Abstract
Background
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease resulting from an intricate interplay between genetics and environmental factors. Many studies have explored living in rural areas as a possible risk factor for ALS, without focusing simultaneously on incidence, age at onset and phenotypic features.
Objective
To evaluate the effect of croplands residential proximity on ALS incidence and phenotype, focusing on age of onset, site of onset and progression rate.
Methods
The address history of ALS patients belonging to the population‐based Piemonte and Valle d'Aosta registry (PARALS), diagnosed between 2007 and 2014, was obtained for the 20 years prior to the onset date. The smoothed ALS incidence per year (i m) was compared with the percentage of area covered by each crop for each municipality. A proximity score was calculated for each cropland by geolocation, measuring the percentage of area surrounding patients’ residence for variable radii, and was used to compare croplands exposure and phenotype.
Results
We observed an increased ALS incidence in the municipalities with a higher percentage of area covered by arable crops (R = 0.191, p < 0.001). Age at onset was significantly lower in those patients who lived near arable crops, with a median anticipation ranging from 1.8 to 3.4 years; using historical data, a significant anticipation was found also for patients living near vineyards.
Discussion
Our study proved a direct association between arable crops and ALS risk and an inverse association between arable crops and vineyards proximity and age at onset, suggesting the possible causative role of specific environmental contaminants.
Keywords: amyotrophic lateral sclerosis, croplands, environmental risk factors, geospatial epidemiology, pesticides
A solid, direct association between arable crops and amyotrophic lateral sclerosis (ALS) risk was found in Northern Italy. ALS age at onset was inversely associated with arable crops and vineyards residential proximity, especially using historical residential exposure assessment. Croplands‐related risk suggests a possible detrimental role of environmental contaminants.
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a fatal disease of unknown origin characterized by the degeneration of upper and lower motor neurons [1]. Its extreme clinical variability in terms of age and site of onset, progression and cognitive impairment is likely underpinned by the presence of different pathogenic mechanisms [2]. Furthermore, its prevalence and distribution vary worldwide, likely reflecting the interaction between a predisposing genetic background and differently distributed environmental factors [3]. Given that a neurotoxic insult could act many years before disease onset, the role of environmental exposures is hard to assess, especially considering the rareness of the disease [4, 5]. Among environmental contaminants, some neurotoxic pesticides have been associated with a higher ALS risk [6]. To date, studies analysing the geographical distribution of ALS cases have not yielded robust evidence on any specific environmental factors [7]. However, environmental factors have been associated with an earlier age at onset and sometimes with faster disease progression, independently from other phenotypic features (i.e., site of onset). This is the case for the ALS cluster in the Western Pacific [8], or the higher risk among Gulf War veterans [9, 10], professional soccer players [11] and National Football League Athletes [12], or head/neck injury‐associated activities [13]. Many studies have described that living in rural areas or agriculture occupational exposure are possible risk factors for ALS [14, 15, 16, 17], but none of them evaluated the effect of croplands exposure on both ALS incidence and phenotype. In the present study we evaluated the effect of residential proximity to different agricultural crops on ALS incidence and phenotype, focusing on age at onset, site of onset and progression rate.
METHODS
ALS patients’ data
All ALS patients included in the Piemonte and Valle d'Aosta ALS Register (PARALS), resident in Piemonte who received a diagnosis between 2007 and 2014 were considered for the study. The PARALS inclusion criteria have been described elsewhere [18] and a brief summary is included in Appendix S1. All clinical data were derived from PARALS as the primary source. Using civil registries, we were also able to obtain each patient's address at diagnosis and in the 20 years prior to disease onset. Each address geolocation (latitude and longitude) was then assessed using the Google Maps services [19]. Data on genetic analysis have been previously reported [20]. Disease progression was assessed using the Revised ALS Functional Rating Scale (ALSFRS‐R) score and calculated according the following formula: (48 – ALSFRS‐R score at diagnosis)/(time expressed in month from onset to diagnosis).
Environmental data
Piemonte covers an area of approximately 25,386,696,869 m2 and, according to the 2011 census, includes a total population of 4,363,916 inhabitants (2,258,928 females and 2,104,988 males). [21] Data on the geographical distribution of various types of crops areas were obtained from the Regional Environmental Protection Agency (Agenzia Regionale per la Protezione Ambientale, ARPA, Piemonte). These data are collected for public health purposes at regular intervals and are publicly available [22]. All available data on crops were retrieved, considering the year 2011 as the reference date (Table S1). Due to the small total area covered, we did not consider citrus and olive groves in the analyses.
ALS smoothed incidence
Municipality and census division resident populations were obtained from the Italian Institute of Statistics [21]. Given the low number of cases, ALS incidence would have been highly variable across municipalities. Thus, we calculated the smoothed ALS incidence per year (i m) between 2007 and 2014 according to the following formula:
where i 1 is the municipality incidence, i 2 is the mean of the neighbours’ municipalities incidence and a 1 is the weight of the index municipality considered; consequently, (1–a 1) is the weight of the neighbours’ municipalities. For the purpose of this analysis, the maximum incidence was set at 10/100,000 person‐years (i max = 80 cases over a 8‐year period) and a 1 at 0.7.
Proximity score calculation
The proximity of patients to each cropland was calculated both at the time of diagnosis and as the cumulative proximity over the 20 years leading up to the onset of the disease. Proximity scores for each environmental factor at the time of diagnosis were calculated by drawing a circle centred on the patient's residence address and then calculating the enclosed percentage covered by the considered agricultural area (Figure 1).
FIGURE 1.
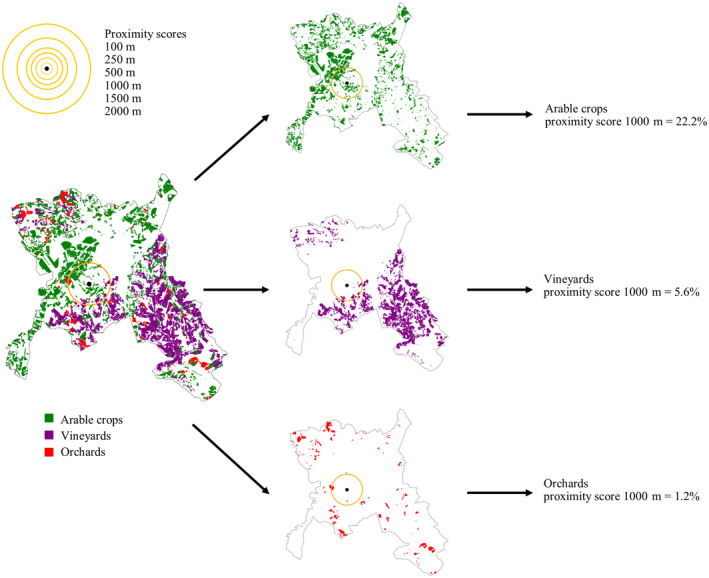
Proximity score calculation. In this figure, we exemplified the proximity score calculation for different radii (100, 250, 500, 1000, 1500, 2000 m). For each patient (black dot) we calculated the percentage of area surrounding his/her residence covered by each specific crop. In this figure, as an example, only arable crops, vineyards and orchards are illustrated and the patient localization is fictitious, to avoid possible privacy issues.
The cumulative proximity score (S h ) was assessed using the same approach for each patient's address and then calculating the weighted sum of his/her exposures during the 20‐year period, using the following formula:
where i refers to each patient's address, s i is the proximity score calculated for that address, d i is the number of the days the patient was resident in that address and n p is the total number of days that the patient lived in Piemonte, during the 20‐year period.
Since we could not acknowledge at which distance pollutants are dangerous, we used different incremental radii (100, 250, 500, 1000, 1500 and 2000 m). Figure 1 illustrates that drawing a circle around a patient's residence may include part of an agricultural area. If it does, the percentage of area covered will be >0% (and the patient is considered as “exposed”); otherwise, it will be 0% (and the patient is considered as “not exposed”). This method allowed us to overcome the limit of considering only small areas around patients' houses [23]. Using different radii, the population of exposed and not exposed patients changed accordingly.
We further explored a strategy to select the most informative radius for each cropland by computing the mean and the signal‐to‐noise ratio (SNR) value for drawing the statistical description of each subfeature, considering that multiple patients may live in the same municipality. Given the i‐th radius, the SNR represents the ratio between the average of the proximity scores in each municipality and the pooled standard deviation (), where N is the number of different municipalities, is the number of patients belonging to the j‐th municipality and is the standard deviation of the proximity scores associated with the j‐th municipality.
Difference between the two groups was calculated using Mann–Whitney U test, considering a p‐value <0.05 as significant for continuous variables (age at onset and Δ‐ALSFRS) and chi‐square test for categorical variable (site of onset). We also performed a time‐to‐event analysis using Kaplan–Meier curves with log‐rank test to compare age at onset as the event in patients exposed or not exposed to every specific crop. All analyses were performed using Python version 3.8 and IBM SPSS Statistics version 28.0 (IBM Corp., Armonk, NY, USA).
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Azienda Ospedaliero‐Universitaria Città della Salute (Prot. N. 0036344, 18‐May‐2011 and 0090754, 20‐Sep‐2016). Informed consent was obtained from all individual participants included in the study.
RESULTS
The main descriptive statistics of the included ALS population are summarized in Table 1.
TABLE 1.
Descriptive statistics.
| Parameter | Patients with residence at diagnosis | Patients included with historical data | p § |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Age at onset (years) | 68.2 (60.3–74.4) | 68.2 (60.2–74.3) | 0.949 |
| Onset–diagnosis interval (months) | 9.0 (5.0–13.1) | 9.0 (5.0–13.1) | 0.194 |
| ΔALSFRS‐R at diagnosis (point loss per month) | 0.649 (0.299–1.323) | 0.642 (0.290–1.323) | 0.220 |
| Survival (years) | 2.8 (1.9–4.0) | 2.3 (1.5–3.7) | 0.853 |
| n (%) | n (%) | p* | |
|---|---|---|---|
| Sex | |||
| Male | 587 (53.5) | 566 (53.4) | >0.999 |
| Female | 511 (46.5) | 494 (46.6) | |
| Site of onset | |||
| Bulbar onset | 376 (34.3) | 362 (34.1) | 0.993 |
| Spinal onset | 702 (63.9) | 678 (64.0) | |
| Respiratory onset | 20 (1.8) | 20 (1.9) | |
| Genetic mutations | |||
| Wild‐type | 816 (74.3) | 787 (74.3) | 0.987 |
| c9orf72, SOD1, TARDBP, FUS | 104 (9.5) | 103 (9.7) | |
| Not assessed | 178 (16.2) | 170 (16.0) | |
| Total | 1098 (100.0) | 1060 (100.0) |
Note: Patients with residence at diagnosis and patients with complete residential historical data were compared using Mann–Whitney U test (§) for continuous variable and chi‐square test for categorical variables (*).
Abbreviations: ALSFRS‐R, Revised Amyotrophic Lateral Sclerosis Functional Rating Scale; IQR, interquartile range.
No statistical significance was found between the whole patients cohort and the cohort with complete historical residence data. The Figure 2a shows the distribution of different crops in Piemonte and in Table S1 we summarize data on the percentage of the total Piemonte area covered by each crop and the number of municipalities with a percentage of area covered by each crop >0 and the relative percentage of total numbers of municipalities.
FIGURE 2.
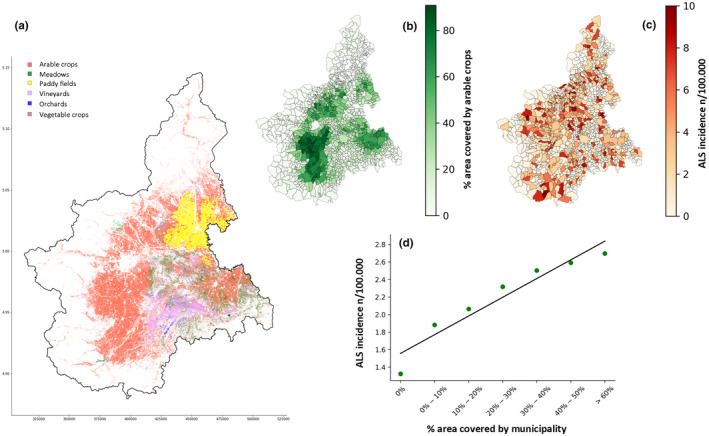
(a) Geographical distribution of the principal crops in the Piemonte region. As is easily seen, arable crops are the most represented and widely distributed of all crops. (b) Piemonte municipalities according to arable crops’ area percentage. Arable crops are mainly distributed in the plains, with the exception of the Vercelli and Novara districts. (c) Piemonte municipalities according to amyotrophic lateral sclerosis (ALS) smoothed incidence. (d) Correlation between ALS smoothed incidence and arable crops’ area percentage. Linear regression model R = 0.191, p < 0.001. Green dots represent the median incidence in all municipalities grouped according to percentage area covered by arable crops. ALS incidence results are significantly higher in municipalities with a larger area covered by arable crops.
Arable crops are clearly the most represented and widely distributed, while other crops showed different distributions that are important to know in order to interpret our results correctly. For example, paddy fields covered a large area of Piemonte (4.08%), but only in 117 municipalities (9.9% of the municipalities of Piemonte), being diffused mainly in the Western plains of Novara and Vercelli. Conversely, vineyards covered only 1.90% of the Piemonte area but were distributed in 747 municipalities (63.3%). This heterogeneity in crop distribution forced us to gather municipalities according to the percentage of area covered and to compare smoothed incidence using nonparametric tests as shown in Table 2.
TABLE 2.
Amyotrophic lateral sclerosis smoothed incidence in different municipalities according to the percentage of area covered by a specific crop.
| Crops (% area covered in municipalities by each specific crop) | Municipalities (N) | Median ALS incidence (IQR) | p § | Mean ALS incidence (SD) | p* |
|---|---|---|---|---|---|
| Arable crops | |||||
| 0% | 13 | 0.75 (0.00–1.26) | <0.001 | 1.32 (2.04) | 0.001 |
| 0%–10% | 423 | 0.79 (0.16–2.50) | 1.88 (2.45) | ||
| 10%–20% | 223 | 0.93 (0.23–3.00) | 2.06 (2.51) | ||
| 20%–30% | 134 | 1.21 (0.47–3.00) | 2.32 (2.58) | ||
| 30%–40% | 122 | 1.25 (0.39–4.10) | 2.50 (2.68) | ||
| 40%–50% | 156 | 1.65 (0.57–4.25) | 2.59 (2.45) | ||
| > 60% | 110 | 1.81 (0.75–4.11) | 2.70 (2.51) | ||
| Vineyards | |||||
| 0% | 434 | 0.81 (0.22–3.00) | <0.001 | 1.99 (2.49) | 0.274 |
| 0%–10% | 631 | 1.34 (0.46–3.42) | 2.43 (2.57) | ||
| >10% | 116 | 0.75 (0.19–2.28) | 1.67 (2.14) | ||
| Meadows | |||||
| 0% | 771 | 1.22 (0.44–3.21) | <0.001 | 2.29 (2.52) | 0.289 |
| 0%–10% | 261 | 1.06 (0.31–3.00) | 2.11 (2.46) | ||
| >10% | 149 | 0.53 (0.15–2.34) | 1.81 (2.59) | ||
| Orchards | |||||
| 0% | 706 | 1.16 (0.40–3.20) | 0.027 | 2.30 (2.58) | 0.537 |
| >0% | 475 | 1.00 (0.28–3.00) | 2.04 (2.42) | ||
| Vegetable crops | |||||
| 0% | 821 | 1.24 (0.44–3.23) | <0.001 | 2.33 (2.56) | 0.569 |
| >0% | 360 | 0.83 (0.21–2.66) | 1.88 (2.40) | ||
| Paddy fields | |||||
| 0% | 1064 | 1.11 (0.34–3.00) | 0.110 | 2.21 (2.51) | 0.527 |
| >0% | 117 | 0.88 (0.24–2.71) | 2.03 (2.55) | ||
| Nurseries | |||||
| 0% | 1057 | 1.06 (0.31–3.00) | 0.018 | 2.14 (2.48) | 0.430 |
| >0% | 124 | 1.45 (0.40–4.31) | 2.67 (2.80) | ||
| Total | 1181 |
Note: Municipalities were grouped differently in order to have a significant number of municipalities for each group. p §, Kruskal–Wallis test and Mann–Whitney U test; p*, linear regression analysis performed using mean incidence in municipalities grouped by increasing percentage of each agricultural area. Significant results (p < 0.05) are in bold type.
Abbreviations: ALS, amyotrophic lateral sclerosis; IQR, interquartile range; SD, standard deviation.
We found significant differences when comparing median incidences in different municipalities grouped according to different percentages of area covered by arable crops, vineyards, meadows, orchards, vegetable crops and nurseries. Considering the previously mentioned distribution of crops areas, a linear regression model resulted valid to compare only the percentage of the area covered by arable crops and the smoothed ALS incidence for each municipality (see Figure 2b,c). The resulting regression coefficient was 0.0076 (p = 0.028) when considering both variables as continuous. In Figure 2d, we confirmed the increasing trend using median incidences, from 0.75 (IQR 0.00–1.26) cases/100,000/year in municipalities with a percentage area covered by arable crops = 0 to 1.81 (IQR 0.75–4.11) cases/100,000/year where arable crops covered more than 60% of the total municipality area (R = 0.191, p < 0.001). No significant results were found for other crops (Table 2).
In Figure S1 we display the mean and the SNR of the proximity scores considering different radii for each cropland. Analysing the proximity scores of arable land, vineyards and meadows we found an increasing pattern, so the variability between patients continues to decrease when considering larger radii. This could be interpreted in the light of the relatively homogenous distribution of these croplands in the Piemonte region. The other croplands revealed different patterns of SNR distribution.
The proximity score analysis results considering only residence at diagnosis are shown in Table S2 and significant results using historical data are shown in Table 3. We were able to obtain historical data for 1060 patients (96.4% of the whole cohort).
TABLE 3.
Age at onset according to the specific crop proximity score, calculated for different radii (100, 250, 500, 1000, 1500, 2000 m radii) using historical residential data.
| Crops | Patients exposed/not exposed (n) | Exposed patients age at onset (years) | Not exposed patients age at onset (years) | Difference in median age at onset | p |
|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | (years) | |||
| Arable crops 100 m radius | 486/574 | 66.7 (59.5–72.9) | 69.2 (60.9–75.3) | 2.5 | 0.0029 |
| Arable crops 250 m radius | 728/332 | 67.3 (59.3–73.4) | 69.6 (62.0–76.3) | 2.3 | 0.0003 |
| Arable crops 500 m radius | 841/219 | 67.4 (59.4–73.6) | 70.1 (62.1–76.8) | 2.7 | 0.0002 |
| Arable crops 1000 m radius | 946/114 | 67.7 (59.8–73.9) | 71.1 (62.5–77.1) | 3.4 | 0.0013 |
| Arable crops 1500 m radius | 989/71 | 67.8 (60.1–74.2) | 69.6 (63.9–77.0) | 1.8 | 0.019 |
| Arable crops 2000 m radius | 1026/34 | 68.2 (60.2–74.3) | 69.6 (61.4–74.6) | 1.4 | 0.296 |
| Vineyards 100 m radius | 60/1000 | 66.3 (57.8–74.1) | 68.3 (60.4–74.3) | 2.0 | 0.096 |
| Vineyards 250 m radius | 158/902 | 67.6 (60.7–73.4) | 68.3 (60.2–74.4) | 0.7 | 0.307 |
| Vineyards 500 m radius | 261/799 | 66.7 (59.0–73.9) | 68.6 (61.0–74.6) | 1.9 | 0.035 |
| Vineyards 1000 m radius | 391/669 | 66.9 (58.9–74.0) | 68.6 (61.5–74.7) | 1.7 | 0.013 |
| Vineyards 1500 m radius | 472/588 | 66.8 (58.4–74.0) | 68.8 (62.0–74.7) | 2.0 | 0.003 |
| Vineyards 2000 m radius | 531/529 | 67.2 (58.9–74.0) | 68.8 (62.0–74.8) | 1.6 | 0.007 |
Note: Data shown for arable crops and vineyards. Patients (N = 1060) were classified as exposed and not exposed patients for each specific crop for different radii. Exposed patients were defined by having a percentage >0 of area covered by the specific crop, while not exposed patients were defined by having a percentage of area covered by the specific crop = 0. Differences were calculated using Mann–Whitney U test and significant results (p < 0.05) are in bold type.
Abbreviation: IQR, interquartile range.
Age at onset was significantly reduced in those patients who were exposed to arable crops when considering both residence at diagnosis (see Table S1) and, more significantly, historical residence data (see Table 3). The difference between exposed and not exposed patients’ median age at onset ranged from 1.8 years (1500 m radius) and 3.4 years (1000 m radius). Kaplan–Meier analysis confirmed an earlier age at onset in patients exposed to arable crops (log‐rank test for 100, 250, 500, 1000 and 1500 m radii p < 0.05, Figure 3 shows the Kaplan–Meier curve for the 1500 m radius curve, log‐rank test p < 0.001).
FIGURE 3.
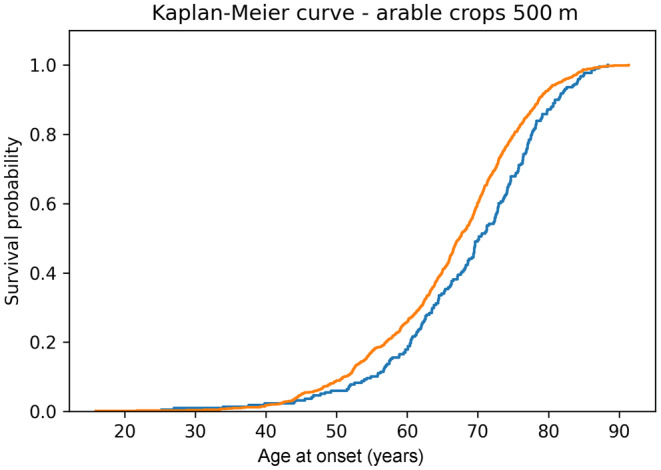
Kaplan–Meier curve showing the significant difference in age at onset for amyotrophic lateral sclerosis (ALS) patients exposed to arable crops versus not exposed patients (considered radius = 500 m) using historical data. Orange line, exposed patients were defined by having a percentage of arable crops >0 in the defined area (N = 841); blue line, not exposed patients were defined by having no arable crops (area = 0%) in the defined area (N = 219). Log‐rank test p < 0.001.
Considering only residence at diagnosis, for patients exposed to vineyards a similar trend of anticipation was observed for 1000, 1500 and 2000 m radii, without reaching significance. Interestingly, using historical data, disease onset occurred in exposed patients from 1.6 to 2.0 years before not exposed patients (considering 500, 1000, 1500 and 2000 m radii, p < 0.05). Kaplan–Meier analysis confirmed the same results with a different methodology (log‐rank test for 500, 1000, 1500 and 2000 m radii p < 0.05). The progression rate (Δ‐ALSFRS) analysis resulted in small differences among exposed and not exposed patients: patients living near to vegetable crops (500, 1000 m radii), meadows (1500, 2000 m radii) and nurseries (500, 1000, 1500 m radii) have a median lower Δ‐ALSFRS than not exposed patients. No significant results for Δ‐ALSFRS were found using historical data.
The site of onset (bulbar vs. spinal versus respiratory) was not significantly related to the proximity scores for different crops and radii (chi‐square test p > 0.05 for all comparisons).
To confirm the effect of historical residential proximity to arable crops and vineyards, we repeated the analysis by subgrouping patients according to sex and site of onset and analysing only wild‐type patients with historical data separately (N = 787, 96.1% of all wild‐type cohort). All the results are described in Table S3. Interestingly, the earlier age at onset was confirmed both in male and female patients exposed to arable crops and vineyards. The difference in anticipation was more evident in males, with differences ranging from 2.90 to 3.70 years for arable crops (significant radii 100, 250, 500, 1000 and 1500 m) and from 2.40 to 2.50 years for vineyards (significant radii 1500 and 2000 m). By stratifying patients for site of onset, we found that for bulbar‐onset patients age at onset was anticipated only by exposure to arable crops (differences ranging from 1.80 to 3.70 years, significant radii 100, 250 and 500 m). For spinal‐onset patients, both living near arable crops (differences ranging from 1.30 to 3.60 years, significant radii 100, 250, 500, 1000 and 1500 m) and vineyards (differences ranging from 1.50 to 2.00 years, significant radii 500, 1000, 1500 and 2000 m) brought disease onset forward. In wild‐type patients, only arable crops proximity resulted in significant differences (differences ranging from 1.90 to 2.50 years, significant radii 250, 500 and 1000 m).
DISCUSSION
We confirmed an increased ALS risk in geographical areas characterized by the presence of arable crops. Moreover, we also observed a significant effect of exposure to arable crops and vineyards on age at onset, which was particularly evident when we considered historical exposure. Our novel approach consists in the stratification of municipalities according to the distribution of different croplands that allowed us to overcome the definition of rural and urban areas, attributing specific weight to different crops exposure. Another novelty of our study consisted in the simultaneous evaluation of the effect on both risk and disease phenotype of the same environmental factor (different croplands proximity) in a 7‐year incident population cohort, using the historical data of the 20 years preceding disease onset. The increased significance obtained by using historical data was in agreement with the presence of possible environmental factors related to specific cropland that can both increase ALS risk and anticipate disease onset in genetically predisposed people. A similar effect has been described in some well‐documented “ALS outbreaks” [8, 9, 11].
Most epidemiological studies performed in different Western and Eastern countries had indicated that living in rural areas or working as farmers or breeders are possible risk factors for ALS [16, 24, 25, 26, 27, 28, 29].
Studies evaluating specifically rural areas and occupational exposure observed that being involved in agriculture more than just living in the countryside was associated with an increased risk of developing ALS [17, 30, 31]. Only one study showed that residency in rural areas as opposed to occupation had a stronger effect on total risk, especially when considering past residence [32]. Nevertheless, most studies defined rural areas simply by using communities' size classes (number of inhabitants), which may have led to some misclassification [17, 30, 31, 32]. Many studies that pointed out agricultural work as a possible risk factor for ALS also showed that most incident ALS cases living in rural areas did not work in agriculture [17].
In our study, by defining the specific use of the agricultural areas surrounding the patients' residence, we explained why in some previous studies, rural areas were equally considered as having a detrimental or no effect. We observed, for example, that living in the countryside but being surrounded by paddy fields or meadows did not confer a higher ALS risk, while living in the proximity of arable crops may play a role. Since population density, especially in a rare disease, can explain a high percentage of rates variance [33], we applied incidence smoothing and considered only agricultural usage data, obtaining data on the correlation between arable crops distribution and ALS incidence.
Few studies have analysed environmental factors in relation to the age at onset: a study performed in Northern Spain found no differences in age at onset between patients living in rural or in urban areas [34], while in another study on a Chinese population, young‐onset ALS patients more frequently lived in rural areas [35]. In the Italian province of Modena, the age‐specific peak ALS frequency in the mountainous area was some 15 years lower in patients living in urban and flat areas (60–64 years old vs. 75–79 years old) [26]. The authors explained this by recognizing that young people may be more exposed to risk factors connected to farming in the mountainous areas than in the flat and urban areas. In our study, we observed a significant difference in age at onset for patients living near arable crops at diagnosis, that become more significant when considering the exposure in the 20 years before diagnosis. Vineyard proximity resulted to differentiate age at onset, but only when considering past exposure. These results, considering that in the historical analysis we took into account crops proximity roughly between 1985 and 2015, can be related to a better classification of real patients' exposure, especially considering that pesticide use has greatly changed over time [36, 37]. Arable crops and vineyards cultivation shared some common pesticide categories, mainly herbicides [23].
Some studies, using self‐reported pesticide exposure [14, 15, 38, 39] or geospatial approaches [6], linked crops and pesticide use with higher ALS risk, while others failed to confirm this association [23, 40]. A population‐based case–control study in two Italian regions found only limited evidence of a dose–response relationship between crop proximity and ALS risk [23]. As compared with our study, they decided to evaluate exposure in a narrow zone of 100 m radius from each subject's residence, reducing both the sensitivity and power of the analysis. In our analysis on age at onset we observed that significant discriminative results were obtained using higher radii (from 100 to 1500 m), confirming that possible chronic exposure to some compounds that can volatilize from crops and soil after application could occur at greater distances than previously considered [41].
Only one study analysed blood concentrations of persistent environmental pollutants in ALS patients, confirming an increased odds for some pesticides, such as organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) [42]. Pesticides have been associated with high probability of many neurological and non‐neurological diseases [43], but they are not the only pollutants related to croplands. Other natural toxins, such as phytotoxins and mycotoxins [44], which have been studied in food and feed for decades, received increased attention as environmental micropollutants, especially for their potential neurotoxic effect in both acute and chronic exposure [45]. Both pesticides [46] and natural toxins, such as ochratoxin A [47], have been detected in blood and urine human samples in different concentrations according to the residence area and independently from occupational exposure, confirming the possibility of chronic environmental contamination.
We did not observe any difference in site of onset and rate of progression at diagnosis related to specific crop proximity, observing that rate of progression appeared to be inversely related to age at onset. This could be explained by the fact that older age is generally negatively related to survival [48] and, even if possible environmental factors related to arable crops and vineyards proximity are able to significantly anticipate disease onset in the exposed population, their effect on neurodegeneration is probably not strong enough to accelerate disease progression. Only one study conducted in a Chinese population reported an association between both rural residence and history of pesticides contact and shorter survival [49]: the authors attribute their finding mainly to the large economic gap between urban and rural areas, which hampers ALS patients living in the countryside from obtaining proper treatment.
We found that arable crops and vineyard proximity showed a higher effect in males than in females. A previous study reported a male predominance of patients with systemic forms of the disease only in rural cases [34]. In the Piemonte population, a risk excess was observed in some rural municipalities, particularly in males aged from 35 to 60 years [50], while in Japan death rates were higher in rural areas for males than for females [51]. Moreover, in a case–control study on occupational risk, agricultural chemicals exposure was associated with ALS with a dose–response trend only in men [52]. In Piemonte, the number of male farmers is higher than female farmers (approximately 72% of the 64,450 agricultural workers) [53]. A cumulative effect of both residential proximity and occupational exposure could explain our sex‐unbalanced results.
Our study is not without limitations. First, the complexity of croplands area distribution determined by analysis of the whole regional area prevented us from using an atmospheric dispersion model [54], which is usually performed to weight aerial transport of pollutants from their source. We minimized possible bias by analysing different increasing radii to avoid an underestimation of the effect, and the SNR analysis confirmed the validity of our results for croplands and vineyards. Second, we considered only the official residence noted in the civil registry, without including domicile or residence time splits (i.e., living portions of the week or year in two different places). However, considering that in Italy the residential mobility among the elderly is lower than in other European countries [55], this confounder should be irrelevant. Third, we did not adjust our analysis for socioeconomic/occupational status or include occupational data for the study subjects since, especially for historical analysis, these data were complete only in a subset of patients.
CONCLUSIONS
Our ALS population‐based data merged with unbiased geospatial data on croplands distribution, analysed in the 20 years before disease onset, returned us a solid, direct association between arable crops occurrence and ALS risk in the same municipalities and an inverse association between arable crops and vineyards proximity and age at onset. Even if our findings cannot prove a causative effect, they highlight the importance of weighting for potential environmental exposures to crop‐related contaminants, and in particular considering historical residential exposure. Future research is needed to clarify the possible causative role of specific environmental contaminants, pinpointing their biological neurotoxic mechanisms and reducing their detrimental effect through prevention or development of new‐targeted drugs.
AUTHOR CONTRIBUTIONS
Umberto Manera: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; validation; writing – original draft. Stefano Callegaro: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; software; validation; writing – original draft. Antonio Canosa: Data curation; investigation; resources; writing – review and editing. Francesca Palumbo: Data curation; investigation; resources; writing – review and editing. Maurizio Grassano: Data curation; investigation; resources; writing – review and editing. Alessandro Bombaci: Data curation; investigation; resources; writing – review and editing. Arianna Dagliati: Formal analysis; software; writing – review and editing. Pietro Bosoni: Formal analysis; software; writing – review and editing. Margherita Daviddi: Data curation; investigation; resources; writing – review and editing. Federico Casale: Data curation; investigation; resources; writing – review and editing. Sara Cabras: Data curation; investigation; resources; writing – review and editing. Enrico Matteoni: Data curation; investigation; resources; writing – review and editing. Fabiola De Marchi: Data curation; investigation; resources; writing – review and editing. Letizia Mazzini: Data curation; investigation; resources; writing – review and editing. Cristina Moglia: Data curation; resources; investigation; writing – review and editing. Rosario Vasta: Conceptualization; investigation; resources; supervision; validation; visualization; writing – review and editing. Andrea Calvo: Funding acquisition; project administration; supervision; validation; writing – review and editing. Adriano Chiò: Supervision; project administration; writing – review and editing; funding acquisition; validation.
FUNDING INFORMATION
The study was supported by the Horizon 2020 project BRAINTEASER (Bringing Artificial Intelligence home for a better care of amyotrophic lateral sclerosis and multiple sclerosis). BRAINTEASER has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No. GA101017598 (start date 01/01/2021); the Italian Ministry of Health (Ministero della Salute, Ricerca Sanitaria Finalizzata, grant RF‐2016‐02362405); the Progetti di Rilevante Interesse Nazionale program of the Ministry of Education, University and Research (grant 2017SNW5MB); the European Commission's Health Seventh Framework Programme (FP7/2007–2013 under grant agreement 259867); and the Joint Programme–Neurodegenerative Disease Research (Strength, ALS‐Care and Brain‐Mend projects), granted by the Italian Ministry of Education, University and Research. This study was performed under the Department of Excellence grant of the Italian Ministry of University and Research to the “Rita Levi Montalcini” Department of Neuroscience, University of Torino, Italy, and to the Department of Health Sciences, University of Eastern Piemonte, Novara, Italy. The funders had no role in data collection or analysis and did not participate in writing or approving the manuscript.
CONFLICT OF INTEREST STATEMENT
Umberto Manera, Stefano Callegaro, Antonio Canosa, Francesca Palumbo, Maurizio Grassano, Alessandro Bombaci, Arianna Dagliati, Pietro Bosoni, Margherita Daviddi, Federico Casale, Sara Cabras, Enrico Matteoni, Fabiola De Marchi, Letizia Mazzini, Cristina Moglia e Rosario Vasta report no disclosures. Adriano Chiò serves on scientific advisory boards for Mitsubishi Tanabe, Biogen, Roche, Denali Pharma, Cytokinetics, Lilly and Amylyx Pharmaceuticals and has received a research grant from Biogen. Andrea Calvo serves on scientific advisory boards for Amylix Pharmaceuticals and has received a research grant from Cytokinetics.
Supporting information
Appendix S1.
ACKNOWLEDGEMENTS
The authors acknowledge the Arpa Piemonte (Regional Environmental Protection Agency, Agenzia Regionale per la Protezione Ambientale) for providing data on environmental exposures, with a special mention to Marcella Alibrandi, Fulvio Raviola, Filippo Richieri, Enrico Bonansea, Gianmario Nava, Luca Forestello, Massimiliano Carrino and Franco Ghione.
Manera U, Callegaro S, Canosa A, et al. Croplands proximity is associated with amyotrophic lateral sclerosis incidence and age at onset. Eur J Neurol. 2025;32:e16464. doi: 10.1111/ene.16464
Umberto Manera and Stefano Callegaro contributed equally to this work and shared first authorship.
Rosario Vasta, Andrea Calvo and Adriano Chiò contributed equally to this work and shared last authorship.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hardiman O, Al‐Chalabi A, Chio A, et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3(1):17071. doi: 10.1038/nrdp.2017.71 [DOI] [PubMed] [Google Scholar]
- 2. Bendotti C, Bonetto V, Pupillo E, et al. Focus on the heterogeneity of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(7–8):485‐495. doi: 10.1080/21678421.2020.1779298 [DOI] [PubMed] [Google Scholar]
- 3. Marin B, Boumédiene F, Logroscino G, et al. Variation in worldwide incidence of amyotrophic lateral sclerosis: a meta‐analysis. Int J Epidemiol. 2016;2:dyw061. doi: 10.1093/ije/dyw061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617‐628. doi: 10.1038/nrneurol.2013.203 [DOI] [PubMed] [Google Scholar]
- 5. Vasta R, Chia R, Traynor BJ, Chiò A. Unraveling the complex interplay between genes, environment, and climate in ALS. EBioMedicine. 2022;75:103795. doi: 10.1016/j.ebiom.2021.103795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrew A, Zhou J, Gui J, et al. Pesticides applied to crops and amyotrophic lateral sclerosis risk in the U.S. Neurotoxicology. 2021;87:128‐135. doi: 10.1016/j.neuro.2021.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vasta R, Canosa A, Manera U, et al. Do ecological factors influence the clinical presentation of amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2021;92(9):1017‐1019. doi: 10.1136/jnnp-2020-325625 [DOI] [PubMed] [Google Scholar]
- 8. Spencer PS. Parkinsonism and motor neuron disorders: lessons from Western Pacific ALS/PDC. J Neurol Sci. 2022;433:120021. doi: 10.1016/j.jns.2021.120021 [DOI] [PubMed] [Google Scholar]
- 9. Kasarskis EJ, Lindquist JH, Coffman CJ, et al. Clinical aspects of ALS in Gulf War veterans. Amyotroph Lateral Scler. 2009;10(1):35‐41. doi: 10.1080/17482960802351029 [DOI] [PubMed] [Google Scholar]
- 10. Re DB, Yan B, Calderón‐Garcidueñas L, Andrew AS, Tischbein M, Stommel EW. A perspective on persistent toxicants in veterans and amyotrophic lateral sclerosis: identifying exposures determining higher ALS risk. J Neurol. 2022;269(5):2359‐2377. doi: 10.1007/s00415-021-10928-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chio A. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(3):472‐476. doi: 10.1093/brain/awh373 [DOI] [PubMed] [Google Scholar]
- 12. Daneshvar DH, Mez J, Alosco ML, et al. Incidence of and mortality from amyotrophic lateral sclerosis in National Football League Athletes. JAMA Netw Open. 2021;4(12):e2138801. doi: 10.1001/jamanetworkopen.2021.38801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feddermann‐Demont N, Junge A, Weber KP, Weller M, Dvořák J, Tarnutzer AA. Prevalence of potential sports‐associated risk factors in Swiss amyotrophic lateral sclerosis patients. Brain Behav. 2017;7(4):e00630. doi: 10.1002/brb3.630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamel F, Umbach DM, Bedlack RS, et al. Pesticide exposure and amyotrophic lateral sclerosis. Neurotoxicology. 2012;33(3):457‐462. doi: 10.1016/j.neuro.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kang H, Cha ES, Choi GJ, Lee WJ. Amyotrophic lateral sclerosis and agricultural environments: a systematic review. J Korean Med Sci. 2014;29(12):1610‐1617. doi: 10.3346/jkms.2014.29.12.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mandrioli J, Biguzzi S, Guidi C, et al. Epidemiology of amyotrophic lateral sclerosis in Emilia Romagna region (Italy): a population based study. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(3–4):262‐268. doi: 10.3109/21678421.2013.865752 [DOI] [PubMed] [Google Scholar]
- 17. Govoni V, Granieri E, Fallica E, Casetta I. Amyotrophic lateral sclerosis, rural environment and agricultural work in the local Health District of Ferrara, Italy, in the years 1964–1998. J Neurol. 2005;252(11):1322‐1327. doi: 10.1007/s00415-005-0859-z [DOI] [PubMed] [Google Scholar]
- 18. Chiò A, Mora G, Moglia C, et al. Secular trends of amyotrophic lateral sclerosis: the Piemonte and Valle d'Aosta register. JAMA Neurol. 2017;74(9):1097‐1104. doi: 10.1001/jamaneurol.2017.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Python Client for Google Maps Services (2021). Version 4.5.3. https://github.com/googlemaps/google‐maps‐services‐python
- 20. Grassano M, Calvo A, Moglia C, et al. Systematic evaluation of genetic mutations in ALS: a population‐based study. J Neurol Neurosurg Psychiatry. 2022;93:1190‐1193. doi: 10.1136/jnnp-2022-328931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. http://dati‐censimentopopolazione.istat.it/Index.aspx
- 22. https://geoportale.arpa.piemonte.it/app/public/
- 23. Vinceti M, Filippini T, Violi F, Rothman KJ, Costanzini S, Malagoli C, Wise LA, Odone A, Signorelli C, Iacuzio L, Arcolin E, Mandrioli J, Fini N, Patti F, Lo Fermo S, Pietrini V, Teggi S, Ghermandi G, Scillieri R, Ledda C, Mauceri C, Sciacca S, Fiore M, Ferrante M. Pesticide exposure assessed through agricultural crop proximity and risk of amyotrophic lateral sclerosis. Environ Health 2017;16(1):91. doi: 10.1186/s12940-017-0297-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Granieri E, Carreras M, Tola R, et al. Motor neuron disease in the province of Ferrara, Italy, in 1964–1982. Neurology. 1988;38(10):1604. doi: 10.1212/WNL.38.10.1604 [DOI] [PubMed] [Google Scholar]
- 25. Rosati G, Pinna L, Granieri E, et al. Studies on epidemiological, clinical and etiological aspects of ALS disease in Sardinia, Southern Italy. Acta Neurol Scand. 1977;55(3):231‐244. doi: 10.1111/j.1600-0404.1977.tb05642.x [DOI] [PubMed] [Google Scholar]
- 26. Mandrioli J, Faglioni P, Merelli E, Sola P. The epidemiology of ALS in Modena, Italy. Neurology. 2003;60:683‐689. [DOI] [PubMed] [Google Scholar]
- 27. Kalfakis N, Vassilopoulos D, Voumvourakis C, Ndjeveleka M, Papageorgiou C. Amyotrophic lateral sclerosis in Southern Greece: an epidemiologic study. Neuroepidemiology. 1991;10(4):170‐173. doi: 10.1159/000110266 [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Liu X, Liang H, Xu S, Wang X, Xu R. Amyotrophic lateral sclerosis in seven provinces of Chinese mainland: a cross‐sectional survey from 2015 to 2016. Front Aging Neurosci. 2022;14:946353. doi: 10.3389/fnagi.2022.946353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Das K, Nag C, Ghosh M. Familial, environmental, and occupational risk factors in development of amyotrophic lateral sclerosis. N Am J Med Sci. 2012;4(8):350‐355. doi: 10.4103/1947-2714.99517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furby A, Beauvais K, Kolev I, Rivain JG, Sébille V. Rural environment and risk factors of amyotrophic lateral sclerosis: a case‐control study. J Neurol. 2010;257:792‐798. doi: 10.1007/s00415-009-5419-5 [DOI] [PubMed] [Google Scholar]
- 31. Bharucha NE, Schoenberg BS, Raven RH, Pickle LW, Byar DP, Mason TJ. Geographic distribution of motor neuron disease and correlation with possible etiologic factors. Neurology. 1983;33(7):911‐915. doi: 10.1212/WNL.33.7.911 [DOI] [PubMed] [Google Scholar]
- 32. Korner S, Kammeyer J, Zapf A, et al. Influence of environment and lifestyle on incidence and progress of amyotrophic lateral sclerosis in a German ALS population. Aging Dis. 2019;10(2):205‐216. doi: 10.14336/AD.2018.0327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scott KM, Abhinav K, Wijesekera L, et al. The association between ALS and population density: a population based study. Amyotroph Lateral Scler. 2010;11(5):435‐438. doi: 10.3109/17482961003754552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riancho J, Lozano‐Cuesta P, Santurtún A, et al. Amyotrophic lateral sclerosis in Northern Spain 40 years later: what has changed? Neurodegener Dis. 2016;16:337‐341. doi: 10.1159/000445750 [DOI] [PubMed] [Google Scholar]
- 35. Wei Q, Chen X, Zheng Z, et al. Clinical features of amyotrophic lateral sclerosis in south‐west China. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(7–8):512‐519. doi: 10.3109/21678421.2015.1069849 [DOI] [PubMed] [Google Scholar]
- 36. https://annuario.isprambiente.it/sys_ind/476
- 37. https://ec.europa.eu/eurostat/statistics‐explained/index.php?title=Agri‐environmental_indicator_‐_consumption_of_pesticides#Analysis_at_EU_and_country_level
- 38. Bonvicini F, Norina M, Mandrioli J, Pietrini V, Vinceti M. Exposure to pesticides and risk of amyotrophic lateral sclerosis: a population‐based case‐control study. Ann Ist Super Sanita. 2010;46(3):284‐287. [DOI] [PubMed] [Google Scholar]
- 39. Malek AM, Barchowsky A, Bowser R, et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case‐control study. Neurodegener Dis. 2014;14(1):31‐38. doi: 10.1159/000355344 [DOI] [PubMed] [Google Scholar]
- 40. Bermudo Fuenmayor S, Serrano Castro PJ, Quiroga Subirana P, López Palmero S, Requena Mullor M, Parrón CT. Environmental exposure to pesticides and amyotrophic lateral sclerosis in the South of Spain. Neurologia. 2021;23:30. doi: 10.1016/j.nrl.2021.01.013 [DOI] [PubMed] [Google Scholar]
- 41. Gunier RB, Ward MH, Airola M, et al. Determinants of agricultural pesticide concentrations in carpet dust. Environ Health Perspect. 2011;119(7):970‐976. doi: 10.1289/ehp.1002532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Su FC, Goutman SA, Chernyak S, et al. Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol. 2016;73(7):803–811. doi: 10.1001/jamaneurol.2016.0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mostafalou S, Abdollahi M. Pesticides: an update of human exposure and toxicity. Arch Toxicol. 2017;91(2):549‐599. doi: 10.1007/s00204-016-1849-x [DOI] [PubMed] [Google Scholar]
- 44. Manera U, Matteoni E, Canosa A, et al. Mycotoxins and amyotrophic lateral sclerosis: food exposure, nutritional implications and dietary solutions. CNS Neurol Disord Drug Targets. 2023;23(5):562–572. doi: 10.2174/1871527323666230817145434 [DOI] [PubMed] [Google Scholar]
- 45. Hoerger CC, Schenzel J, Strobel BW, Bucheli TD. Analysis of selected phytotoxins and mycotoxins in environmental samples. Anal Bioanal Chem. 2009;395(5):1261‐1289. doi: 10.1007/s00216-009-3088-y [DOI] [PubMed] [Google Scholar]
- 46. Wielgomas B, Piskunowicz M. Biomonitoring of pyrethroid exposure among rural and urban populations in northern Poland. Chemosphere. 2013;93(10):2547‐2553. doi: 10.1016/j.chemosphere.2013.09.070 [DOI] [PubMed] [Google Scholar]
- 47. Lino CM, Baeta ML, Henri M, Dinis AMP, Pena AS, Silveira MIN. Levels of ochratoxin a in serum from urban and rural Portuguese populations and estimation of exposure degree. Food Chem Toxicol. 2008;46(3):879‐885. doi: 10.1016/j.fct.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 48. Chiò A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10(5–6):310‐323. doi: 10.3109/17482960802566824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen L, Zhang B, Chen R, et al. Natural history and clinical features of sporadic amyotrophic lateral sclerosis in China. J Neurol Neurosurg Psychiatry. 2015;86(10):1075‐1081. doi: 10.1136/jnnp-2015-310471 [DOI] [PubMed] [Google Scholar]
- 50. Migliaretti G, Berchialla P, Dalmasso P, Cavallo F, Chiò A. Amyotrophic lateral sclerosis in Piedmont (Italy): a Bayesian spatial analysis of the incident cases. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(1):58‐65. doi: 10.3109/21678421.2012.733401 [DOI] [PubMed] [Google Scholar]
- 51. Imaizumi Y. Mortality rate of amyotrophic lateral sclerosis in Japan: effects of marital status and social class, and geographical variation. Japanese J Human Genet. 1986;31(2):101‐111. doi: 10.1007/BF01871404 [DOI] [PubMed] [Google Scholar]
- 52. McGuire V, Longstreth WT, Nelson LM, et al. Occupational exposures and amyotrophic lateral sclerosis. A population‐based case‐control study. Am J Epidemiol. 1997;145(12):1076‐1088. doi: 10.1093/oxfordjournals.aje.a009070 [DOI] [PubMed] [Google Scholar]
- 53. https://www.istat.it/it/files//2013/02/Agricoltura‐Piemonte.pdf
- 54. Teggi S, Costanzini S, Ghermandi G, Malagoli C, Vinceti M. A GIS‐based atmospheric dispersion model for pollutants emitted by complex source areas. Sci Total Environ. 2018;610‐611:175‐190. doi: 10.1016/j.scitotenv.2017.07.196 [DOI] [PubMed] [Google Scholar]
- 55. Angelini V, Laferrère A. Residential mobility of the European elderly. CESifo Econ Stud. 2012;58(3):544‐569. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


