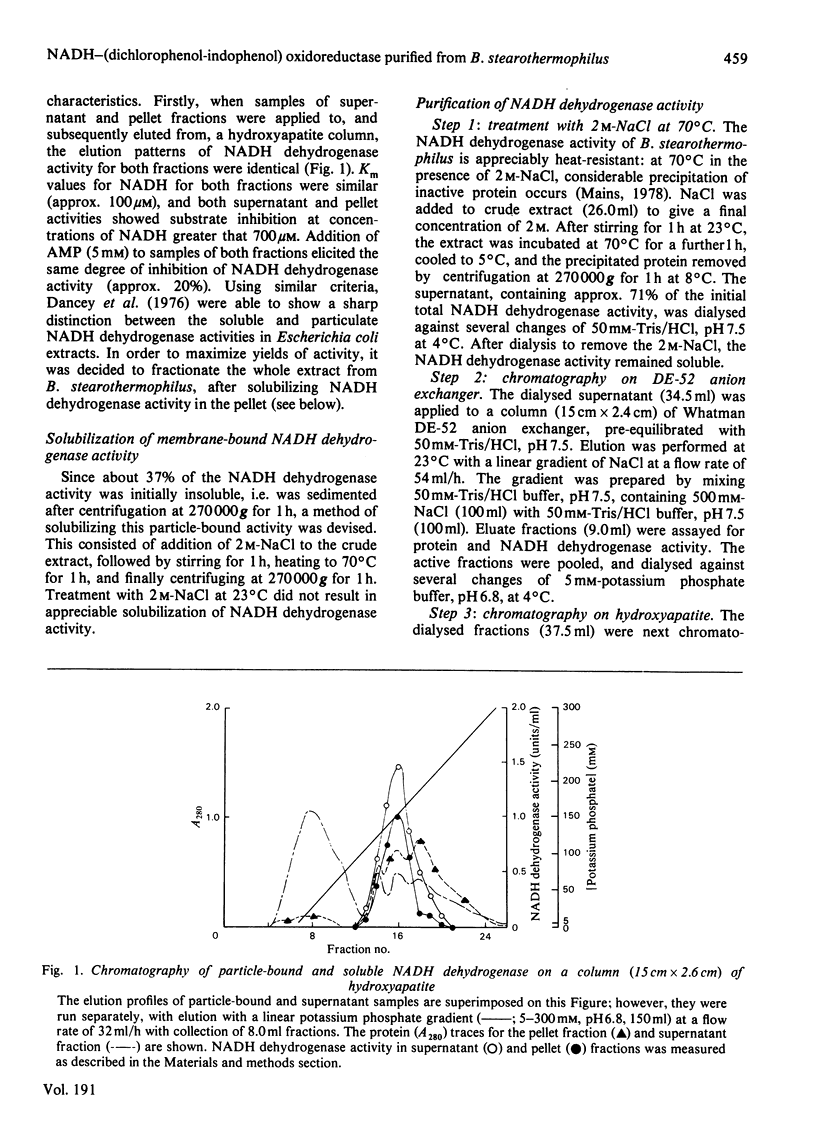
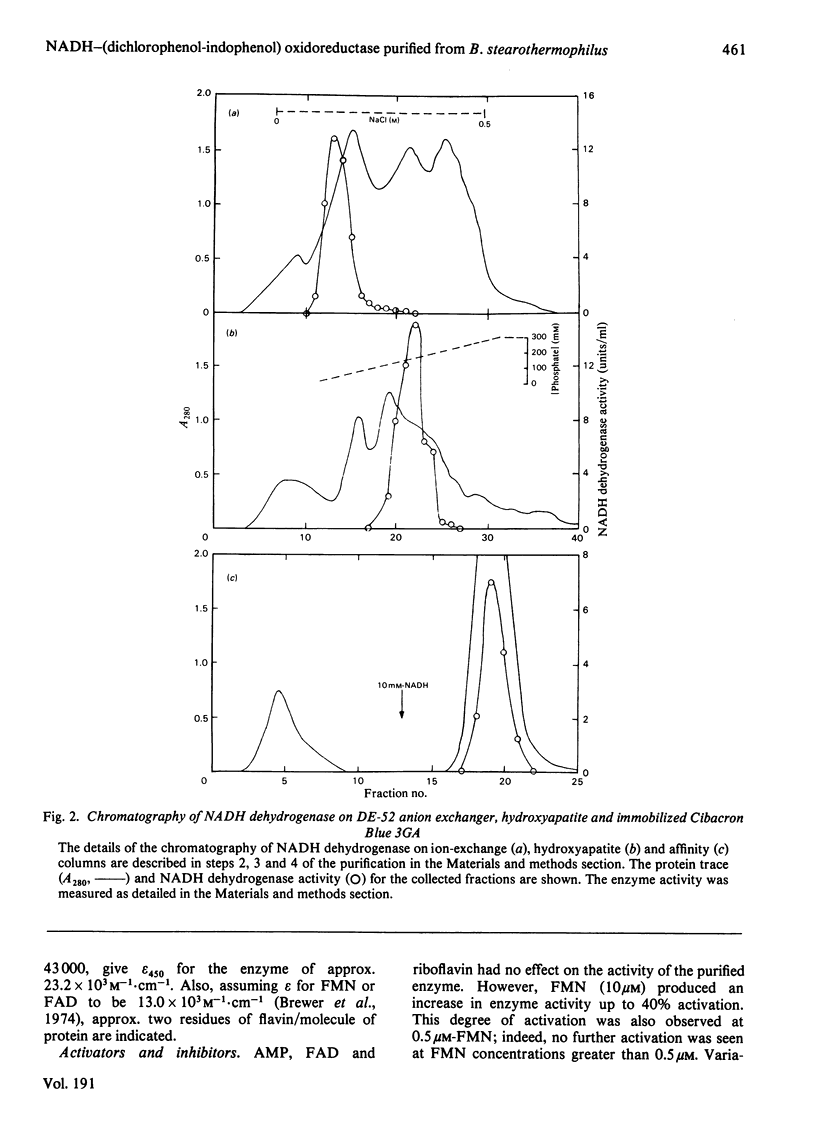
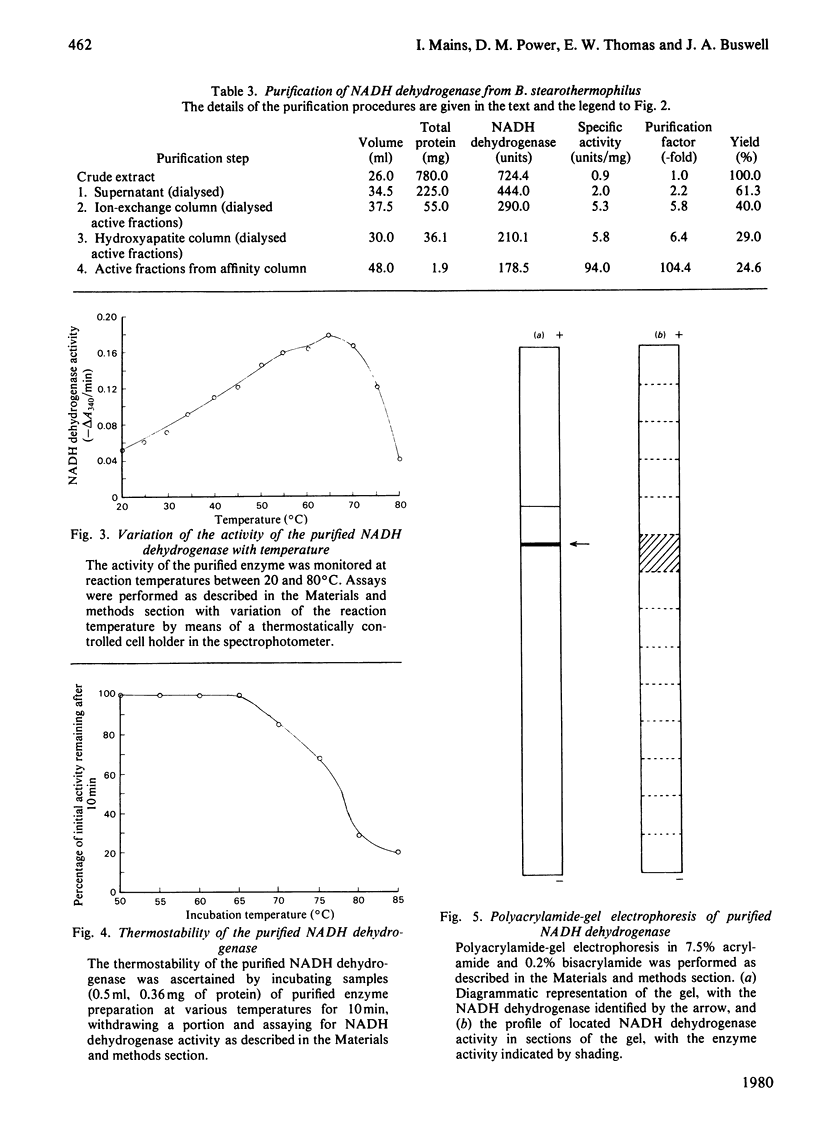
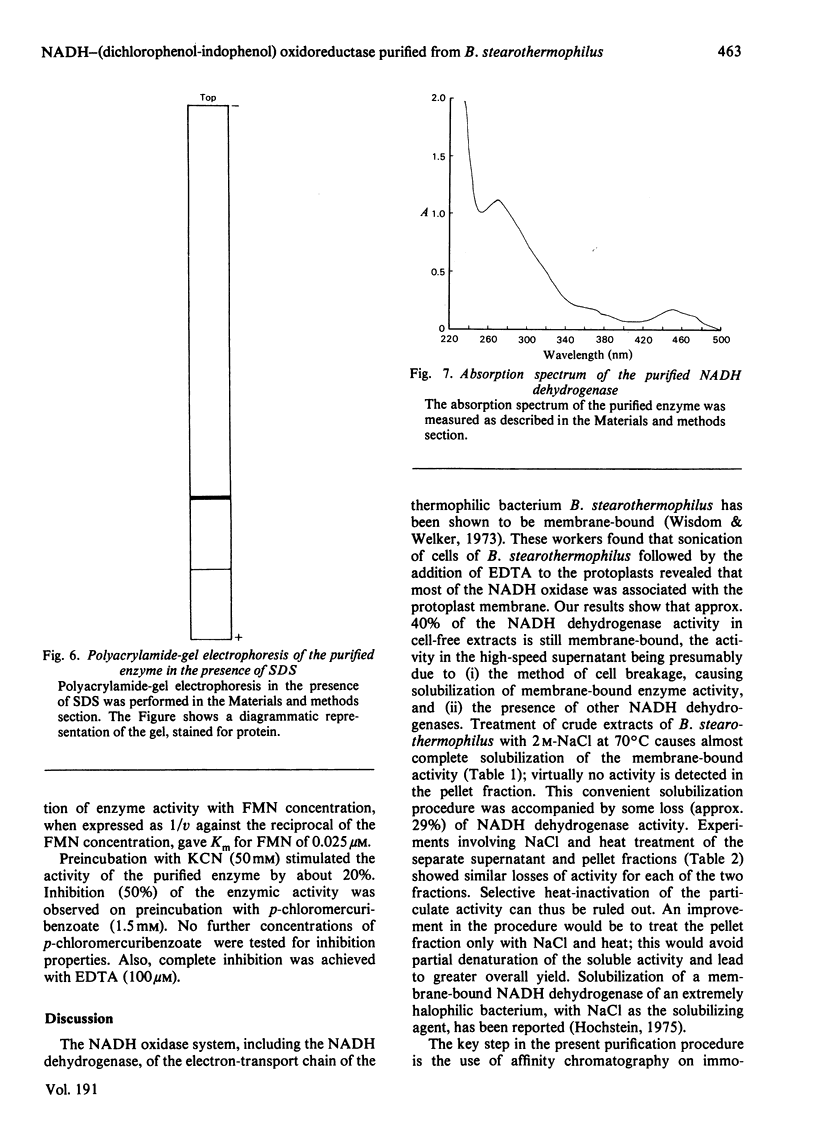
Abstract
An NADH-(dichlorophenol-indophenol) oxidoreductase was purified 104-fold and in 25% overall yield from the thermophilic bacterium Bacillus stearothermophilus, strain PH24. After solubilization in 2M-NaCl at 70 degrees C, the enzyme was purified by ion-exchange and hydroxyapatite chromatography, followed by affinity chromatography on immobilized Cibacron Blue 3GA. The purified enzyme had a mol.wt. of 43 000 and had an absorption spectrum characteristic of flavoprotein. The enzyme activity was enhanced by FMN and by CN-. The enzyme was inhibited by EDTA and by rho-chloromercuribenzoic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amelunxen R., Lins M. Comparative thermostability of enzymes from Bacillus stearothermophilus and Bacillus cereus. Arch Biochem Biophys. 1968 Jun;125(3):765–769. doi: 10.1016/0003-9861(68)90512-2. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buswell J. A. Metabolism of phenol and cresols by Bacillus stearothermophilus. J Bacteriol. 1975 Dec;124(3):1077–1083. doi: 10.1128/jb.124.3.1077-1083.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dancey G. F., Levine A. E., Shapiro B. M. The NADH dehydrogenase of the respiratory chain of Escherichia coli. I. Properties of the membrane-bound enzyme, its solubilization, and purification to near homogeneity. J Biol Chem. 1976 Oct 10;251(19):5911–5920. [PubMed] [Google Scholar]
- Dean P. D., Brown P., Leyland M. J., Watson D. H., Angal S., Harvey M. J. Electrophoretic desorption of affinity adsorbents [proceedings]. Biochem Soc Trans. 1977;5(4):1111–1113. doi: 10.1042/bst0051111a. [DOI] [PubMed] [Google Scholar]
- Heyns W., De Moor P. A 3(17)beta-hydroxysteroid dehydrogenase in raterythrocytes. Conversion of 5alpha-dihydrotestosterone into 5alpha-androstane-3beta,17beta-diol and purification of the enzyme by affinity chromatography. Biochim Biophys Acta. 1974 Jul 17;358(1):1–13. doi: 10.1016/0005-2744(74)90251-4. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I., Dalton B. P. Studies of a halophilic NADH dehydrogenase. I. Purification and properties of the enzyme. Biochim Biophys Acta. 1973 Apr 12;302(2):216–228. doi: 10.1016/0005-2744(73)90150-2. [DOI] [PubMed] [Google Scholar]
- Hochstein L. I. Studies of a halophilic NADH dehydrogenase. II. Kinetic properties of the enzyme in relation to salt activation. Biochim Biophys Acta. 1975 Sep 22;403(1):58–66. doi: 10.1016/0005-2744(75)90008-x. [DOI] [PubMed] [Google Scholar]
- Jinks D. C., Matz L. L. Purification of the reduced nicotinamide adenine dinucleotide dehydrogenase from membranes of Acholeplasma laidlawii. Biochim Biophys Acta. 1976 Nov 8;452(1):30–41. doi: 10.1016/0005-2744(76)90055-3. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, D'Eustachio A. J., Hardy R. W. Flavodoxin: a flavoprotein with ferredoxin activity from Clostrium pasteurianum. Biochim Biophys Acta. 1966 Mar 7;113(3):626–628. doi: 10.1016/s0926-6593(66)80025-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W. S., Armstrong D. A., Gaucher G. M. Formation and repair of papain sulfenic acid. Can J Biochem. 1975 Mar;53(3):298–307. doi: 10.1139/o75-042. [DOI] [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- SWOBODA B. E., MASSEY V. PURIFICATION AND PROPERTIES OF THE GLUCOSE OXIDASE FROM ASPERGILLUS NIGER. J Biol Chem. 1965 May;240:2209–2215. [PubMed] [Google Scholar]
- Singer T. P., Gutman M. The DPNH dehydrogenase of the mitochondrial respiratory chain. Adv Enzymol Relat Areas Mol Biol. 1971;34:79–153. doi: 10.1002/9780470122792.ch3. [DOI] [PubMed] [Google Scholar]
- Thompson S. T., Stellwagen E. Binding of Cibacron blue F3GA to proteins containing the dinucleotide fold. Proc Natl Acad Sci U S A. 1976 Feb;73(2):361–365. doi: 10.1073/pnas.73.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wisdom C., Welker N. E. Membranes of Bacillus stearothermophilus: factors affecting protoplast stability and thermostability of alkaline phosphatase and reduced nicotinamide adenine dinucleotide oxidase. J Bacteriol. 1973 Jun;114(3):1336–1345. doi: 10.1128/jb.114.3.1336-1345.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


