Abstract
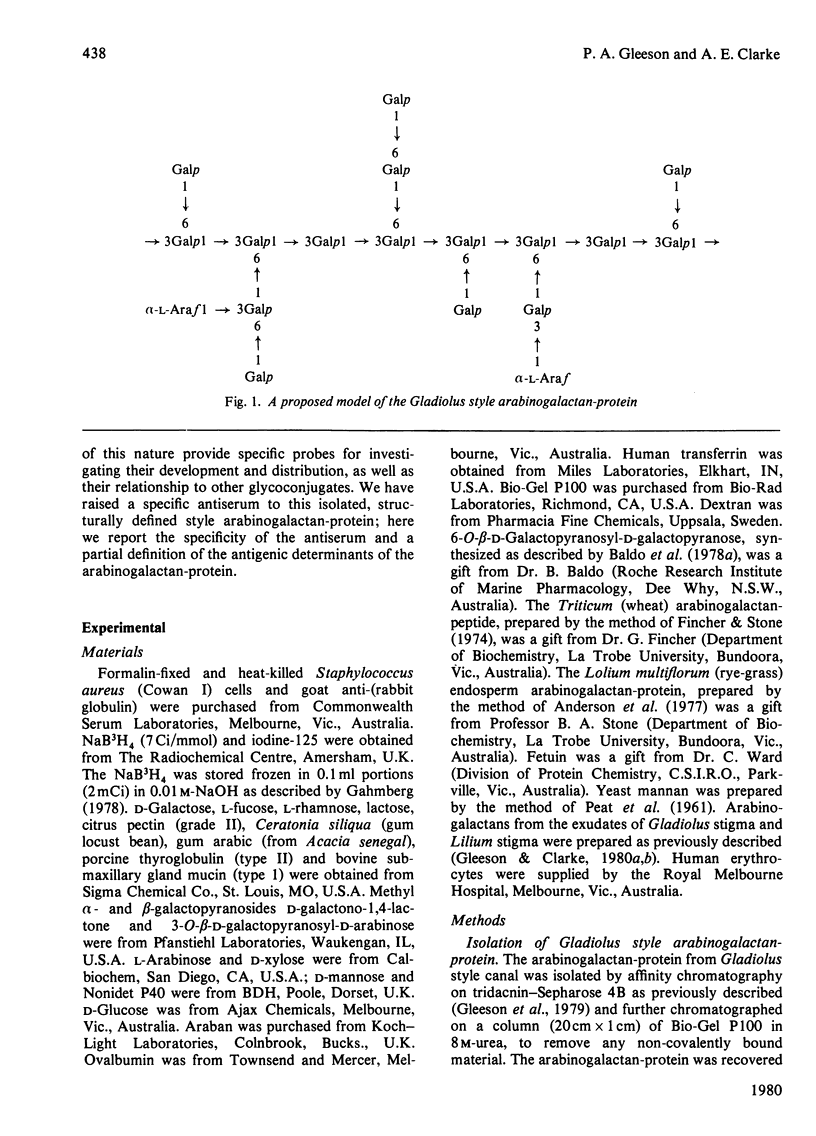
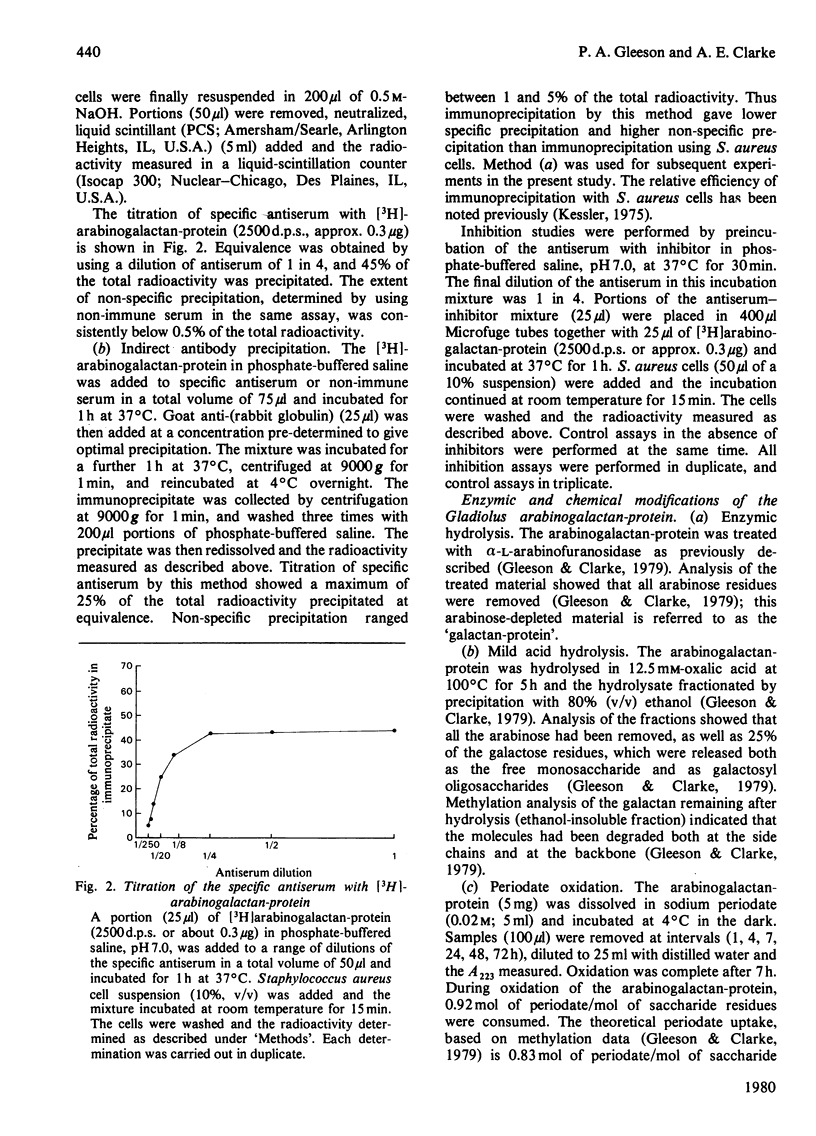
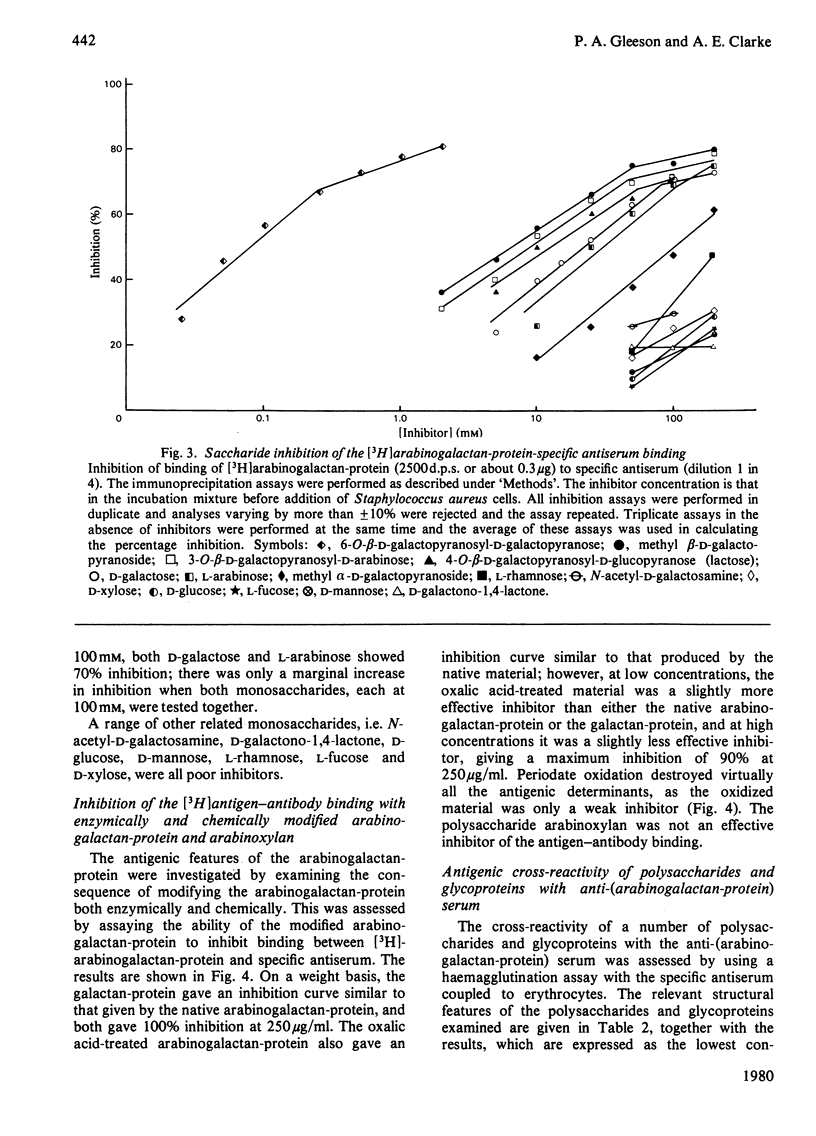
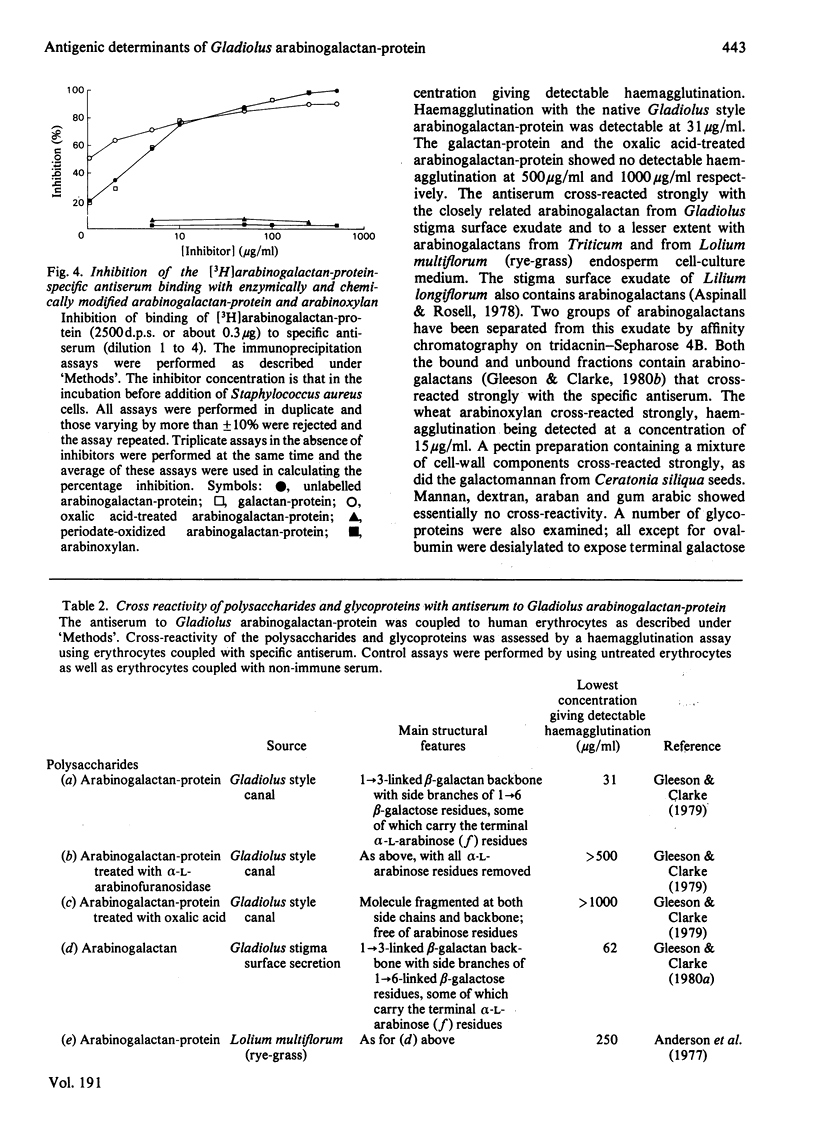
Antiserum has been raised to the arabinogalactan-protein of Gladiolus style mucilage. This macromolecule has been characterized and has a structure consistent with a 1 leads to 3-linked beta-galactan backbone with side branches of 1 leads to 6-linked beta-galactosyl residues, some of which carry terminal alpha-L-arabinofuranoside residues [Gleeson & Clarke (1979) Biochem. J. 181, 607-621]. The specificity of the antiserum has been investigated by immunoprecipitation with [3H]arabinogalactan-protein. THe 3H label was introduced into the arabinogalactan-protein by oxidation of the terminal galactose residues with galactose oxidase, followed by reduction with NaB3H4. The antigenic specificity of the antiserum was shown to be directed towards the carbohydrate component of the arabinogalactan-protein. D-galactose and L-arabinose were the most effective hapten inhibitors of the antiserum; other monosaccharides, N-acetyl-D-galactono-1,4-lactone, D-glucose, D-mannose, L-rhamnose. L-fucose and D-xylose, were all poor inhibitors. The antiserum showed preference for beta-galactosides over alpha-galactosides. Of the haptens examined, the disaccharide 6-O-beta-D-galactopyranosyl-D-galactopyranose was the most potent inhibitor. The antigenic features of the arabinogalactan-protein were investigated by examining the interaction of the antiserum with chemically and enzymically modified arabinogalactan-protein. Also, the cross-reactivity of structurally related polysaccharides and glycoproteins with the specific antiserum was assessed by a haemagglutination assay using erythrocytes coupled with specific antiserum. The results indicate that the dominant antigenic determinants of the arabinogalactan-protein are probably the side branches of 1 leads to 6 -linked beta-galactose residues bearing the terminal alpha-L-arabinose residues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
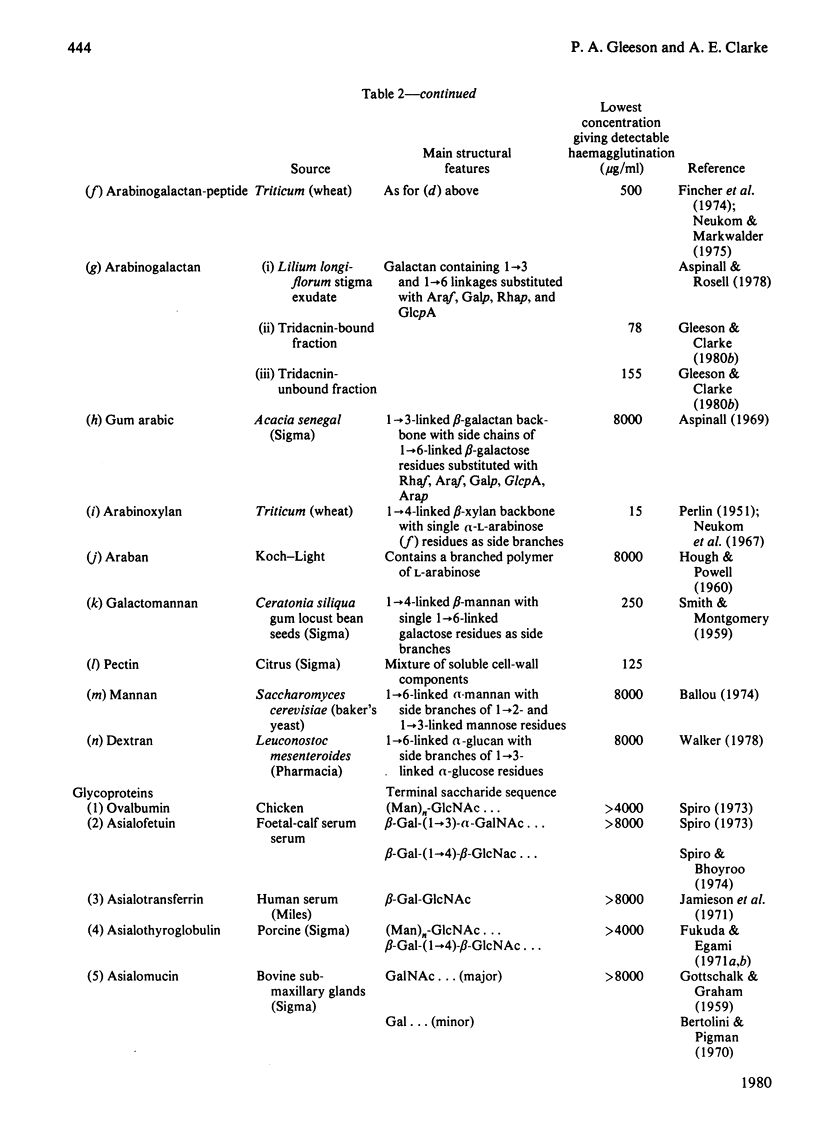
- Aspinall G. O. Gums and mucilages. Adv Carbohydr Chem Biochem. 1969;24:333–379. doi: 10.1016/s0065-2318(08)60353-4. [DOI] [PubMed] [Google Scholar]
- Baldo B. A., Sawyer W. H., Stick R. V., Uhlenbruck G. Purification and characterization of a galactan-reactive agglutinin from the clam Tridacna maxima (Röding) and a study of its combining site. Biochem J. 1978 Nov 1;175(2):467–477. doi: 10.1042/bj1750467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou C. E. A study of the immunochemistry of three yeast mannans. J Biol Chem. 1970 Mar 10;245(5):1197–1203. [PubMed] [Google Scholar]
- Ballou C. E. Some aspects of the structure, immunochemistry, and genetic control of yeast mannans. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):239–270. doi: 10.1002/9780470122853.ch6. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Johnson L. N., Mair G. A., North A. C., Phillips D. C., Sarma V. R. Crystallographic studies of the activity of hen egg-white lysozyme. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):378–388. doi: 10.1098/rspb.1967.0035. [DOI] [PubMed] [Google Scholar]
- Clarke A. E., Knox R. B. Plants and immunity. Dev Comp Immunol. 1979 Fall;3(4):571–589. doi: 10.1016/s0145-305x(79)80053-1. [DOI] [PubMed] [Google Scholar]
- Clarke A., Gleeson P., Harrison S., Knox R. B. Pollen-stigma interactions: Identification and characterization of surface components with recognition potential. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3358–3362. doi: 10.1073/pnas.76.7.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher G. B., Sawyer W. H., Stone B. A. Chemical and physical properties of an arabinogalactan-peptide from wheat endosperm. Biochem J. 1974 Jun;139(3):535–545. doi: 10.1042/bj1390535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W., Glaser L. Surface components and cell recognition. Annu Rev Biochem. 1979;48:491–523. doi: 10.1146/annurev.bi.48.070179.002423. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Egami F. The structure of a glycopeptide purified from porcine thyroblobulin. Biochem J. 1971 Jul;123(3):415–420. doi: 10.1042/bj1230415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK A., GRAHAM E. R. 6-alpha-D-Sialyl-N-acetyl-galactosamine: the neuraminidase-susceptible prosthetic group of bovine salivary mucoprotein. Biochim Biophys Acta. 1959 Aug;34:380–391. doi: 10.1016/0006-3002(59)90290-2. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Tritium labeling of cell-surface glycoproteins and glycolipids using galactose oxidase. Methods Enzymol. 1978;50:204–206. doi: 10.1016/0076-6879(78)50020-7. [DOI] [PubMed] [Google Scholar]
- Glaudemans C. P. The interaction of homogeneous, murine myeloma immunoglobulins with polysaccharide antigens. Adv Carbohydr Chem Biochem. 1975;31:313–346. doi: 10.1016/s0065-2318(08)60299-1. [DOI] [PubMed] [Google Scholar]
- Gleeson P. A., Clarke A. E. Structural studies on the major component of Gladiolus style mucilage, an arabinogalactan-protein. Biochem J. 1979 Sep 1;181(3):607–621. doi: 10.1042/bj1810607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson P. A., Jermyn M. A., Clarke A. E. Isolation of an arabinogalactan protein by lectin affinity chromatography on tridacnin-sepharose 4B. Anal Biochem. 1979 Jan 1;92(1):41–45. doi: 10.1016/0003-2697(79)90622-5. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Jett M., Jamieson G. A., DeBernardo S. L. The carbohydrate sequence of the glycopeptide chains of human transferrin. J Biol Chem. 1971 Jun 10;246(11):3686–3693. [PubMed] [Google Scholar]
- Jolley M. E., Glaudemans C. P., Rudikoff S., Potter M. Structural requirements for the binding of derivatives of D-galactose to two homogeneous murine immunoglobulins. Biochemistry. 1974 Jul 16;13(15):3179–3184. doi: 10.1021/bi00712a028. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. The nature of an antigenic determinant. J Immunol. 1966 Jul;97(1):1–11. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kieda C. M., Bowles D. J., Ravid A., Sharon N. Lectins in lymphocyte membranes. FEBS Lett. 1978 Oct 15;94(2):391–396. doi: 10.1016/0014-5793(78)80985-5. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Kabat E. A., Layug E. J., Gruezo F. Immunochemical studies on blood groups. XXXIV. Structures of some oligosaccharides produced by alkaline degradation of blood group A, B, and H substances. Biochemistry. 1966 May;5(5):1489–1501. doi: 10.1021/bi00869a007. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish C. R., McKenzie I. F. A sensitive rosetting method for detecting subpopulations of lymphocytes which react with alloantisera. J Immunol Methods. 1978;20:173–183. doi: 10.1016/0022-1759(78)90254-5. [DOI] [PubMed] [Google Scholar]
- Potter M., Mushinski E. B., Glaudemans C. P. Antigen-binding IgA myeloma proteins in mice: specificities to antigens containing -D 1 leads to 6 linked galactose side chains and a protein antigen in wheat. J Immunol. 1972 Feb;108(2):295–300. [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Watkins W. M. Genetics and biochemistry of some human blood groups. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):31–53. doi: 10.1098/rspb.1978.0056. [DOI] [PubMed] [Google Scholar]
- Winand R. J., Kohn L. D. Relationships of thyrotropin to exophthalmic-producing substance. Purification of homogeneous glycoproteins containing both activities from [3H]-labeled pituitary extracts. J Biol Chem. 1970 Mar 10;245(5):967–975. [PubMed] [Google Scholar]


