits cultivated in different provinces, with fruits from Long An exhibiting the highest values (14.00 ± 0.50 °Bx) and fruits from Binh Thuan exhibited the lowest values (12.50 ± 0.50 °Bx). The cause of such variances was most likely due to the disparity in their sugar content. Conversely, the relatively high difference between total dissolved solids content and sugars content of all samples suggested that in addition to sugars, the juices from red-fleshed dragon fruits cultivated in Vietnam also contain prominent amounts of other phytochemical compounds, which may provide certain health benefits to humans.
Quantitative analysis of bioactive compounds in juice samples of red-fleshed dragon fruit showed that vitamin C content was notably higher in fruits from Long An, at 273.86 ± 16.81 ppm, compared to the value of 229.34 ± 17.78 ppm in fruits from Binh Thuan. This variation could be due to different levels of exposure to sunlight, or other differences in agricultural practices that affect the synthesis of ascorbic acid in the fruit. 25 Analyzed betacyanins and anthocyanins contents, which contribute to antioxidant properties and the visual appeal of commercial fruit juices, also showed variabilities between samples. In particular, the levels of both betacyanins and anthocyanins were highest in fruits from Binh Thuan (12.05 ± 0.49 mg/100g and 212.77 ± 12.95 mg/100g, respectively), indicating that some specific climatic or soil conditions in this province were more favorable for pigment synthesis. Conversely, the levels of betacyanins and anthocyanins were both lowest in fruits from Long An, underscoring regional differences in secondary metabolite production.
Interestingly, polyphenols contents and DPPH radical scavenging activities of the samples also showed significant variances, with fruits from Long An exhibited the lowest polyphenols level (572.88 ± 9.93 ppm) but the highest DPPH radical scavenging activity (46.52 ± 1.75%), while fruits from Binh Thuan possessed the highest polyphenols level (625.69 ± 14.88 ppm) but the lowest DPPH radical scavenging activity (42.78 ± 1.21%). This discrepancy suggests that polyphenols content alone does not directly correlate with antioxidant efficiency, and other constituents may also play crucial roles in free radical scavenging — such as vitamin C, a well-known antioxidant.
The pH levels, which affect the sourness and potential microbial stability of fruit juices, varied from 4.52 ± 0.09 in fruits from Long An to 4.90 ± 0.14 in fruits from Binh Thuan. Similar results were received from total acidity analysis, with the highest value recorded in fruits from Long An (5.89 ± 0.29 g/L), and the lowest value recorded in fruits from Binh Thuan (4.76 ± 0.32 g/L). Based on these results, it can be concluded that the quality and bioactive profiles of red-fleshed dragon fruits cultivated in Vietnam were deeply impacted by geographical and environmental factors. Understanding these variations is crucial for optimizing agricultural practices in order to enhance the nutritional quality of the fruits and achieve better commercial success of fruit-cultivating operations.
However, it should also be noted that most quality and bioactive properties of the samples investigated were within the range of values for red-fleshed dragon fruits presented in existing literature.26–28
Optimization of enzymatic pectin hydrolysis parameters
Within the scope of this study, the effects of pH condition, incubation temperature, incubation duration, and initial enzyme concentration on the clarification efficiency of enzymatic pectin hydrolysis for red-fleshed dragon fruit were investigated, with optimization done based on the results received from Box–Behnken design model. Details on experimental design matrix, measured responses, data fitting, model selection, and statistical analysis for model confirmation were presented in the attached Supplementary Materials 01.
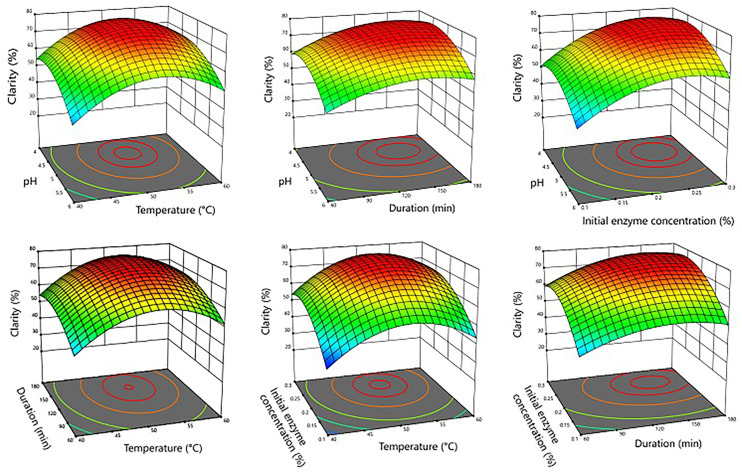
In order to optimize enzymatic pectin hydrolysis parameters for maximum clarity of treated red-fleshed dragon fruit juice, analyses were made on 3D response surface graphs illustrating interactions between these parameters and clarity (see Figure 1). Particularly, the analytical results suggested that hydrolysis activity of the pectinase enzyme was highly dependent on the pH of the medium, with the highest efficiency achieved when the pH value ranging from 4.3 to 4.8, which align with previous literatures specifying the optimal pH operational range of pectinase enzyme to be between 4.0 and 5.0. 29 When allowed to operate within its optimal acidic conditions, pectinase enzyme can promote more robust and complete breakdown of pectin structures, leading to better clarity of filtered juices. Conversely, the significant drop in hydrolysis activity at pH values higher than 5.0 underscored the enzyme's sensitivity to pH variations. Coincidentally, the pH values of red-fleshed dragon fruit juices from Vietnam also fell within this optimal range, indicating that the enzymatic treatment for pectin hydrolysis of such juices would require minimal use of pH buffers to regulate the acidity of the medium.
Figure 1.
3D response surface graphs illustrating interactions between enzymatic pectin hydrolysis parameters and clarity of treated red-fleshed dragon fruit juice.
Temperature also plays a crucial role in the enzymatic hydrolysis of pectin. The results indicated that hydrolysis activity of pectinase enzyme increased significantly as the temperature rose from 40°C to 50°C, with the treated juice achieving peak clarity when temperature ranging from 50°C to 55°C. This enhancement can be attributed to the increased kinetic energy available at higher temperatures, which facilitates more interactions between pectinase enzyme and the substrate.30,31 However, hydrolysis activity of the pectinase enzyme began to sharply decline as temperature rose above 55°C, likely due to enzyme denaturation. These results are consistent with the general behavior of pectinase enzyme, which is known to be active within the temperature range of 30°C to 50°C. 29
Likewise, the clarity of treated juices significantly improved as the incubation duration increased from 60 to 135 min, then remained relatively unchanged when the incubation duration extended from 135 to 180 min. This result suggested that longer exposure to the pectinase enzyme would allow for more extensive pectin breakdown. However, extending the hydrolysis beyond 135 min did not produce significant improvements, indicating that a plateau in efficiency of enzymatic hydrolysis was reached, beyond which no further clarity would be gained. Additionally, extending the enzymatic pectin hydrolysis duration could also negatively impact microbiological safety and bioactive contents of the treated dragon fruit juice, leading to reduced health benefits and the necessity of more stringent sterilization procedures, which would further reduce health benefits of the final juice products. Overall, these findings emphasized the importance of optimizing incubation duration to enhance efficiency and productivity without unnecessary prolongation of the process.
Similarly, the clarity of treated juices significantly improved as the initial enzyme concentration increased from 0.10% to 0.23%, then remained relatively unchanged when the initial enzyme concentration further increased from 0.23% to 0.30%. The cause of this phenomenon was most likely due to the enzyme concentration reaching its saturation point where all available substrate sites were occupied, therefore adding more enzymes would not further enhance the reaction rate. 29
With the aim of achieving maximum clarity for treated red-fleshed dragon fruit juice, optimization calculations were conducted, resulting in optimal levels of various parameters as follows: pH = 4.6, temperature = 53 °C, duration = 145 min, and initial enzyme concentration = 0.24%.
At these enzymatic pectin hydrolysis parameters, the clarity of treated red-fleshed dragon fruit juice was predicted to be 78.53%. Three confirmation experiments were conducted at calculated optimal enzymatic pectin hydrolysis parameters, with the average clarity of treated red-fleshed dragon fruit juice determined to be 78.767% ± 0.496%. This result was within 95% of predicted values. However, it should be noted that these optimal enzymatic pectin hydrolysis parameters were only valid within the specified range of values investigated. Additional experiments must be made to confirm any other extrapolations/interpolations built upon these experimental data.
Impacts of sterilization parameters on microbial control and betacyanin stability in red-fleshed dragon fruit juice
Nowaday, sterilization has become an important step in the production of commercial beverages. Through the sterilization process, beverages not only become more safe for consumption, but their shelf-life can also be extended significantly. In this study, a comprehensive assessment was conducted to investigate the impacts of sterilization parameters, specifically temperature and duration, on microbial activity and betacyanins content of juice samples from red-fleshed dragon fruit cultivated in Vietnam. Experimental results were presented in Table 2.
Table 2.
Impacts of sterilization parameters on microbial density and betacyanin content in red-fleshed dragon fruit juice.
| Sterilization parameters | Microbial density | Betacyanins content (mg/100g) | Compliance with standards (QCVN 6-2:2010/BYT) | ||
|---|---|---|---|---|---|
| Temperature (°C) | Duration (min) | Aerobic bacteria (CFU/mL) | Yeast and mold (CFU/mL) | ||
| 85 | 10 | 591; 509; 664 | 4454; 3364; 4091 | 10.33 | No |
| 90 | 9; 36; 73 | 818; 545; 273 | 8.87 | Partial | |
| 95 | 0; 0; 0 | 0; 0; 0 | 7.22 | Yes | |
| 95 | 4 | 27; 73; 100 | 91; 455; 91 | 10.81 | No |
| 6 | 9; 18; 0 | 0; 0; 0 | 9.17 | Yes | |
| 8 | 0; 0; 0 | 0; 0; 0 | 8.45 | Yes | |
Results shown in Table 2 clearly showed a significant reduction in microbial activity with increasing sterilization temperatures and durations. For example, the microbial counts (including both aerobic bacteria and yeast/mold) after sterilization at 85°C and 10 min were considerably high, indicating insufficient sterilization. Such outcomes demonstratedly failed to meet the safety standards set by local regulation QCVN 6-2:2010/BYT for non-alcoholic beverages. As sterilization temperature increased, marked decreases in microbial counts were observed, with readings for both aerobic bacteria and yeast/mold growth reaching zero colony-forming units per milliliter (CFU/mL) at the sterilization temperature of 95°C. These findings align with existing literature, which suggests that higher temperatures are generally more effective at reducing microbial viability. The complete eradication of microbes at 95°C supports the hypothesis that certain critical temperatures are necessary to ensure microbial safety in beverage processing. 32
Similarly, microbial activity of sterilized samples showed profound reductions as the sterilization duration rose from 4 min to 10 min, with no aerobic bacteria and yeast/mold growth observed when the sterilization duration reached 8 min. These results indicated that in order to completely eradicate microbes in juices from red-fleshed dragon fruit, the sterilization process needed to be conducted at an appropriate temperature, and for an appropriate duration.
However, the success of beverage products relies not only on their compliance with safety standards, but also on their nutrition content and visual appeal. In particular, betacyanins — key pigments presented in red-fleshed dragon fruit — were known for their antioxidant properties and their role in elevating the aesthetic factor of food products. Experimental results presented in Table 2 showed a significant decline in betacyanins content as sterilization temperature increased. The betacyanins content of juice samples decreased from its original value of 12.05 mg/100g to 10.33 mg/100g after sterilization at 85°C for 10 min (14.27% decrease), and further dropped to 8.87 mg/100g (26.39% decrease) and 7.22 mg/100g (40.08% decrease) when the sterilization temperature raised to 90°C and 95°C, respectively. These results align with the understanding that higher temperatures accelerate the degradation of thermal sensitive compounds like betacyanins, and indicate that lower sterilization temperatures are preferable for preserving nutrition contents of juice-based products. 33
Similar changes in betacyanins content were also observed when the sterilization duration increased from 4 min to 10 min. The reduction in pigment stability at higher temperatures is a critical consideration, as it impacts not only the nutritional value and antioxidant capacity of the produced beverages, but also their marketability color intensity, sensory attributes, and overall appearance. Optimal sterilization conditions, while effective at ensuring microbial safety, were demonstrated to significantly compromise the integrity of important nutritional components in the juice of red-fleshed dragon fruit. Therefore, in the future, other sterilization methods such as microwave sterilization, ultrasonic sterilization, high-pressure sterilization, and so forth should also be considered, so that both microbial safety and nutrients content of the treated dragon fruit juice could be well-sustained.34,35
Optimizing formulation for dragon fruit energy drink
In order to counterbalance the negative impacts caused by the sterilization process, while also elevating the visual appeal and functional aspects, it is necessary to supplement some food additives to the beverages containing red-fleshed dragon fruit extract. Within the scope of this study, the effects of added sugar, citric acid, and energy drink flavor on the sensory quality of dragon fruit energy drink were investigated, with optimization done based on the results received from Box–Behnken design model. Details on experimental design matrix, measured responses, data fitting, model selection, and statistical analysis for model confirmation were presented in the attached Supplementary Materials 01.
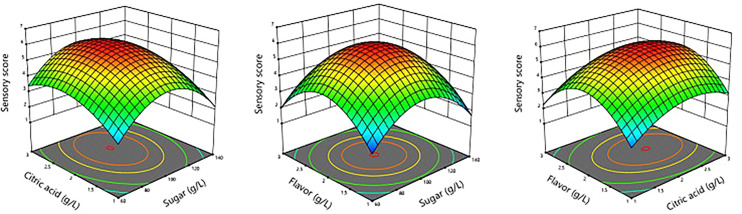
In order to optimize the amount of supplemental additives for maximum sensory score of dragon fruit energy drink, analyses were made on 3D response surface graphs illustrating interactions between these parameters and sensory score (see Figure 2). Particularly, sensory evaluation revealed that energy drink samples would receive the highest median preference score from assessors for scenarios in which the amount of added sugar being around 100 g/L. This result revealed that consumers would prefer energy drinks with balanced sweetness, while also less favorable toward both under-sweetened or over-sweetened products. Moreover, the optimal amount of added sugar determined in this study were much lower than the sugars content of most energy drinks currently commercialized on the market, suggesting that the high sugars content of unprocessed dragon fruit extracts would played an important role in reducing the need to supplement refined sugar to these type of beverages, thus alleviating their health implications. 24
Figure 2.
3D response surface graphs illustrating interactions between amount of supplemental additives and sensory score of dragon fruit energy drink.
Likewise, the optimal amount of added citric acid was determined to be around 2.25 g/L. Normally, citric acid is supplemented to beverage products for the purpose of adjusting the acidity and enhancing their taste. At the optimal amount, added citric acid would provide pleasant hints of tartness without overpowering the characteristic flavors of natural dragon fruit juice. However, amounts above such threshold would lead to an undesirable increase in sourness, negatively impacting the sensory appeal of the drink. Similar impacts on sensory aspects of dragon fruit beverages were also observed with added energy drink flavor. In particular, at amounts around 2.0 g/L, added energy drink flavor would provide a distinct yet balanced profile, enhancing the overall taste of the beverage without being too overwhelming. However, higher amounts were less preferred due to a strong flavor intensity that could detract the drink's refreshment quality.
With the aim of achieving maximum sensory score for dragon fruit energy drink, optimization calculations were conducted, resulting in optimal levels of various parameters as follows: amount of added sugar = 99 g/L, amount of added citric acid = 2.25 g/L, and amount of added energy drink flavor = 2.00 g/L.
At these amount of supplemental additives, the sensory score of dragon fruit energy drink was predicted to be 6.01. Three confirmation experiments were conducted at calculated optimal amount of supplemental additives, with the sensory score of dragon fruit energy determined to be 6.067 ± 0.118. This result was within 95% of predicted values. However, it should be noted that these optimal enzymatic pectin hydrolysis parameters were only valid within the specified range of values investigated. Additional experiments must be made to confirm any other extrapolations/interpolations built upon these experimental data.
Conclusion
This study effectively explored the variances in phytochemicals content and radical scavenging activity of red-fleshed dragon fruit samples collected from four provinces in Vietnam, and highlighted the importance of cultivation conditions and geological origin on fundamental characteristics of red-fleshed dragon fruit. This study also successfully optimized the pectinase enzymatic hydrolysis and thermal sterilization of dragon fruit juice, and the formulation for dragon fruit energy drink — so that both nutrient content, microbial safety, and consumer acceptance of the product could be achieved.
In conclusion, this study bridged the gap between scientific exploration and practical application, providing valuable insights for the beverage industry in the quest to innovate its products. Findings from this study not only advanced the understanding of the production process for dragon fruit beverages, but also underscore the potential for broader applications of dragon fruit in creating fruit-based functional drinks that are both beneficial to human health and sensory appeal. Future studies should expand on integrating non-thermal sterilization technologies and advanced enzymatic treatments to further refine the manufacturing process, so that consumer demands for nutritious and safe functional beverages could be met.
Supplemental Material
Supplemental material, sj-docx-1-sci-10.1177_00368504241300854 for Enhancing the quality and consumer satisfaction of dragon fruit beverage production: The effects of geological origin and processing conditions by Thinh Van Pham, Thuy Xuan Cao, Ngoc Thi Hong Le, Ai Minh Pham, Hung Tuan Trinh, Dat Tien Nguyen, Anh Le Tuan Hoang, Minh Quang Bui, Tung Ngoc Nguyen, Minh Ngoc Truong and Tao Minh Hoang in Science Progress
Supplemental material, sj-docx-2-sci-10.1177_00368504241300854 for Enhancing the quality and consumer satisfaction of dragon fruit beverage production: The effects of geological origin and processing conditions by Thinh Van Pham, Thuy Xuan Cao, Ngoc Thi Hong Le, Ai Minh Pham, Hung Tuan Trinh, Dat Tien Nguyen, Anh Le Tuan Hoang, Minh Quang Bui, Tung Ngoc Nguyen, Minh Ngoc Truong and Tao Minh Hoang in Science Progress
Acknowledgments
The authors gratefully acknowledge the support provided by the Faculty of Food Science and Technology at Ho Chi Minh City University of Industry and Trade, which supplied the necessary facilities for this research.
Footnotes
Authors’ contribution: PVT, CXT, and HMT contributed to conceptualization; NTD and HMT contributed to methodology; TTH contributed to software;, HLTA, BQM, and NNTcontributed to validation; LTHN and PMAcontributed to formal analysis; TNM, LTHN, and PMAcontributed to investigation; CXT, PVT, HLTA, BQM, and NNT contributed to resources; TTH contributed to data curation; TNM, HMT, and TTH contributed to writing–original draft preparation; TNM, HMT, and TTH contributed to writing–review and editing; TTH contributed to visualization; PVT, NTD, and TNM contributed to supervision; HMT and NTD contributed to project administration; HMT contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data availability statement: The original contributions presented in the study are included in the article and supplementary materials, further inquiries can be directed to the corresponding author.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was funded by Vietnam Academy of Science and Technology (VAST), grant number KHCBHH.02/23-25.
Institutional review board statement: The study was conducted in accordance with the Declaration of Helsinki, with approval from the Center for High Technology Research and Development's Scientific Advisory Committee being obtained prior to commencement of the study (Summary of the Extraordinary Meeting on 10/01/2024).
All experiments were performed in accordance with the approved guidelines, and participants were informed about the risks associated with participating in the study, which include a detailed list of ingredients used to prepare samples and their respective safety data.
ORCID iD: Tao Minh Hoang https://orcid.org/0000-0002-6326-5087
Supplemental material: Supplemental material for this article is available online.
References
- 1.Ruxton CHS, Myers M. Fruit juices: are they helpful or harmful? An evidence review. Nutrients 2021; 13: 1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IndustryARC. Fruit and vegetable juice market – forecast (2024–2030). 2023.
- 3.Stintzing FC, Schieber A, Carle R. Phytochemical and nutritional significance of cactus pear. Eur Food Res Technol 2001; 212: 396–407. [Google Scholar]
- 4.Wu LC, Hsu HW, Chen YC, et al. Antioxidant and antiproliferative activities of red pitaya. Food Chem 2005; 95: 319–327. [Google Scholar]
- 5.Azeredo HMC. Betalains: properties, sources, applications, and stability – a review. Int J Food Sci Technol 2008; 44: 2365–2376. [Google Scholar]
- 6.Herbach KM, Stintzing FC, Carle R. Betalain stability and degradation – structural and chromatic aspects. J Food Sci 2006; 71: 41–50. [Google Scholar]
- 7.Cai Y, Luo Q, Sun M, et al. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004; 74: 2157–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinelo M, Rubilar M, Sineiro J, et al. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem 2003; 85: 267–273. [Google Scholar]
- 9.Codex Alimentarius Commission. CODEX Standard for pitahayas CXS 237-2003 (adopted in 2003, amended in 2005, 2011). 2011.
- 10.Skrovankova S, Sumczynski D, Mlcek J, et al. Bioactive compounds and antioxidant activity in different types of berries. Int J Mol Sci 2015; 16: 24673–24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng S, Xiang S, Bian X, et al. Quantitative analysis of total acidity in aqueous lactic acid solutions by direct potentiometric titration. Microchem J 2020; 157: 105049. [Google Scholar]
- 12.Kapur A, Hasković A, Čopra-Janićijević A, et al. Spectrophotometric analysis of total ascorbic acid contetnt in various fruits and vegetables. Bull Chem Technol Bosnia Herzegovina 2012; 38: 39–42. [Google Scholar]
- 13.Phebe D, Chew MK, Suraini AA, et al. Red-fleshed pitaya (Hylocereus polyrhizus) fruit colour and betacyanin content depend on maturity. Int Food Res J 2009; 16: 233–242. [Google Scholar]
- 14.Wang WD, Xu SY, Jin MK. Effects of different maceration enzymes on yield, clarity and anthocyanin and other polyphenol contents in blackberry juice. Int J Food Sci Technol 2008; 44: 2342–2349. [Google Scholar]
- 15.Taghavi T, Patel H, Rafie R. Comparing pH differential and methanol-based methods for anthocyanin assessments of strawberries. Food Sci Nutr 2021; 10: 2123–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamuela-Raventós RM. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In: Apak R, Capanoglu E, Shahidi F. (eds) Measurement of antioxidant activity & capacity: recent trends and applications. Oxford: John Wiley & Sons, 2017, pp.107–115. doi: 10.1002/9781119135388.ch6 [DOI] [Google Scholar]
- 17.Gusakov AV, Kondratyeva EG, Sinitsyn AP. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int J Anal Chem 2011; 2011: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Floegel A, Kim DO, Chung SJ, et al. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 2011; 24: 1043–1048. [Google Scholar]
- 19.Walkling-Ribeiro M, Noci F, Cronin DA, et al. Shelf life and sensory evaluation of orange juice after exposure to thermosonication and pulsed electric fields. Food Bioprod Process 2008; 87: 102–107. [Google Scholar]
- 20.Evans M. Optimization of manufacturing processes: a response surface approach. London: Carlton House Terrace, 2003. doi: 10.1201/9780138744885 [DOI] [Google Scholar]
- 21.Montgomery DC. Design and analysis of experiments. 5th ed. Singapore: Wiley, 2004. [Google Scholar]
- 22.Chapai A, Upadhayaya KP, Adhikari S, et al. Dragon fruit farming in Nepal: a comprehensive review. Int J Hortic 2024; 14: 156–162. [Google Scholar]
- 23.Kosiyachinda S. Quality management of dragon fruits: A Case Study of an Amateur Orchard in Thailand. Acta Hortic 2015; 1088: 267–272. [Google Scholar]
- 24.Arshad S, Rehman T, Saif S, et al. Replacement of refined sugar by natural sweeteners: focus on potential health benefits. Heliyon 2022; 8: e10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somers GF, Hamner KC, Kelly WC. Further studies on the relationship between illumination and the ascorbic acid content of tomato fruits. J Nutr 1950; 40: 133–143. [DOI] [PubMed] [Google Scholar]
- 26.Attar ŞH, Gündeşli MA, Urün I, et al. Nutritional analysis of red-purple and white-fleshed pitaya (Hylocereus) species. Molecules 2022; 27: 08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paśko P, Galanty A, Zagrodzki P, et al. Dragon fruits as a reservoir of natural polyphenolics with chemopreventive properties. Molecules 2021; 26: 2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Mekhlafi NA, Mediani A, Ismail NH, et al. Metabolomic and antioxidant properties of different varieties and origins of dragon fruit. Microchem J 2020; 160: 105687. [Google Scholar]
- 29.Sharma N, Rathore M, Sharma M. Microbial pectinase: sources, characterization and applications. Rev Environ Sci Bio/Technol 2012; 12: 45–60. [Google Scholar]
- 30.Ninga KA, Sengupta S, Jain A, et al. Kinetics of enzymatic hydrolysis of pectinaceous matter in guava juice. J Food Eng 2017; 221: 158–166. [Google Scholar]
- 31.Ortega N, De Diego S, Rodríguez-Nogales JM, et al. Kinetic behaviour and thermal inactivation of pectinlyase used in food processing. Int J Food Sci Technol 2004; 39: 631–639. [Google Scholar]
- 32.Johnson PT, Toledo RT. Fundamentals of food process engineering. 4th ed. Cham: Springer, 2015. doi: 10.1007/978-3-319-90098-8 [DOI] [Google Scholar]
- 33.Rodriguez-Amaya DB. A guide to carotenoid analysis in foods. Washington, DC: ILSI Press, 2016. [Google Scholar]
- 34.Zhu D, Zhang Y, Kou C, et al. Ultrasonic and other sterilization methods on nutrition and flavor of cloudy apple juice. Ultrason Sonochem 2022; 84: 105975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma T, Wang J, Wang L, et al. Ultrasound-combined sterilization technology: an effective sterilization technique ensuring the microbial safety of grape juice and significantly improving its quality. Foods 2020; 9: 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sci-10.1177_00368504241300854 for Enhancing the quality and consumer satisfaction of dragon fruit beverage production: The effects of geological origin and processing conditions by Thinh Van Pham, Thuy Xuan Cao, Ngoc Thi Hong Le, Ai Minh Pham, Hung Tuan Trinh, Dat Tien Nguyen, Anh Le Tuan Hoang, Minh Quang Bui, Tung Ngoc Nguyen, Minh Ngoc Truong and Tao Minh Hoang in Science Progress
Supplemental material, sj-docx-2-sci-10.1177_00368504241300854 for Enhancing the quality and consumer satisfaction of dragon fruit beverage production: The effects of geological origin and processing conditions by Thinh Van Pham, Thuy Xuan Cao, Ngoc Thi Hong Le, Ai Minh Pham, Hung Tuan Trinh, Dat Tien Nguyen, Anh Le Tuan Hoang, Minh Quang Bui, Tung Ngoc Nguyen, Minh Ngoc Truong and Tao Minh Hoang in Science Progress