Abstract
Methionine oxidation is involved in multiple biological processes including protein misfolding and enzyme regulation. However, it is often challenging to measure levels of methionine oxidation by mass spectrometry, in part due to the prevalence of artifactual oxidation that occurs during the sample preparation and ionization steps of typical proteomic workflows. Isotopically labeled hydrogen peroxide (H218O2) can be used to block unoxidized methionines and enables accurate measurement of in vivo levels of methionine oxidation. However, H218O2 is an expensive reagent that can be difficult to obtain from commercial sources. Here, we report a method for synthesizing H218O2 in-house. Glucose oxidase catalyzes the oxidation of β-d-glucose and produces hydrogen peroxide in the process. We took advantage of this reaction to enzymatically synthesize H218O2 from 18O2 and assessed its concentration, purity, and utility in measuring methionine oxidation levels by mass spectrometry.
Keywords: Mass Spectrometry (MS), Glucose Oxidase (GOx), Methionine Oxidation
Introduction
Side chains of methionines are susceptible to oxidation by reactive oxygen species (ROS) or monooxygenases.1−6 This post-translational modification converts the nonpolar methionine residues (Met) to polar methionine sulfoxide residues (MetO).1−4 MetO formation can induce protein misfolding and has been linked to a number of neurodegenerative disorders and pathological aging.1−4,6 Additionally, regulated methionine oxidation can modulate diverse cellular processes and signaling pathways.6 Due to its involvement in protein damage and functional regulation, global quantification of methionine oxidation can provide important insights into a number of diverse biological processes.
It is challenging to measure levels of in vivo methionine oxidation by mass spectrometry in part because unoxidized methionines can become spontaneously oxidized during typical proteomic workflows.1−4 Selective blocking of unoxidized methionines can prevent this artifactual oxidation and allow for more accurate quantitation of methionine oxidation. An example of such an approach is methionine oxidation by blocking (MObB).1−4 In MObB, unoxidized methionines within denatured proteins are fully oxidized with isotopically labeled hydrogen peroxide (H218O2) and blocked from spontaneous oxidation in subsequent steps of bottom-up proteomic workflows.1,2 Relative levels of 16O- and 18O-modified peptides can then be measured and used to determine levels of endogenously oxidized methionines.1,2 Furthermore, isotopically labeled hydrogen peroxide can be utilized to assess protein stability and protein–ligand binding using approaches such as stability of proteins from rates of oxidation (SPROX).7 In addition to its utility in quantifying methionine oxidation, H218O2 has been employed in other mass spectrometric applications including quantitation of H2O2-producing reactions, analysis of the effects of H2O2 on metabolite synthesis, and quantitation of H2O2-induced oxidation of macromolecules.8−10
Despite its usefulness in diverse mass spectrometric applications, H218O2 is expensive and can be difficult to obtain from commercial sources. For example, the sale of H218O2 was entirely discontinued between 2021 and 2024, and currently there is only a single supplier for this reagent (Sigma). Traditionally, hydrogen peroxide is synthesized through the hydrogenation and subsequent oxidation of anthraquinone, an aromatic organic compound that acts as a catalyst in this reaction.11,12 In the most popular synthetic methods, anthraquinone is hydrogenated by a trickle bed with a palladium catalyst. The hydrogenated anthraquinone is then oxidized by O2 through a bubble column, restoring anthraquinone and producing hydrogen peroxide in the process.11 The synthesized hydrogen peroxide is extracted using a sieve-plate extraction tower before it is purified via distillation. Alternative synthesis methods involving electrodes, biochemical approaches, or electrosynthesis reactions with different catalysts have also been reported.12
Enzymatic synthesis provides a more practical approach for in-house generation of H218O2 in typical biochemical laboratories. Glucose oxidase (GOx), an oxidoreductase that originates from insects and fungi, has been used in multiple industries including pharmaceuticals, food, textiles, and biofuels.13−17 GOx catalyzes the oxidation of β-d-glucose to d-glucono-δ-lactone using a FAD cofactor and generates H2O2 by reduction of molecular oxygen (O2).5,13−15,17−19 Previously, GOx from Aspergillus niger has been used to generate ∼11 mM H2O2 for use in textile bleaching studies.16 In this study, we have optimized this enzymatic reaction and used 18O2 as the substrate to generate ∼200 mM H218O2 with high isotopic purity. We further demonstrated the efficacy of in-house generated H218O2 in conducting quantitative mass spectrometric analyses of methionine oxidation levels.
Results and Discussion
Formation and Characterization of H218O2 Generated by GOx
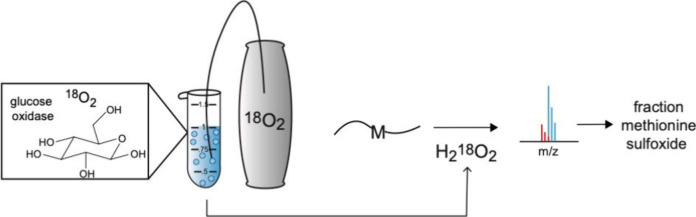
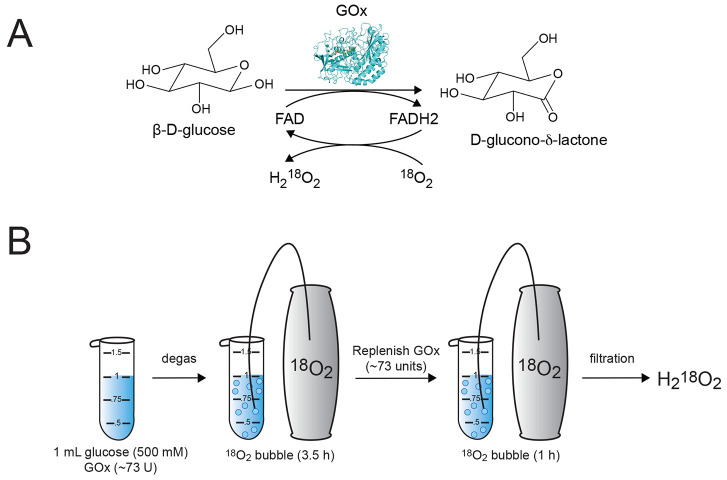
The mechanism for production of hydrogen peroxide by glucose oxidase is illustrated in Figure 1A. We utilized this enzymatic reaction to synthesize H218O2 from 18O2 as described in detail in the Experimental Section (Figure 1B). In brief, the enzymatic conversion of β-d-glucose to d-glucono-δ-lactone was carried out in the presence of a slow flow of 18O2 into an initially degassed solution containing GOx. In initial experiments we observed that the evolution of H2O2 deactivates GOx over time. Thus, to maximize the yield of H2O2, the reaction was supplemented with additional GOx after an initial generation period of 3.5 h, allowing further generation of H2O2 for an additional hour. Following the reaction, GOx was removed from the mixture by filtration.
Figure 1.
Reaction of glucose oxidase (PDB: 1GAL)19 with β-d-glucose and 18O2 generates H218O2. (A) Reaction mechanism of glucose oxidase. (B) Experimental protocol used for generation of H218O2. Details of the protocol are described in the Experimental Section.
The purity and concentration of generated H2O2 was measured by mass spectrometry. An unoxidized synthetic peptide was fully oxidized with the generated H218O2 or commercially obtained H216O2 (Figure 2A). Relative levels of unmodified, 16O-modified and 18O-modified peptides were measured by mass spectrometry (Figure 2B). The peptide oxidized with in-house generated H218O2 contained ∼94% 18O-labeled methionines, indicative of the isotopic purity of the oxidant. In a second experiment, the peptide was partially oxidized with known concentrations of H216O2, and the resulting oxidation levels, as measured by mass spectrometry, were compared to peptides oxidized with various dilutions of in-house generated H218O2. This comparison indicated that the in-house generated H218O2 had a concentration of ∼230 mM (Figure 2C).
Figure 2.
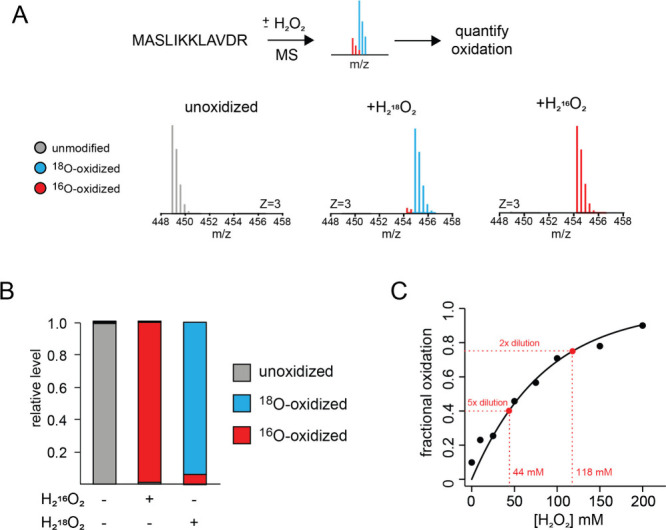
Synthesized H218O2 has high isotopic purity and concentration. (A, B) Synthetic peptide was oxidized with the in-house generated H218O2 (A) and shown to be 94% 18O-labeled (B). (C) Concentration of in-house generated H218O2 was determined to be ∼230 mM by comparing its efficiency in oxidizing a model peptide with known concentrations of the H216O2 ladder.
Generated H218O2 Can Be Used to Accurately Measure Methionine Oxidation Levels
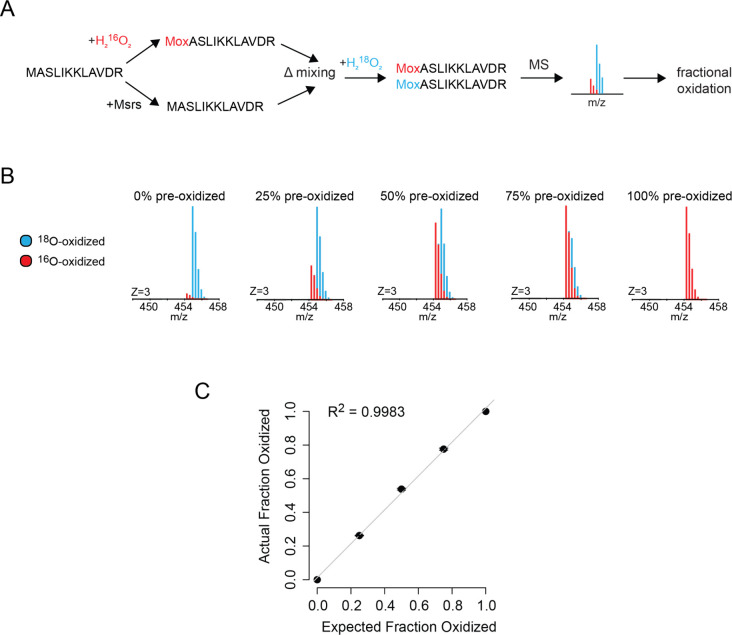
Next, we demonstrated that the generated H218O2 can be used as an effective blocking agent, enabling the accurate measurement of methionine oxidation levels. A synthetic peptide was fully reduced by methionine sulfoxide reductase A and methionine sulfoxide reductase B (Msrs) or fully oxidized with H216O2. Oxidized peptide was mixed with Msr-treated unoxidized peptide at variable ratios to generate mixtures with known predetermined methionine oxidation levels. These mixtures were then oxidized with diluted H218O2, resulting in 18O-oxidation of the previously unoxidized methionines. Relative 16O-oxidation levels of each peptide mixture were determined by measuring the fractional populations of 16O- and 18O-oxidized peptides. The pairwise comparison of expected versus measured 16O-oxidation levels of peptide mixtures is shown in Figure 3. This analysis demonstrates that in-house generated H218O2 can be effectively used as an isotopically labeled blocking reagent for accurate measurement of methionine oxidation levels.
Figure 3.
Synthesized H218O2 can be used to accurately measure levels of methionine oxidation by mass spectrometry. (A, B) Preoxidized peptide mixtures containing different levels of 16O-methionines (0%, 25%, 50%, 75%, 100%) were fully oxidized with H218O2 (A) and analyzed by mass spectrometry (B). (C) Fractional oxidation with 16O was measured and normalized to unoxidized and oxidized controls. The pairwise plot shows the correlation between measured and expected 16O-oxidation levels for each mixture. The error bars indicate standard deviations of two replicate experiments.
In-house synthesis also provides a more cost-effective approach for obtaining 18O-labeled hydrogen peroxide (at the time of writing this manuscript, it reduced costs by approximately 50% in comparison to commercial sources). However, although the described method for in-house synthesis of hydrogen peroxide is straightforward and accessible, there are two important caveats that require special consideration. First, the isotopic purity of the H218O2 produced is dependent on the purity of the dissolved 18O2 in the reaction buffer. Thus, to obtain isotopically pure H218O2, removal of 16O2 by careful initial degassing, and subsequent use of highly pure 18O2 as a substrate is required. Second, the H218O2 generated using the described protocol will also contain buffer components (in this case, sodium acetate) and glucose in oxidized and unoxidized forms. These impurities were inconsequential to the methionine blocking applications investigated in this study. However, if downstream applications require chemically pure H218O2, further purification of the generated product may be required.
Conclusions
This study describes a protocol that employs a widely available enzyme, glucose oxidase, for generation of concentrated and isotopically enriched 18O-labeled hydrogen peroxide. The synthesized H218O2 can be used to block unoxidized methionines and facilitate the measurement of methionine oxidation levels in mass spectrometric workflows.
Experimental Section
To generate H218O2, 1.5 mL of H218O (Cambridge Isotope Laboratories, OLM-240-10G) was degassed in a 2 mL Eppendorf tube inside of a sealed vacuum flask connected to a vacuum. Glucose and sodium acetate were added to 1 mL of H218O to attain final concentrations of 500 mM and 50 mM, respectively. Activity units (72.8) of glucose oxidase (Sigma, G2133-10KU) was added to the solution, then 18O2 (Sigma, 602892-1L) was slowly bubbled from a pipet tip attached to tubing connected to a 1 L gas tank for 4.5 h at 35 °C. Note that one activity unit is defined as 1.0 μmole of hydrogen peroxide per minute at 35 °C and pH 5.1. After 3.5 h, another 72.8 units of glucose oxidase was added to the tube and the slow bubble of 18O2 continued for another hour. After incubation, the hydrogen peroxide was purified through centrifugation at 14,000g for 20 min at 4 °C in a 0.5 mL Amicon filter (3 kDa MWCO) to remove the glucose oxidase. The synthesized H218O2 was aliquoted and stored at −20 °C until use. Additional experimental information related to the determination of the purity and concentration of H218O2 and mass spectrometric analyses are provided in the Supporting Information.
Glossary
Abbreviations
- ROS
reactive oxygen species
- Msrs
methionine sulfoxide reductases
- MObB
methionine oxidation by blocking
- GOx
glucose oxidase
- FAD
flavin adenine dinucleotide
- H2O2
hydrogen peroxide
- Met
methionine
- MetO
methionine sulfoxide
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jasms.4c00326.
Experimental details, including determination of the purity and concentration of H218O2, generation of peptide mixtures with different oxidation levels, and mass spectrometry workflow (PDF)
Author Contributions
The study concept was conceived by M.H., R.T., and S.G. The experiments were carried out by M.H., I.M., and R.T. Mass spectrometry was performed by K.W., K.S., and J.H. Data analysis was conducted by M.H., R.T., and S.G. The initial draft of the manuscript was written by M.H. and S.G.
This work was supported by grants from the National Institutes of Health to S.G. (R35 GM119502 and S10 OD025242) and the Beckman Foundation (Beckman Scholars Program) to M.H.
The authors declare no competing financial interest.
Supplementary Material
References
- Bettinger J. Q.; Welle K. A.; Hryhorenko J. R.; Ghaemmaghami S. Quantitative Analysis of in Vivo Methionine Oxidation of the Human Proteome. J. Proteome Res. 2020, 19 (2), 624–633. 10.1021/acs.jproteome.9b00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettinger J. Q.; Simon M.; Korotkov A.; Welle K. A.; Hryhorenko J. R.; Seluanov A.; Gorbunova V.; Ghaemmaghami S. Accurate Proteomewide Measurement of Methionine Oxidation in Aging Mouse Brains. J. Proteome Res. 2022, 21 (6), 1495–1509. 10.1021/acs.jproteome.2c00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Ponniah G.; Neill A.; Patel R.; Andrien B. Accurate Determination of Protein Methionine Oxidation by Stable Isotope Labeling and LC-MS Analysis. Anal. Chem. 2013, 85 (24), 11705–11709. 10.1021/ac403072w. [DOI] [PubMed] [Google Scholar]
- Shipman J. T.; Go E. P.; Desaire H. Method for Quantifying Oxidized Methionines and Application to HIV-1 Env. J. Am. Soc. Mass Spectrom. 2018, 29 (10), 2041–2047. 10.1007/s13361-018-2010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe K. The Effect of Hydrogen Peroxide on Glucose Oxidase from Aspergillus Niger *. Biochemistry 1966, 5 (1), 139–143. 10.1021/bi00865a018. [DOI] [PubMed] [Google Scholar]
- Hoshi T.; Heinemann S. H. Regulation of Cell Function by Methionine Oxidation and Reduction. J. Physiol 2001, 531 (1), 1–11. 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeArmond P. D.; West G. M.; Huang H.-T.; Fitzgerald M. C. Stable Isotope Labeling Strategy for Protein–Ligand Binding Analysis in Multi-Component Protein Mixtures. J. Am. Soc. Mass Spectrom. 2011, 22 (3), 418–430. 10.1007/s13361-010-0060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Cooper D. E.; Cluntun A. A.; Warmoes M. O.; Zhao S.; Reid M. A.; Liu J.; Lund P. J.; Lopes M.; Garcia B. A.; Wellen K. E.; Kirsch D. G.; Locasale J. W. Acetate Production from Glucose and Coupling to Mitochondrial Metabolism in Mammals. Cell 2018, 175 (2), 502–513. 10.1016/j.cell.2018.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermilyea A. W.; Dixon T. C.; Voelker B. M. Use of H2(18)O2 to Measure Absolute Rates of Dark H2O2 Production in Freshwater Systems. Environ. Sci. Technol. 2010, 44 (8), 3066–3072. 10.1021/es100209h. [DOI] [PubMed] [Google Scholar]
- Hofer T.; Badouard C.; Bajak E.; Ravanat J.-L.; Mattsson Å.; Cotgreave I. A. Hydrogen Peroxide Causes Greater Oxidation in Cellular RNA than in DNA. Biol. Chem. 2005, 386 (4), 333–337. 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- Chen Q. Development of an Anthraquinone Process for the Production of Hydrogen Peroxide in a Trickle Bed Reactor—From Bench Scale to Industrial Scale. Chemical Engineering and Processing: Process Intensification 2008, 47 (5), 787–792. 10.1016/j.cep.2006.12.012. [DOI] [Google Scholar]
- Perry S. C.; Pangotra D.; Vieira L.; Csepei L.-I.; Sieber V.; Wang L.; Ponce de León C.; Walsh F. C. Electrochemical Synthesis of Hydrogen Peroxide from Water and Oxygen. Nat. Rev. Chem. 2019, 3 (7), 442–458. 10.1038/s41570-019-0110-6. [DOI] [Google Scholar]
- Bauer J. A.; Zámocká M.; Majtán J.; Bauerová-Hlinková V. Glucose Oxidase, an Enzyme “Ferrari”: Its Structure, Function, Production and Properties in the Light of Various Industrial and Biotechnological Applications. Biomolecules 2022, 12 (3), 472. 10.3390/biom12030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankar S. B.; Bule M. V.; Singhal R. S.; Ananthanarayan L. Glucose Oxidase — An Overview. Biotechnology Advances 2009, 27 (4), 489–501. 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Khatami S. H.; Vakili O.; Ahmadi N.; Soltani Fard E.; Mousavi P.; Khalvati B.; Maleksabet A.; Savardashtaki A.; Taheri-Anganeh M.; Movahedpour A. Glucose Oxidase: Applications, Sources, and Recombinant Production. Biotechnology and Applied Biochemistry 2022, 69 (3), 939–950. 10.1002/bab.2165. [DOI] [PubMed] [Google Scholar]
- Tzanov T.; Costa S. A.; Gübitz G. M.; Cavaco-Paulo A. Hydrogen Peroxide Generation with Immobilized Glucose Oxidase for Textile Bleaching. J. Biotechnol. 2002, 93 (1), 87–94. 10.1016/S0168-1656(01)00386-8. [DOI] [PubMed] [Google Scholar]
- Dubey M. K.; Zehra A.; Aamir M.; Meena M.; Ahirwal L.; Singh S.; Shukla S.; Upadhyay R. S.; Bueno-Mari R.; Bajpai V. K. Improvement Strategies, Cost Effective Production, and Potential Applications of Fungal Glucose Oxidase (GOD): Current Updates. Front Microbiol 2017, 8, 1032. 10.3389/fmicb.2017.01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J.; Furumoto K.; Yoshimoto M.; Fukunaga K.; Nakao K. Competitive Inhibition by Hydrogen Peroxide Produced in Glucose Oxidation Catalyzed by Glucose Oxidase. Biochemical Engineering Journal 2003, 13 (1), 69–72. 10.1016/S1369-703X(02)00120-1. [DOI] [Google Scholar]
- Hecht H. J.; Kalisz H. M.; Hendle J.; Schmid R. D.; Schomburg D. Crystal Structure of Glucose Oxidase from Aspergillus Niger Refined at 2·3 Å Reslution. J. Mol. Biol. 1993, 229 (1), 153–172. 10.1006/jmbi.1993.1015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.