Abstract
Introduction:
Prenatal and early-life exposure to air pollution and extreme temperatures are associated with childhood asthma and wheeze. However, potential windows of susceptibility and their sex-specific and interactive effects have not been fully elucidated. We aimed to identify critical windows of susceptibility and evaluate sex-specific effects in these associations, and evaluate exposure interactions.
Methods:
We analyzed data from 468 mother–child pairs enrolled in the PROGRESS birth cohort in Mexico City. Daily residential levels of PM2.5, NO2, and temperature were generated from our validated spatiotemporally resolved models from conception to age 4 years. Childhood asthma and wheeze outcomes were collected at 4–6 and 7–8 years. Distributed lag nonlinear models (DLNMs) were used to identify susceptible windows for prenatal weekly-specific and postnatal monthly-specific associations of air pollution and temperature with respiratory outcomes adjusting for covariates. To evaluate sex-specific effects, DLNMs were stratified. Joint effects were assessed using relative excess risk due to interaction and attributable proportion.
Results:
Mid-gestation was a critical window for both PM2.5 (weeks 20–28, cumulative OR: 1.18 [95% CI: 1.01, 1.37]; weeks 19–26, cumulative OR: 1.18 [95% CI: 1.02, 1.36]) and NO2 (weeks 18–25, cumulative OR: 1.16 [95% CI: 1.02, 1.31]) exposure, associated with higher odds of wheeze. Postnatal exposure to PM2.5 and NO2 during the first year of life was also linked to higher odds of wheeze. The warmer and colder temperatures showed mixed effects on respiratory outcomes. We observed a synergistic interaction between high PM2.5 and high temperature exposure during the first year of life, associated with higher odds of current wheeze. The associations of prenatal air pollution and temperature exposure with respiratory outcomes were more pronounced in males.
Conclusions:
Early-life air pollution exposure contributes to the development of childhood asthma and wheeze, while exposure to temperature showed mixed associations with respiratory outcomes.
Keywords: Air pollution, Temperature, Asthma, Wheeze, Susceptible window
1. Introduction
Asthma and wheeze are chronic respiratory conditions that significantly impact the health and quality of life of children worldwide. Childhood asthma is a major public health concern, affecting approximately 14 % of children globally (Asher and Pearce, 2014). Wheezing refers to whistling-like sounds during exhalation and is a common symptom of asthma. Wheezing can also be indicative of other respiratory conditions (Ducharme et al., 2014) such as lower airway infections. Recurrent wheeze is characterized by breathlessness and coughing, which can impair a child’s physical activity, sleep quality, and overall well-being (Nurmagambetov et al., 2018). Over the past decades, the prevalence of childhood asthma has seen a substantial rise, with indications of stabilization in some developed areas (Khreis et al., 2017). However, low- and middle-income nations have experienced a sharp increase in recent times (Asher et al., 2006; Song et al., 2022). Although the precise drivers behind these trends remain largely unidentified, scientists postulate that simultaneous shifts in environmental exposures could be significant contributors (Gaffin et al., 2014).
There is increasing evidence suggesting that pre- and postnatal exposure to environmental stressors may play a significant role in the etiology of childhood asthma and wheeze. Air pollution and temperature have been linked to the onset and exacerbation of childhood respiratory conditions (Cisneros et al., 2021; Hertz-Picciotto et al., 2007; Hu et al., 2022; Ibrahim et al., 2021; Khreis et al., 2017; Liu et al., 2016; Parker et al., 2009). Consistent evidence has linked air pollutants, such as nitrogen dioxide (NO2) and particulate matter ≤ 2.5 μm in diameter (PM2.5), to an elevated risk of asthma and wheeze in children (Anenberg et al., 2022; Brunst et al., 2015; Gehring et al., 2015). Moreover, exposure to extreme temperatures and temperature fluctuations have been associated with adverse respiratory outcomes (Hu et al., 2022; Xu et al., 2012). The fetal and early life periods represent crucial developmental windows, during which environmental insults can disrupt the normal maturation of the respiratory system inhibiting growth and predisposing the child to respiratory disease later in life (Kajekar, 2007; Wright, 2010). Although several studies have reported significant associations of prenatal exposure to air pollution with higher odds of childhood asthma and wheeze (Clark et al., 2010; Leon Hsu et al., 2015; Pedersen et al., 2023; Tian et al., 2024), other studies have reported null or even protective effects (Aguilera et al., 2013; Fuertes et al., 2013; Hazlehurst et al., 2024; Madsen et al., 2017). Furthermore, most previous studies have relied on trimester averages of air pollution and temperature exposure, while the true susceptible window could be shorter (e.g., weekly specific) and warrants more precise definition (Wilson et al., 2017). The auto-correlation of air pollution and temperature across adjacent periods also presents a challenge in accurately identifying a true sensitive window. In addition to the necessity of identifying susceptible windows of prenatal exposure, Bettiol et al. (2021) emphasized the need to confirm vulnerable time windows after birth through studies with more precise exposure assessment.
Furthermore, the joint effects of air pollutants and temperature remain largely unexplored (Buckley and Richardson, 2012; Hu et al., 2022). Previous research (D’Amato et al., 2010) has identified a link between air pollution and ambient temperature, indicating potential temperature–pollutant interactions (Reinmuth-Selzle et al., 2017). Given the potential for synergistic interactions between these environmental factors, it is essential to investigate their combined effects on childhood asthma and wheeze (Lu et al., 2023; Lu et al., 2022). To date, evidence on associations of pre- and postnatal air pollution and temperature exposure with childhood asthma and wheeze in Mexico City is limited. This megacity, where air pollution levels routinely exceed World Health Organization (WHO) guidelines, offers a unique study environment. Its geographical location in a valley exacerbates pollution, especially during cold weather thermal inversions. These factors, along with the distinct climate and socio-demographic profile, highlight the need for investigations in populations with specific environmental conditions, potentially informing targeted interventions in highly polluted urban areas. Additionally, males and females exhibit distinct patterns in lung growth and airway development (Bolte et al., 2021; Carey et al., 2007b; Torday et al., 1981), suggesting differential responses to environmental stressors may be operating (Guilbert et al., 2023b; Hsu et al., 2015). Therefore, investigating sex-specific windows of susceptibility to these exposures is critical.
We leveraged an ongoing longitudinal birth cohort study with highly temporally and spatially resolved air pollution and temperature data to identify prenatal and postnatal windows of susceptibility to these exposures and odds of asthma and wheeze in childhood, and explore sex-specific effects. We additionally investigated potential joint effects of air pollution and temperature.
2. Materials and methods
2.1. Study population
The ongoing prospective birth cohort, Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS), was initiated in Mexico City in 2007 (Burris et al., 2013). From July 2007 to February 2011, pregnant women who were receiving prenatal care through the Instituto Mexicano del Seguro Social (IMSS), the Mexican Social Security Institute, were enrolled in the study. Eligibility criteria for participation included: gestational age less than 20 weeks, aged 18 or older, completion of primary education, intention to reside in Mexico City for the following 3 years, access to a telephone, and absence of a history of kidney or heart disease, daily alcohol consumption, or use of steroid or anti-epilepsy medications. In Mexico, ultrasounds are not routinely performed as standard of care, therefore gestational age was based on last menstrual period (LMP) and by a standardized physical examination to determine gestational age at birth (Capurro et al., 1978). If the physical examination assessment of gestational age differed by more than 3 weeks from the gestational age based on LMP, the physical exam gestational age was used instead of the gestational age determined by LMP. The study protocol was approved by the institutional review boards of the Mexican National Institute of Public Health and the Icahn School of Medicine at Mount Sinai. All participants provided written informed consent during the study visits. Among the 948 mothers who gave birth to a live infant, 681 mother–child pairs were followed longitudinally. The selected sample included children with gestational ages ≥ 37 weeks and with complete exposure, outcome and covariate data. A flow diagram is provided in Supplemental Fig. S1. No material differences were seen between the excluded and included subsamples (Supplemental Table S1).
2.2. Ambient air pollution and temperature exposure assessment
Validated hybrid spatiotemporal resolved model, previously described in detail (Gutiérrez-Avila et al., 2022; He et al., 2023; Just et al., 2015), were employed to estimate prenatal and postnatal air pollutant concentrations to PM2.5 and NO2. For PM2.5 we used both extreme gradient boosting and inverse-distance weighting techniques that incorporate aerosol optical depth, meteorology, and land-use variables to generate daily residence predictions at 1 km resolutions. For NO2 we applied a unique hybrid ensemble model which incorporates machine-learning (XGBOOST, RF), geo-statistics and remote sensing approaches. Daily temperature data were obtained using a calibrated model that integrated satellite-derived surface temperature readings with ground-based air temperature measurements, employing land use regression techniques for adjustment (Gutiérrez-Avila et al., 2021; Politis et al., 2024). Model performance was assessed using monitor-level leave-one-out cross-validation. The R2 values ranged from 0.64 to 0.86 for PM2.5 and 0.78 to 0.95 for temperature across years. For NO2, the cross-validated R2 of RF and XGBOOST models were 0.75 and 0.86, respectively, indicating strong predictive performance across all parameters. Daily estimates of PM2.5, NO2, and temperature were generated at a 1 × 1 km spatial resolution from conception up to 4 years postnatal at each participant’s address obtained at enrollment and updated following any relocation. For prenatal models, daily exposures were averaged into weekly measures and for postnatal models, daily measures were averaged into monthly measurements (Daouda et al., 2024; Guilbert et al., 2023a). Additionally, exposure was averaged into clinically relevant exposure periods: (1st trimester: 1–13 weeks, 2nd trimester: 14–27 weeks, 3rd trimester: 28 weeks–delivery) (McGuinn et al., 2020; Rosa et al., 2019). For the postnatal exposure assessment, air pollutant concentrations were averaged: from birth to 12 months (the first year of life), from birth to 24 months (the first 2 years of life), and from birth to 48 months (the first 4 years of life) (Tian et al., 2024).
2.3. Child asthma and wheeze outcome assessment
At the 4–6 and 7–8 years of age visits, the validated Spanish version of the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire was administered (Mata Fernández et al., 2005). During both visits, the respiratory outcomes including ever wheeze, current wheeze, and asthma were assessed. Ever wheeze was defined as a caregiver’s affirmative response to the question, “Has your child ever experienced wheezing or whistling in the chest at any time in the past?” Current wheeze was determined based on the caregiver’s response to the question, “In the past year, did your child have chest wheezing or whistling?” Asthma was defined by asking caregivers, “Has your child ever had asthma in their life?”.
2.4. Covariates
Covariates were considered based on previous research of factors influencing child respiratory health, which might be associated with air pollution and temperature exposures but not on the causal pathway (for instance, gestational age or birth weight). The selection of covariates was confirmed using a Directed Acyclic Graph (DAG; Supplemental Fig. S2). The main models were adjusted for the minimally sufficient adjustment sets, which included maternal age (continuous, years), pre-pregnancy body mass index (BMI) (continuous, kg/m2), education level (< high school, high school, > high school), parity (primiparous vs. multiparous), environmental tobacco smoke (ETS) (yes vs. no), child sex and age (continuous, years) at the time of visit, and seasonality of PM2.5, NO2, temperature exposure, which was defined as sine and cosine of time of year (Rice et al., 2016; Stolwijk et al., 1999). Information on maternal age, education level, and parity was gathered via questionnaires administered at enrollment. ETS was defined as the presence of any household smoker reported during the second or third trimester of pregnancy. Child sex was obtained from delivery records. Women’s pre-pregnancy BMI (kg/m2) was estimated as previously described (Soria-Contreras et al., 2020). Socioeconomic status (SES) was assessed using a six-level index developed by the Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública (AMAI), which was based on 13 variables obtained from the prenatal questionnaire (Politis et al., 2024). These levels were then grouped into lower, medium, and higher SES categories (Rosa et al., 2019). The season of visit and birth was defined according to weather patterns in Mexico City as dry cold (January–February; November–December), dry warm (March–April), and rainy (May–October) (Politis et al., 2024). Information on maternal asthma history (yes vs. no) was also collected at the time of enrollment.
2.5. Statistical analysis
Summary statistics were calculated for all sociodemographic characteristics and respiratory outcomes, and the continuous variables were evaluated for normality using visual inspection of histograms. For normally distributed variables, we reported mean values with standard deviations (SD); for skewed distributions, we presented medians and interquartile ranges (IQR). Categorical data were summarized as counts (n) and percentages (%). Spearman’s correlations between different time periods for PM2.5, NO2, and temperature measures were calculated.
To assess the exposure-lag-response associations and identify susceptibility windows, distributed lag nonlinear models (DLNMs) were applied (Gasparrini, 2014). The exposure period was set from the 1st to 37th gestational weeks (the minimum gestational age of term births) to facilitate the assessment of prenatal susceptible windows according to previous studies (Chen et al., 2023; Yitshak-Sade et al., 2021). For each respiratory outcome and exposure indicator, two exposure periods were examined separately: (1) prenatal exposure, in weeks, from the 1st to 37th gestational weeks; and (2) postnatal exposure, in months, from the 1st to 48th months postpartum. The analysis was restricted to full-term births (Carlson et al., 2023). All DLNMs were adjusted for maternal age, pre-pregnancy BMI, education level, parity, ETS, child sex and age, seasonality of exposure, and the same exposure indicator in the other exposure period. We modeled PM2.5 and NO2 effects on respiratory outcomes assuming linear relationships, employing natural cubic splines with varying degrees of freedom (2–6) for the lag function (Jakpor et al., 2020). Given the often non-linear nature of temperature-respiratory outcome associations (Agache et al., 2024), we modeled the exposure–response association using natural cubic splines with knots positioned at the 10th, 50th, and 90th percentiles of the temperature distribution for each relevant lag period. For the lag-response association, we applied natural cubic splines with one knot centered on the full lag period specific to each outcome. We compared warmer (95th percentile) and colder (5th percentile) temperatures exposure against median temperature exposure, plotting lag response curves with odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) (Guilbert et al., 2023b). The optimal degrees of freedom for PM2.5 and NO2 were determined by minimizing the Akaike Information Criterion (AIC). Effect estimates were presented as ORs and 95 % CIs by using logistic regression models combined with DLNMs per 5 μg/m3 increment in PM2.5 and 5 ppb increment in NO2. Susceptible exposure windows were identified as the weeks/months during which the 95 % CI did not include 1. DLNM also enables for the estimation of cumulative effects of exposure across several weeks/months by summing the coefficients from individual time intervals (lags) within the specified period (Carlson et al., 2023). We computed the cumulative effect of weekly/monthly increases in each exposure for significant windows. We then examined effect modification by stratifying DLNMs by sex.
Additionally, multivariable logistic regression models were employed to investigate the associations of air pollution and temperature exposure with childhood asthma and wheeze across various time windows after adjusted the aforementioned covariates. For logistic regression models without time series data, seasonality was accounted for by incorporating season of visit in prenatal models and season of birth in postnatal models. Associations were calculated as ORs with 95 % CIs for each 5 μg/m3 increase in PM2.5, 5 ppb increase in NO2, and per 1 °C increase in temperature. We assessed effect modification by including an interaction term between exposure variables and child sex in our models. To investigate potential sex-specific effects of air pollution and temperature exposure on childhood asthma and wheeze, we added a cross-product term between each exposure variable and child sex, considering interactions with p < 0.10 as statistical significance (Tillaut et al., 2023). Subsequently, we conducted sex-stratified analyses to examine whether the observed associations were qualitatively similar between males and females.
To investigate potential additive interactions between PM2.5, NO2, and temperature exposure on the risk of childhood asthma and wheeze, these exposures were dichotomized within each exposure window based on their median values. Exposures were categorized as high (1) if they exceeded the median, and low (0) if they were less than or equal to the median (Chen et al., 2023; Lu et al., 2022; Xiao et al., 2024). Dummy variables were created by combining the two air pollutants with temperature (P1T0/N1T0: exposure to high PM2.5/NO2 and low temperature; P0T1/N0T1: exposure to low PM2.5/NO2 and high temperature; P1T1/N1T1: exposure to high PM2.5/NO2 and high temperature), with P0T0/N0T0 as the reference. The relative excess risk due to interaction (RERI) and attributable proportion (AP) were calculated to assess the joint effects of exposures. A positive RERI indicates synergistic effects, while a negative RERI suggests antagonistic effects. AP, calculated as RERI divided by the OR of co-exposure, represents the proportion of the risk attributable to the interaction. The absence of interactions is indicated by an RERI or AP equal to zero. Logistic regressions were fitted, and ORs with corresponding 95 % CIs were calculated using the bootstrap percentile method (Chen et al., 2023).
To evaluate the stability of our main results, we performed additional sensitivity analyses including: 1) an expanded model incorporating additional adjustments for maternal asthma history and SES; 2) a multi-exposure model simultaneously adjusting for PM2.5, NO2, and temperature within the same exposure window; 3) a model adjusting for the same exposure in other exposure windows; 4) considering the causal relationships among temperature, PM2.5/NO2, and respiratory outcomes, previous research has indicated that ambient temperature influences PM2.5/NO2 concentrations, rather than the reverse (Buckley et al., 2014). Therefore, we additionally adjusted for weekly temperature when examining the associations between prenatal PM2.5 and NO2 exposure and childhood asthma and wheeze; and 5) a model using natural cubic splines with degrees of freedom selected based on the AIC for both exposure–response and lag-response relationships in prenatal temperature exposure analyses.
All statistical analyses were performed using R software (version 4.3.3), with the DLNM implemented via the “dlnm” package (version 2.4.7).
3. Results
3.1. Characteristics of the study participants
Characteristics of the study population are presented in Table 1. Mothers’ mean age (SD) was 27.6 (5.63) years, with a pre-pregnancy BMI of 26.5 (4.17) kg/m2. Maternal education levels varied: 40.4 % had not finished high school, 37.8 % had completed high school, and 21.8 % had attained more than a high school degree. A total of 324 (69.2 %) mothers did not report ETS. Among the mothers, 282 (60.3 %) were primiparous, and 186 (39.7 %) were multiparous. Overall, only three (0.6 %) mothers had a history of asthma. Child sex was evenly split with 235 (50.2 %) males and 233 (49.8 %) females. Child age was 4.8 (0.55) and 6.7 (0.54) years at 4–6 years and 7–8 years study visits, respectively. At the 4–6 years of age study visit, 123 (26.3 %) children had ever wheeze, 60 (12.8 %) had current wheeze, and 14 (3.0 %) had asthma. The distribution of respiratory outcomes at the 7–8 years of age study visit was as follows: 75 (16.0 %) were classified as ever wheeze, 27 (5.8 %) as current wheeze, and 21 (4.5 %) as asthma.
Table 1.
PROGRESS participant characteristics (N = 468).
| Characteristics | Total sample | Males (n = 235) | Females (n = 233) |
|---|---|---|---|
| Maternal age (years), mean (SD) | 27.6 (5.63) | 27.9 (5.46) | 27.2 (5.78) |
| Maternal education, n (%) | |||
| < High school | 189 (40.4) | 103 (43.8) | 86 (36.9) |
| High school | 177 (37.8) | 82 (34.9) | 95 (40.8) |
| > High school | 102 (21.8) | 50 (21.3) | 52 (22.3) |
| Maternal ever asthma, n (%) | |||
| Yes | 3 (0.6) | 2 (0.9) | 1 (0.4) |
| No | 465 (99.4) | 233 (99.1) | 232 (99.6) |
| ETS, n (%) | |||
| Yes | 144 (30.8) | 72 (30.6) | 72 (30.9) |
| No | 324 (69.2) | 163 (69.4) | 161 (69.1) |
| Maternal pre-pregnancy BMI, mean (SD) | 26.5 (4.19) | 26.8 (4.05) | 26.1 (4.30) |
| Parity, n (%) | |||
| Primiparous | 282 (60.3) | 148 (63.0) | 134 (57.5) |
| Multiparous | 186 (39.7) | 87 (37.0) | 99 (42.5) |
| Child age at 4–6 years visit, mean (SD) | 4.8 (0.55) | 4.8 (0.54) | 4.8 (0.56) |
| Child age at 7–8 years visit, mean (SD) | 6.7 (0.54) | 6.7 (0.55) | 6.7 (0.53) |
| Respiratory outcomes | |||
| (4–6 years of age), n (%) | |||
| Ever wheeze | 123 (26.3) | 68 (28.9) | 55 (23.6) |
| Current wheeze | 60 (12.8) | 37 (15.7) | 23 (9.9) |
| Ever asthma | 14 (3.0) | 8 (3.4) | 6 (2.6) |
| Respiratory outcomes | |||
| (7–8 years of age), n (%) | |||
| Ever wheeze | 75 (16.0) | 41 (17.4) | 34 (14.6) |
| Current wheeze | 27 (5.8) | 16 (6.8) | 11 (4.7) |
| Ever asthma | 21 (4.5) | 14 (6.0) | 7 (3.0) |
Abbreviations: SD, standard deviation; ETS, environmental tobacco smoke; BMI, body mass index.
The mean prenatal and postnatal ambient air pollution and temperature exposures during the whole pregnancy, specific trimesters, first year, first two years, and first four years of life ranged from 22.4 to 23.2 μg/m3 for PM2.5, 32.4 to 33.6 ppb for NO2, and 14.8 to 15.2 °C for temperature (Table 2). There was a moderate to high correlation between the different exposure time periods of exposure measures (Supplemental Fig. S3).
Table 2.
Distribution of ambient air pollution and temperature.
| Exposure and window | Mean | SD | Min. | Q1 | Median | Q3 | Max. |
|---|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | |||||||
| Whole pregnancy | 22.8 | 2.9 | 16.4 | 20.4 | 23 | 24.9 | 30.3 |
| First trimester | 22.8 | 4.9 | 12.6 | 18.8 | 21.8 | 26.8 | 34.3 |
| Second trimester | 22.4 | 5.1 | 11.5 | 18 | 21.3 | 26.9 | 32.8 |
| Third trimester | 23.2 | 5.8 | 11.9 | 18.1 | 22.5 | 28.3 | 37.5 |
| First year | 22.6 | 2.5 | 17.6 | 20.3 | 23.4 | 24.6 | 26.7 |
| First two years | 22.4 | 1.2 | 20.5 | 21.6 | 22.3 | 23 | 26.1 |
| First four years | 22.5 | 1 | 20 | 21.7 | 22.2 | 23.3 | 26.3 |
| NO2 (ppb) | |||||||
| Whole pregnancy | 33.1 | 5.6 | 17.8 | 29 | 31.9 | 36.9 | 49.7 |
| First trimester | 33.1 | 7.7 | 16.6 | 27.1 | 31.1 | 37.5 | 60.7 |
| Second trimester | 32.8 | 7.2 | 20.1 | 27 | 31.7 | 36.9 | 56.5 |
| Third trimester | 33.6 | 7.6 | 16.4 | 27.7 | 33.1 | 38.2 | 63.7 |
| First year | 32.7 | 5.4 | 17.7 | 28.6 | 30.4 | 37 | 48.9 |
| First two years | 32.4 | 5.2 | 17.3 | 28.5 | 30.3 | 36.1 | 46.9 |
| First four years | 32.1 | 5.2 | 17 | 28.4 | 30 | 35.6 | 46.5 |
| Temperature (°C) | |||||||
| Whole pregnancy | 15 | 1.3 | 11.6 | 14.2 | 15 | 16 | 18 |
| First trimester | 15.2 | 2 | 10 | 13.9 | 15.3 | 16.6 | 19.5 |
| Second trimester | 15 | 2.1 | 10.1 | 13.6 | 14.9 | 16.6 | 19.2 |
| Third trimester | 14.8 | 2.2 | 9.3 | 13.2 | 14.7 | 16.5 | 20.6 |
| First year | 15.1 | 1.2 | 11.5 | 14.2 | 15.2 | 16.1 | 17.8 |
| First two years | 15.2 | 1.2 | 11.6 | 14.4 | 15.2 | 16.2 | 17.7 |
| First four years | 15.2 | 1.2 | 11.5 | 14.4 | 15.2 | 16.2 | 17.6 |
Abbreviations: SD, standard deviation; NO2, nitrogen dioxide; PM2.5, particulate matter ≤ 2.5 μm in diameter.
3.2. Windows of susceptibility of prenatal and postnatal PM2.5, NO2, and temperature
As shown in Fig. 1, prenatal PM2.5 exposure (per 5 μg/m3 increase) was associated with higher odds of ever wheeze at 4–6 years of age during the 20th to 28th gestational weeks (Fig. 1A) and ever wheeze at 7–8 years of age during the 19th to 26th gestational weeks (Fig. 1D). The cumulative ORs for the sensitive windows were 1.18 (95 % CI: 1.01, 1.37) and 1.18 (95 % CI: 1.02, 1.36) for ever wheeze at 4–6 and 7–8 years of age, respectively. Prenatal NO2 exposure during the 18th to 25th gestational weeks (per 5 ppb increase) was associated with higher odds of ever wheeze at 7–8 years of age (Fig. 2D). The cumulative OR for the sensitive windows was 1.16 (95 % CI: 1.02, 1.31). Prenatal warmer temperature exposure was associated with higher odds of current wheeze at 4–6 years of age during the 27th to 37th gestational weeks and ever wheeze at 7–8 years of age during the 26th to 37th gestational weeks, with cumulative OR of 20.01 (95 % CI: 1.35, 295.98) and 21.04 (95 % CI: 1.42, 312.46) respectively (Fig. 3B and 3D). However, no susceptible windows were observed for colder temperature exposure (5th percentile of temperature, 11 °C) with respiratory outcomes compared to the median temperature (50th percentile of temperature, 15 °C) (Fig. 4).
Fig. 1.
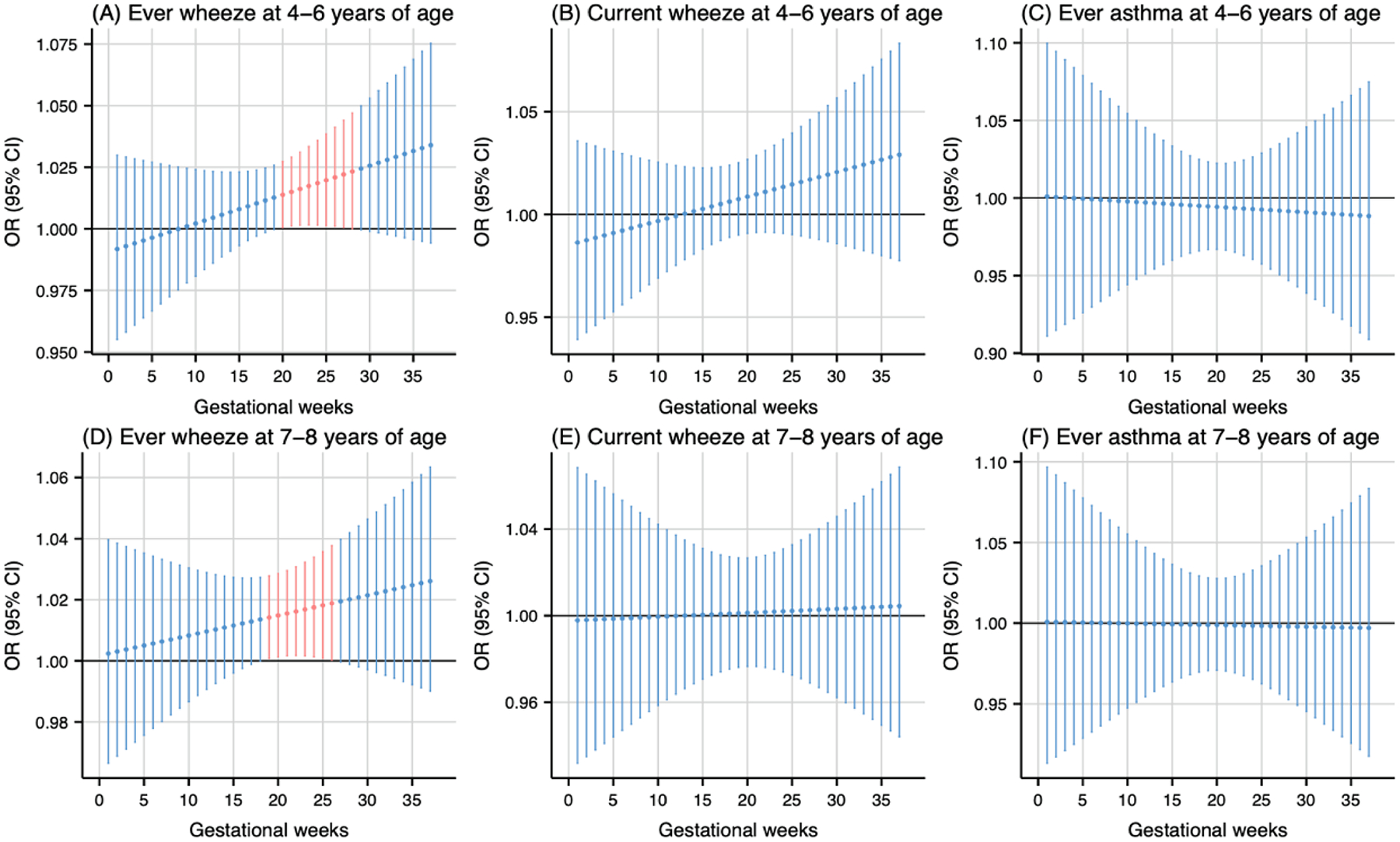
Odds ratio (95 % CI) of childhood asthma and wheeze in association with weekly-specific PM2.5 exposure during 1–37 weeks of pregnancy. Critical windows are highlighted in red. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and postnatal year 4 average PM2.5.
Fig. 2.
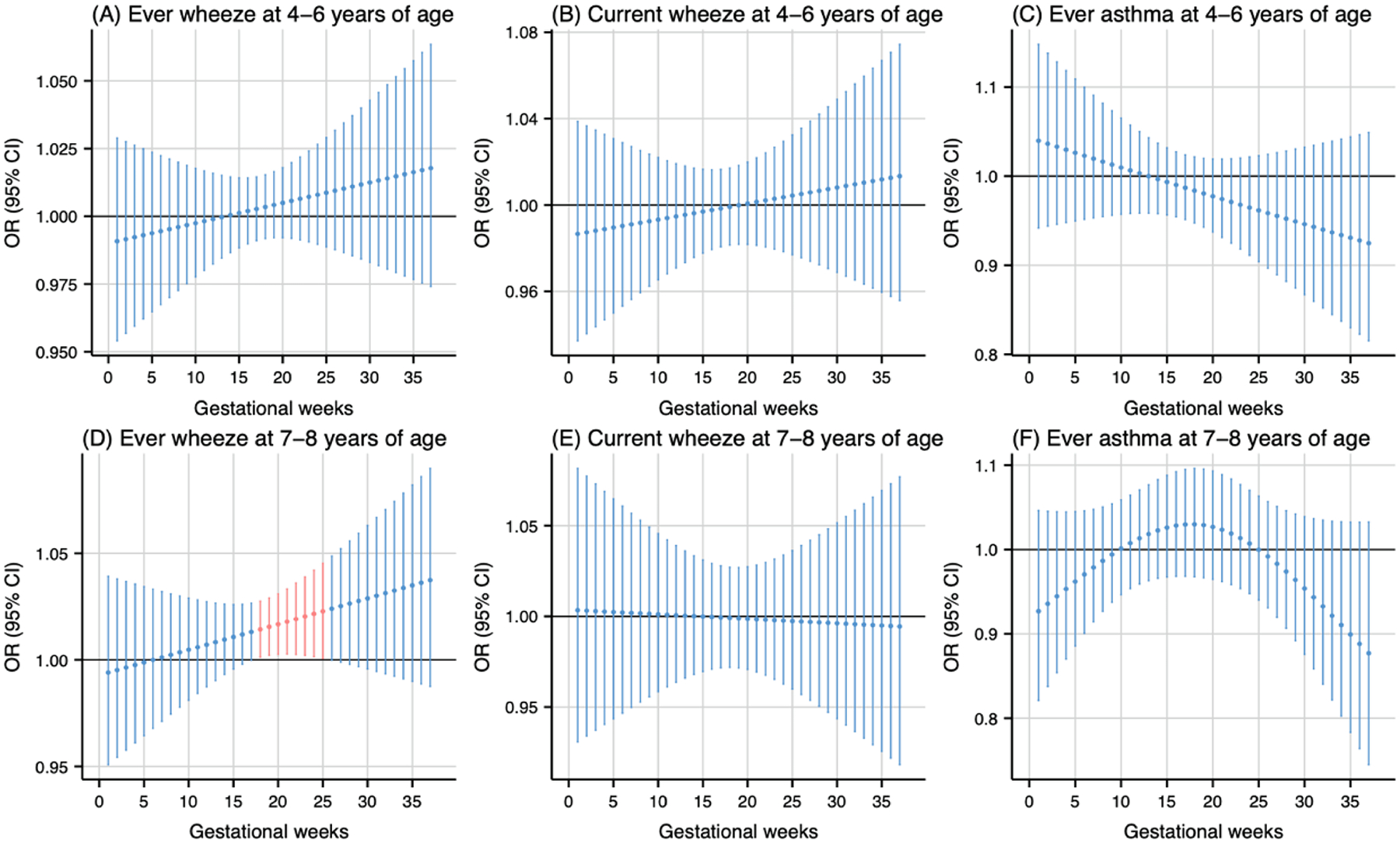
Odds ratio (95 % CI) of childhood asthma and wheeze in association with weekly-specific NO2 exposure during 1–37 weeks of pregnancy. Critical windows are highlighted in red. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and postnatal year 4 average NO2.
Fig. 3.
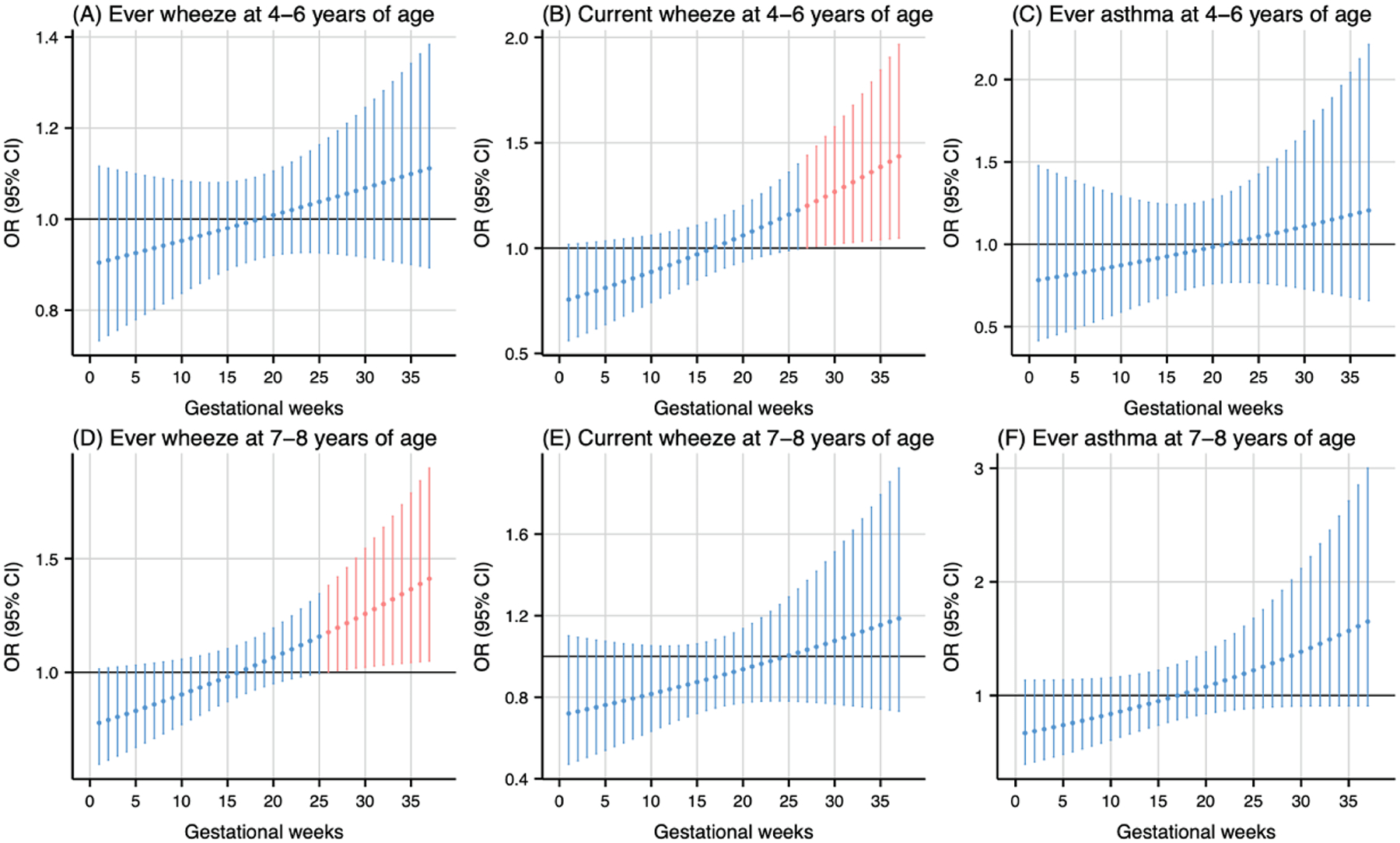
Odds ratio (95 % CI) of childhood asthma and wheeze in association with temperature at 95th percentile (19 °C), relative to the 50th percentile (15 °C) during 1–37 weeks of pregnancy. Critical windows are highlighted in red. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and postnatal year 4 average temperature.
Fig. 4.
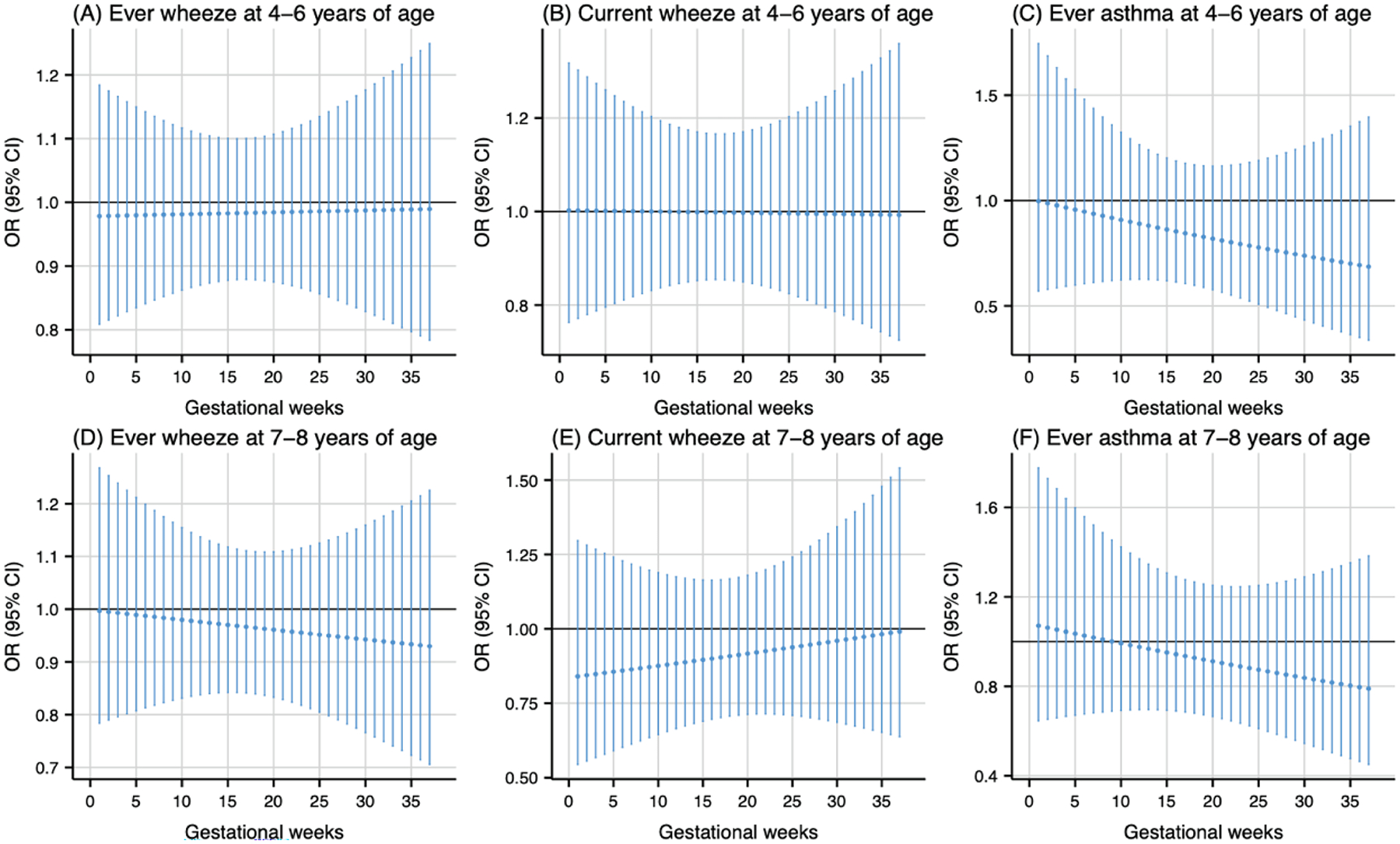
Odds ratio (95 % CI) of childhood asthma and wheeze in association with temperature at 5th percentile (11 °C), relative to the 50th percentile (15 °C) during 1–37 weeks of pregnancy. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and postnatal year 4 average temperature.
For postnatal exposure, PM2.5 (per 5 μg/m3 increase) exposure during the early months of life was associated with higher odds of wheeze (Fig. 5). Exposure during the 1st to 19th months postpartum was associated with higher odds of ever wheeze at 4–6 years of age (cumulative OR: 2.55, 95 % CI: 1.25, 5.20) (Fig. 5A), while exposure during the 1st to 6th months postpartum was associated with higher odds of ever wheeze at 7–8 years of age (cumulative OR: 1.62, 95 % CI: 1.12, 2.34) (Fig. 5D). Additionally, exposure during the 1st to 10th months (cumulative OR: 8.05, 95 % CI: 2.75, 23.60) and the 41st to 48th months (cumulative OR: 4.98, 95 % CI: 1.33, 18.56) postpartum was associated with higher odds of current wheeze at 7–8 years of age (Fig. 5E).
Fig. 5.
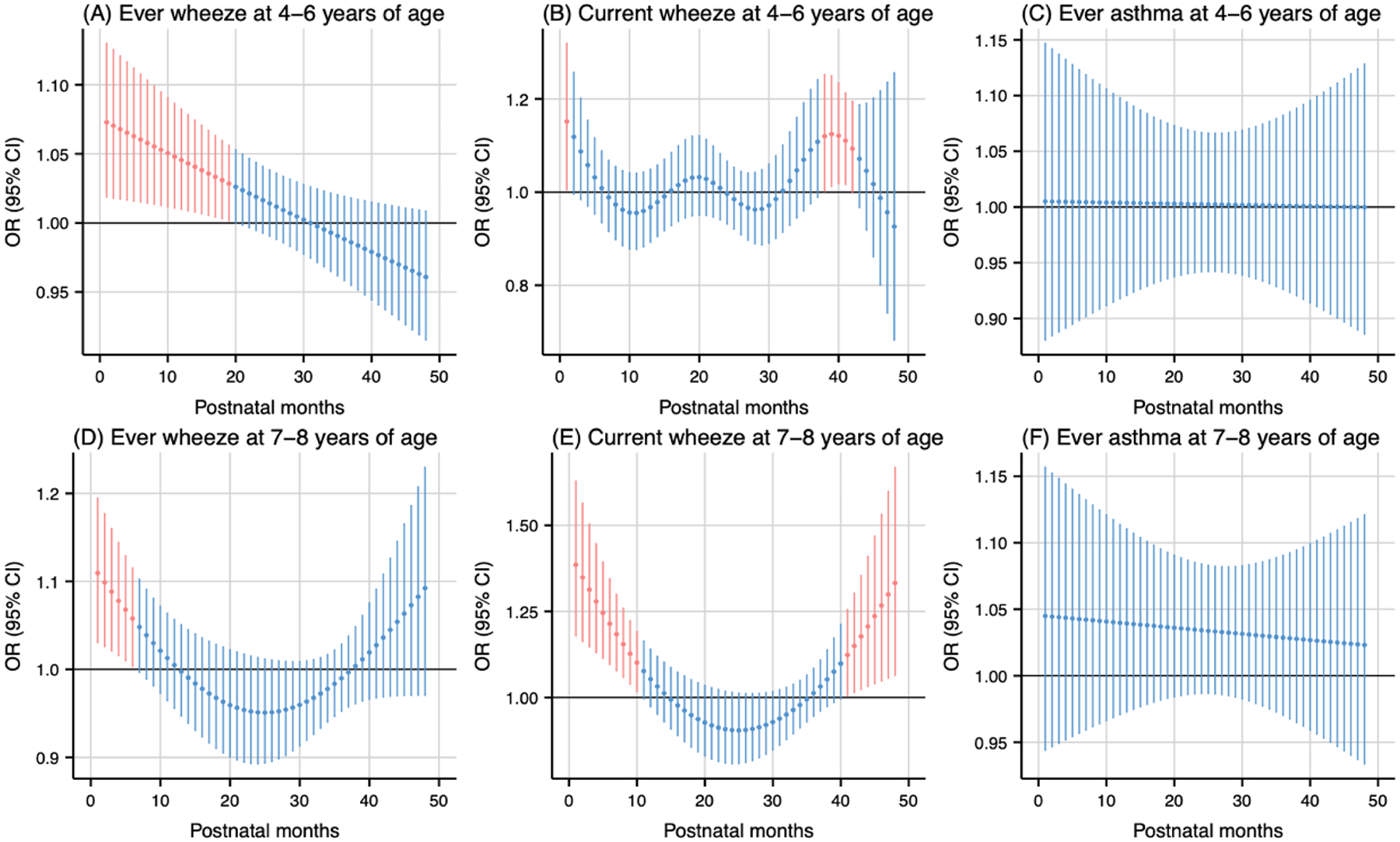
Odds ratio (95 % CI) of childhood asthma and wheeze in association with monthly-specific PM2.5 exposure during postpartum 1–48 months. Critical windows are highlighted in red. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and prenatal average PM2.5.
Postnatal NO2 exposure (per 5 ppb increase) was associated with higher odds of wheeze at various exposure windows (Fig. 6). Exposure during the 1st to 10th months postpartum was associated with higher odds of ever wheeze at 4–6 years of age (cumulative OR: 1.74, 95 % CI: 1.14, 2.65) (Fig. 6A). Similarly, exposure during the 15th to 25th months postpartum was associated with higher odds of current wheeze at 4–6 years of age (cumulative OR: 1.23, 95 % CI: 1.03, 1.46) (Fig. 6B). Postnatal NO2 exposure showed divergent associations with ever wheeze at 7–8 years of age. Higher exposure was linked to increased odds during the 1st to 8th months (cumulative OR: 1.83, 95 % CI: 1.18, 2.83), but decreased odds during the 21st to 36th months postpartum (cumulative OR: 0.44, 95 % CI: 0.23, 0.83) (Fig. 6D). The odds of current wheeze at 7–8 years of age was increased by exposure during two periods: the 2nd to 14th months (cumulative OR: 6.29, 95 % CI: 2.19, 18.09) and the 43rd to 48th months (cumulative OR: 4.99, 95 % CI: 1.54, 16.14), while they decreased during the 25th to 37th months postpartum (cumulative OR: 0.14, 95 % CI: 0.04, 0.51) (Fig. 6E).
Fig. 6.
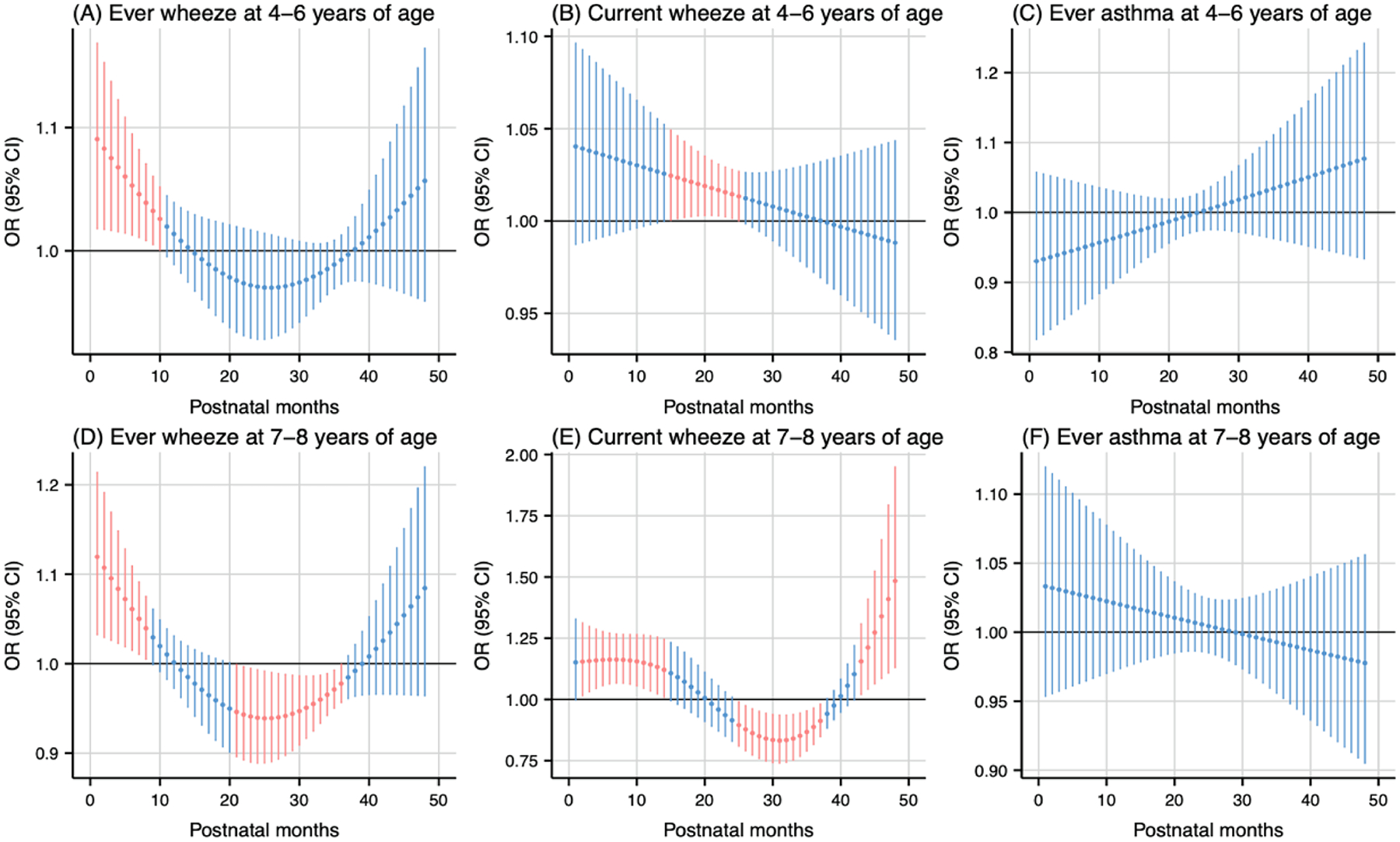
Odds ratio (95 % CI) of childhood asthma and wheeze in association with monthly-specific NO2 exposure during postpartum 1–48 months. Critical windows are highlighted in red. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and prenatal average NO2.
Postnatal temperature exposure analysis revealed temperature-dependent associations with respiratory outcomes. Warmer temperature, compared to the median, during the 12th to 23rd months postpartum were associated with lower odds of current wheeze at 4–6 years of age (cumulative OR: 0.09, 95 % CI: 0.01, 0.89) (Fig. 7B). Similarly, colder temperature during the 15th to 27th months postpartum were associated with lower odds of ever asthma at 7–8 years of age (cumulative OR: 0.01, 95 % CI: 0.00, 0.53) (Fig. 8F).
Fig. 7.
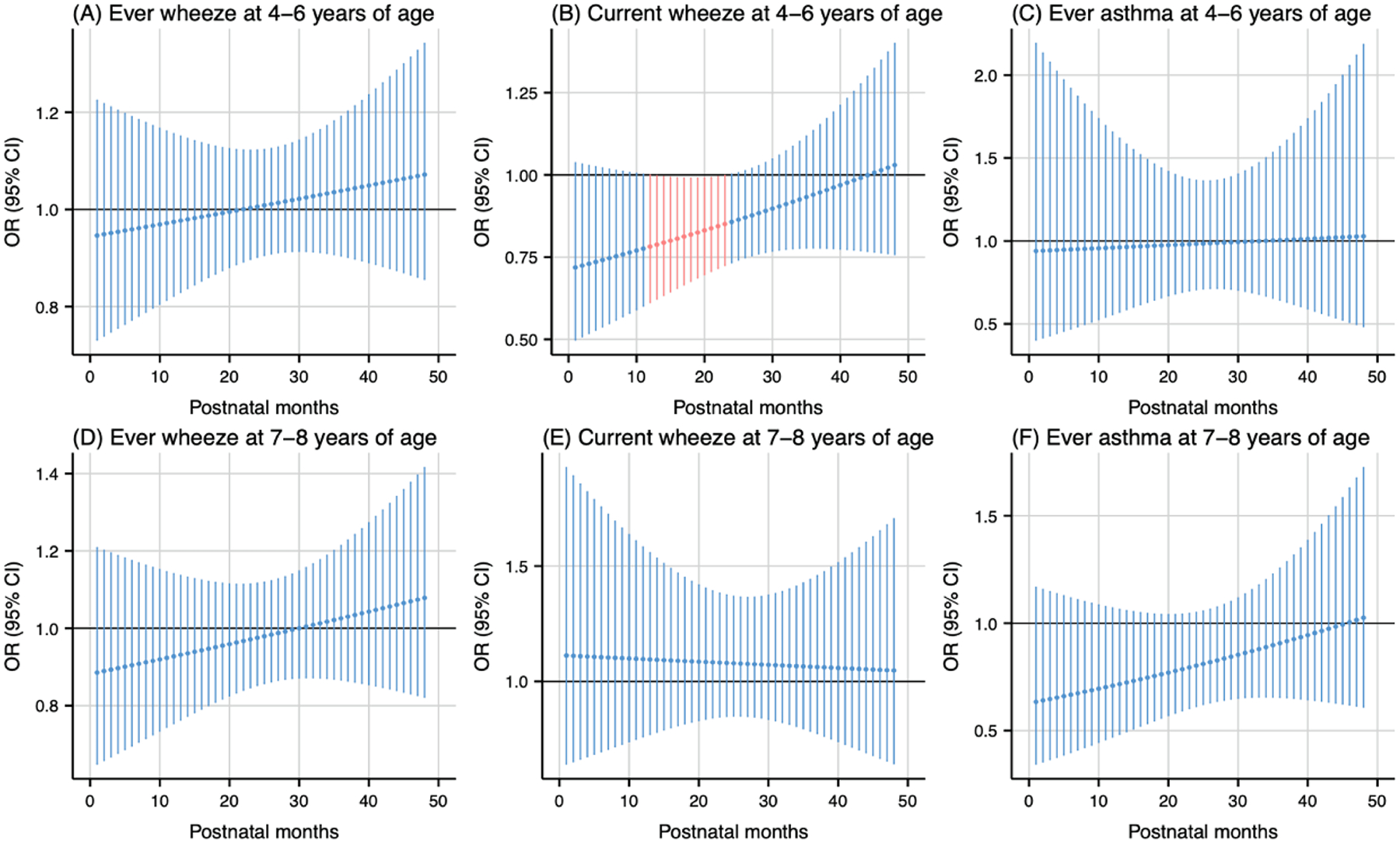
Odds ratio (95 % CI) of childhood asthma and wheeze in association with temperature at the 95th percentile (19 °C), relative to the 50th percentile (15 °C) during postpartum 1–48 months. Critical windows are highlighted in red. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and prenatal average temperature.
Fig. 8.
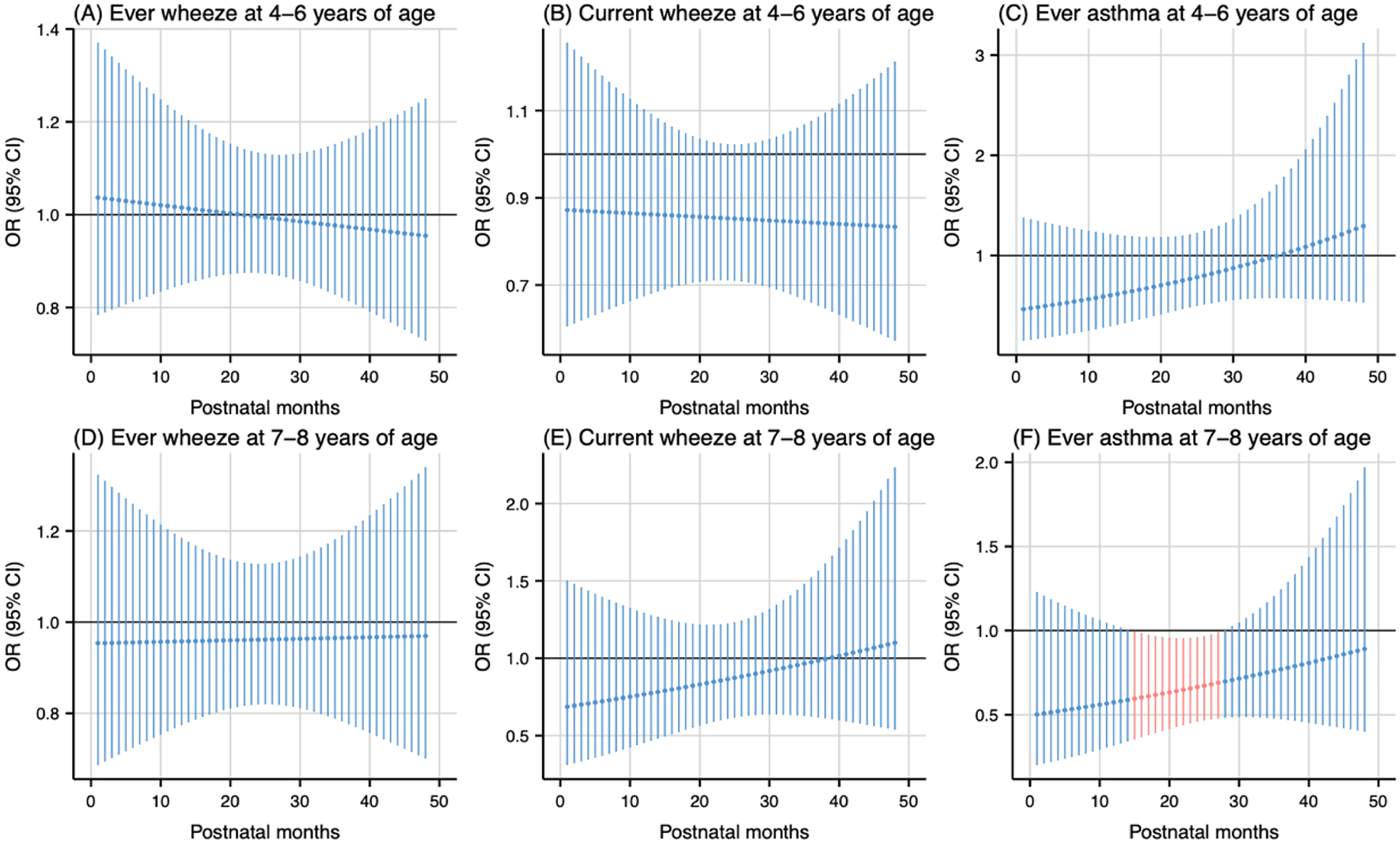
Odds ratio (95 % CI) of childhood asthma and wheeze in association with temperature at the 5th percentile (11 °C), relative to the 50th percentile (15 °C) during postpartum 1–48 months. Critical windows are highlighted in red. Models were adjusted for maternal age, pre-pregnancy body mass index, education level, parity, environmental tobacco exposure, child sex and age, seasonality, and prenatal average temperature.
Sex-stratified analyses of prenatal exposure to PM2.5, NO2, and temperature revealed evidence of effect modification by child sex. The results indicated that the majority of significant associations were observed in males versus females. Specifically, prenatal PM2.5 exposure was significantly associated with increased odds of ever wheeze at both 4–6 and 7–8 years of age exclusively in males. Prenatal NO2 exposure was linked to higher odds of ever wheeze at 7–8 years of age in both sexes, albeit with differing windows of susceptibility. Regarding temperature effects, warmer temperature exposure was associated with higher odds of ever wheeze at 7–8 years of age in males, while colder temperature exposure was associated with lower odds of ever asthma at 4–6 years of age, also in males. (Supplemental Fig. S4–S7).
3.3. Associations of average air pollution and temperature measures with childhood asthma and wheeze
Temperature exposure during the whole pregnancy (OR: 1.20, 95 % CI: 1.00, 1.43), first trimester (OR: 1.12, 95 % CI: 1.00, 1.25) and second trimester (OR: 1.14, 95 % CI: 1.00, 1.29) was associated with higher odds of ever wheeze at 4–6 years of age (Supplemental Fig. S8). No associations were found between exposure to PM2.5, NO2, or temperature at any period and either current wheeze or asthma at 4–6 years of age (Supplemental Fig. S8). For respiratory outcomes at 7–8 years of age, PM2.5 exposure during the first year (OR: 6.65, 95 % CI: 2.34, 22.95), first two years (OR: 8.08, 95 %CI: 1.46, 44.33), and first four years (OR: 7.27, 95 %CI: 1.08, 47.45) of life, as well as NO2 exposure during the first years of life (OR: 1.56, 95 %CI: 1.09, 2.24), were linked to higher odds of current wheeze at 7–8 years of age (Supplemental Fig. S9).
We observed statistical interactions between child sex and average PM2.5 exposure during the whole pregnancy (Pinteraction = 0.09) in association with ever asthma at 7–8 years of age, NO2 exposure during the first four years of life (Pinteraction = 0.07) in association with ever wheeze at 7–8 years of age, and temperature exposure during the third trimester (Pinteraction = 0.05) in association with current wheeze at 7–8 years of age. Furthermore, sex-stratified analyses of the associations between air pollution exposure at various exposure windows and outcomes of interest provided evidence of sex-specific effects, indicating that these effects were more evident in males. For instance, PM2.5 exposure during the first year, first two years, and first four years of life were associated with higher odds of current wheeze at 7–8 years of age, in males but not for females. Similarly, NO2 exposure during the first year of life was linked to higher odds of current wheeze and ever asthma at 7–8 years of age for males, but not for females (Supplementary Fig. S10–S11). Temperature exposure during the first trimester was found to be associated with higher odds of ever asthma at 7–8 years of age in females only (Supplementary Fig. S12).
3.4. Additive interactions between PM2.5, NO2, and temperature on childhood asthma and wheeze
No significant additive interaction patterns were observed for the majority of the air pollutant/temperature-outcome-exposure window combinations (Supplemental Table S2–S7). However, a significant additive effect was found for high PM2.5 exposure and high temperature during the first year postpartum (OR: 7.02, 95 % CI: 2.24, 27.51; RERI: 5.32, 95 % CI: 0.10, 36.90; AP: 0.76, 95 % CI: 0.01, 1.07) on current wheeze at 7–8 years of age.
3.5. Sensitivity analysis
In sensitivity analyses, models additionally incorporating adjustments for alternative prenatal exposure windows or postnatal exposure periods, as well as multipollutant models, generally yielded results consistent with the primary analysis (Supplemental Table S8–S13). Our prenatal air pollution exposure analyses when included weekly temperature as a covariate showed similar shapes but associations are attenuated with the inclusion of weekly temperature measures (Supplemental Fig. S13–S14). We additionally found that prenatal colder temperature exposure was associated with lower risk of ever wheeze at 7–8 years of age, while no sensitive windows were identified for prenatal warmer temperature exposure in comparison with our main analyses when modeled the dose–response and lag-response relationships using natural cubic splines with degrees of freedom selected based on the AIC (Supplemental Fig. S15–S16).
4. Discussion
In this prospective birth cohort study, we identified windows of susceptibility during mid-gestation and early postnatal life for exposure to PM2.5 and NO2, and higher odds of wheeze in early and mid-childhood. Late-gestation was identified as a susceptibility window for warmer temperature, primarily linked to increased odds of childhood wheeze. In contrast, exposure to warmer and colder temperatures around 2–3 years of age was associated with lower odds of wheeze and asthma, respectively. In addition, there was evidence of an interaction between PM2.5 exposure and temperature, with higher exposure to both during the first year of life being associated with higher odds of current wheeze in mid-childhood. Our results also indicated sex-specific effects of prenatal air pollution exposure, with PM2.5 and NO2 both associated with higher odds of ever wheeze in males, while NO2 exposure was additionally linked to higher odds of asthma in males.
We found consistent associations with wheeze outcomes but not with asthma. These results were in line with the Hong Kong Chinese Birth Cohort study, which found that NOx exposure in utero, 0–2, and 3–8 years of age, were also associated with wheezing but not asthma at ~17.5 years (He et al., 2019). Additionally, a previous meta-analysis found that NO2 exposure during the entire pregnancy was associated with higher risk of wheezing, but not asthma, in offspring (Hua et al., 2023). One plausible explanation is that some early life wheezing can be temporary in nature, while persistent wheeze is indicative of impaired lung function and a higher risk of developing asthma later in childhood (Hazlehurst et al., 2024). Moreover, our asthma outcome was based on ever report and was not based on physician diagnosis which might differ from other studies (Lee et al., 2018; Zanobetti et al., 2024). The identified critical windows at mid-gestation for PM2.5 and NO2 exposure are in line with prior studies (Jung et al., 2019; Lavigne et al., 2018; Lavigne et al., 2019; Lee et al., 2018) that focused on the effects of PM2.5 and NOx exposure during pregnancy on childhood asthma development. Exposure to both PM2.5 and NO2 during the first year of life was identified as a consistent critical window of exposure across multiple outcomes. These results were partly supported by previous studies, such as Rancìere et al. (2017) and Nordling et al. (2008) found NOx exposure during the first year of life was linked to higher risk of persistent wheezing at 4 years; Gehring et al. (2010) observed annual average PM2.5 exposure estimated at birth address was linked to higher risk of early transient and late onset wheezing.
In contrast to PM2.5 and NO2, relatively few studies have investigated the long-term effect of temperature extremes, especially for the prenatal period on childhood asthma and wheeze (D’Amato et al., 2010; Hu et al., 2022). Previous time-series studies often focused on postnatal exposure and assessed the short-term effect of temperature on asthma hospital admissions or outpatient/emergency department visits (Agache et al., 2024; Qiu et al., 2015). Temperature extremes showed complex associations with wheeze outcomes in this study, varying by timing and intensity of exposure. While increased average temperature exposure during pregnancy generally increased the odds of ever wheeze, both warmer and colder temperatures exposure during late-gestation was linked to higher odds of wheeze outcomes. Postnatal exposure to warmer temperature was linked to lower odds of current wheeze, while exposure to colder temperature was linked to lower odds of ever asthma. We acknowledge that the linear assumption for temperature in our logistic regression models is a simplification that may not fully capture complex non-linear relationships. Our main findings from non-linear DLNM analyses should be considered more representative of the true temperature-health associations. These findings generally indicate that the relationship between temperature and respiratory health in children is not linear and depends on the specific developmental window of exposure. Our warmer temperature (19 °C) is similar to the Generation R study (Granés et al., 2024) with heat exposure of 20.2 °C and ENVI-RONAGE birth cohort study (Martens et al., 2019) with heat threshold of 19.5 °C. This is consistent with previous studies suggesting a non-linear relationship between temperature and respiratory health, with both extreme cold and heat potentially having adverse effects on the respiratory system (Hu et al., 2020; Xu et al., 2013). Several recent literature reviews (Hu et al., 2022; Xu et al., 2012) indicated that exposure to temperature extremes, both cold and heat, could increase asthma risk through various biological pathways including cold temperatures can induce bronchoconstriction and increase airway inflammation, while heat can lead to dehydration of the airways, altering mucus properties and potentially triggering bronchospasms. Additionally, both extremes can affect the immune system, increasing susceptibility to respiratory infections and allergens, which are known asthma triggers. Complementing these findings, experimental research using a mouse model of asthma revealed a U-shaped relationship between temperature and inflammatory markers. The study observed peak levels of immune proteins and pro-inflammatory factors at 24 °C, suggesting that temperatures deviating from this point in either direction could exacerbate airway inflammation (Deng et al., 2020). Overall, human epidemiological and animal experimental evidence indicates that there exists an optimum range within which the impact of temperature on respiratory health is minimal.
Lung growth and development is a critical process that starts as early as 3–4 weeks of gestation and progresses into early adulthood, with the most rapid growth occurring during the early stages of life, highlighting the crucial role of early lung development in shaping future respiratory health (Martinez, 2016; Stocks et al., 2013). The potential mechanisms explaining the association between early life environmental stressors, such as air pollution and temperature, and childhood asthma and wheeze may be attributed to multiple factors. Firstly, exposure to air pollutants and extreme temperatures during the critical developmental period of the lungs in infancy and early childhood may alter lung function and structure, leading to increased susceptibility to respiratory disorders (Bose et al., 2018; Cai et al., 2020; Guilbert et al., 2023b). Secondly, these environmental stressors may trigger oxidative stress and inflammatory responses in the airways, which are key pathways in the development of asthma and wheeze (Lu et al., 2023; Riedl, 2008; Xu et al., 2023). Moreover, air pollution and temperature variations may modulate the immune system, influencing the balance between T-helper cell types and promoting allergic sensitization (Aguilera et al., 2023; Glencross et al., 2020; Morgenstern et al., 2008; Sampath et al., 2023; Tuazon et al., 2022). Early-life environmental influences may induce epigenetic modifications, including alterations in DNA methylation patterns and histone modifications, potentially contributing to the development of respiratory conditions like asthma and wheeze in children (Ji et al., 2016a; Yang et al., 2017). Lastly, the interaction between environmental factors and genetic predisposition likely plays a role in how early life environmental stressors affect respiratory health outcomes in childhood (Gref et al., 2017; Ji et al., 2016b; Peden, 2005).
The observed sex-specific effects of air pollution and temperature exposure on respiratory outcomes may be attributed to inherent differences in fetal lung development between males and females, which are largely influenced by sex hormones (Guilbert et al., 2023b). These differences are characterized by distinct patterns of pulmonary growth and airway formation. Specifically, female fetuses typically develop smaller lungs and alveolar surface areas compared to males, but possess larger-caliber airways (Carey et al., 2007a; Liptzin et al., 2015). Furthermore, lung surfactant production begins earlier in female fetuses than in males. This advanced surfactant development may contribute to higher airflow rates and lower airway resistance in girls (Carey et al., 2007a). These physiological distinctions could potentially explain the differential susceptibility to environmental exposures observed between sexes during critical periods of lung development. The identified prenatal sensitive window aligns with the canalicular phase of fetal lung development, a critical period for tissue formation and functional differentiation (Hsu et al., 2015). During this stage (weeks 17–26), significant changes occur, including continued airway development, emergence of capillaries and alveolar structures, and the initiation of type II to type I cell differentiation, leading to surfactant production (Burri, 1984; Kajekar, 2007; Miller and Marty, 2010). The airway epithelium formed in this phase plays a crucial role in innate immunity, potentially influencing later respiratory conditions like asthma and wheezing (Kato et al., 2007). The sensitivity observed in the first year of life may be attributed to infants’ heightened vulnerability to environmental factors (Zhao et al., 2021). This susceptibility stems from their unique physiological characteristics, including higher oxygen needs, proportionally smaller lung surface area, more permeable airways, and still-developing pulmonary defense mechanisms (Salvi, 2007).
This study had several strengths. The PROGRESS cohort study included a relatively large sample size of mother–child dyads with longitudinal data collected rigorously over several years, allowing us to assess childhood asthma and wheeze at 4–6 and 7–8 years of age study visits using a well-validated instrument (Mallol et al., 2010). We employed validated exposure models with high spatial and temporal resolution and investigated associations of respiratory outcomes with two air pollutants, temperature, and their interaction effects. Exposure windows were assessed based not only on the biological stages of prenatal lung formation and clinically defined trimesters but also confirmed using the flexible DLNMs. We considered exposure in both prenatal and postnatal periods, covering the first 1000 days of life (from conception to the 2nd year of life), which might be particularly relevant for understanding the long-term impact of environmental stressors on child health.
We also acknowledged potential limitations. Following standard practices in air pollution research, we estimated personal exposure to air pollutants and temperature based on residential addresses. This approach does not account for indoor environments or time spent at non-residential locations. As with most similar studies, we cannot rule out exposure misclassification or confounding by indoor air, which may have attenuated the reported results. Nevertheless, the mild climate in Mexico City led to nearly all participants (≥ 94 %) keeping their windows open throughout the day, reducing the potential for indoor-outdoor pollution/temperature ratio discrepancies that often pose a challenge in studies conducted in developed countries (Hsu et al., 2024). The respiratory outcomes we studied were based on caregiver reports, which may introduce potential recall bias. Nevertheless, it is worth noting that parent-reported asthma and wheeze are a common and accepted practice in epidemiological research on childhood respiratory health (Hazlehurst et al., 2024; Zhang et al., 2021). We acknowledge that some effect estimates for late pregnancy, although not reaching statistical significance, were notably strong. This pattern warrants careful interpretation. The widening of confidence intervals at the extremes of the exposure period is a known limitation of DLNM, which may reduce statistical power to detect significant associations in early and late pregnancy. The potentially important role of late pregnancy exposures should not be overlooked. However, we did not find significant associations for third trimester air pollutant exposure. These findings highlight the complex nature of fetal development and underscore the need for future studies with larger sample sizes to better characterize exposure impacts across different gestational periods. As with any observational study, residual confounding due to unmeasured factors that could confound and/or modify the effect of air pollution and temperature exposure on childhood wheeze and asthma cannot be completely ruled out. Finally, given the extensive number of models fitted, it is plausible that some of the observed associations could be attributable to chance. Instead of applying corrections for multiple testing, which might increase the risk of type II error, we chose to concentrate on identifying consistent patterns of relationships across the various analyses (Althouse, 2016; Fandiño-Del-Rio et al., 2022).
5. Conclusions
Our results provide evidence that exposure to air pollution and temperature, in utero and in early life is associated with childhood asthma and wheeze. We identified susceptibility windows primarily during mid-gestation and early postnatal life for air pollution and late-gestation and 2–3 years for warmer and colder temperatures. We also found the effect estimates were more evident in males versus females. Furthermore, interactions were observed between high PM2.5 exposure and high temperature during the first year of life, resulting in a significantly increased odds of wheeze outcome. The implications of these findings, in conjunction with existing evidence, suggest that reducing early life air pollution exposure could potentially lower the risk of developing childhood asthma and wheeze.
Supplementary Material
Acknowledgment
We are grateful to the PROGRESS participants and staff at the National Institute of Public Health/Ministry of Health of Mexico and the National Institute of Perinatology. We thank the ABC (American British Cowdray Medical Center) in Mexico for providing some of the needed research facilities.
Funding
This work was supported by the Excellence in Doctoral Education and Training Enhancement Program for visiting researcher role at the Icahn School of Medicine at Mount Sinai (Hu, C-Y). The National Institute of Environmental Health Sciences grants, R00ES027496 and R01ES033245 (Rosa MJ, PI). The PROGRESS project has been supported by the following grants; R01ES014930, R01ES013744, R24ES028522, P30ES023515 (Wright RO, PI) and R01ES021357 (Baccarelli A and Wright RO, MPI). CSA was supported by National Institute of Environmental Health Sciences grant K99ES035894. This work was also supported in part through the computational and data resources and staff expertise provided by Scientific Computing and Data at the Icahn School of Medicine at Mount Sinai and supported by the Clinical and Translational Science Award (CTSA) grant UL1TR004419 from the National Center for Advancing Translational Sciences.
Footnotes
CRediT authorship contribution statement
Cheng-Yang Hu: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. Ivan Gutierrez-Avila: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. Mike Z. He: Writing – review & editing, Formal analysis, Conceptualization. Éric Lavigne: Writing – review & editing, Methodology, Formal analysis. Cecilia S. Alcala: Writing – review & editing, Writing – original draft, Methodology, Formal analysis, Conceptualization. Maayan Yitshak-Sade: Writing – review & editing, Supervision, Conceptualization. Hector Lamadrid-Figueroa: Writing – review & editing, Supervision, Conceptualization. Marcela Tamayo-Ortiz: Writing – review & editing, Supervision, Conceptualization. Adriana Mercado-Garcia: Writing – review & editing, Supervision, Conceptualization. Allan C. Just: Writing – review & editing, Methodology. Chris Gennings: Writing – review & editing, Methodology. Martha M Téllez-Rojo: Writing – review & editing, Supervision, Conceptualization. Robert O. Wright: Writing – review & editing, Supervision, Project administration, Funding acquisition. Rosalind J. Wright: Writing – review & editing, Supervision, Conceptualization. Maria José Rosa: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2024.109122.
Data availability
The data that has been used is confidential.
References
- Agache I, et al. , 2024. The impact of outdoor pollution and extreme temperatures on asthma-related outcomes: a systematic review for the EAACI guidelines on environmental science for allergic diseases and asthma. Allergy. [DOI] [PubMed] [Google Scholar]
- Aguilera I, et al. , 2013. Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study. Environ. Health Perspect 121, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera J, et al. , 2023. Editorial: the impact of climate change on allergic disease. Front. Allergy 4, 1246899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althouse AD, 2016. Adjust for multiple comparisons? it’s not that simple. Ann. Thorac. Surg 101, 1644–1645. [DOI] [PubMed] [Google Scholar]
- Anenberg SC, et al. , 2022. Long-term trends in urban NO2 concentrations and associated paediatric asthma incidence: estimates from global datasets. The Lancet Planetary Health. 6, e49–e58. [DOI] [PubMed] [Google Scholar]
- Asher MI, et al. , 2006. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368, 733–743. [DOI] [PubMed] [Google Scholar]
- Asher I, Pearce N, 2014. Global burden of asthma among children. Int. J. Tuberc. Lung Dis 18, 1269–1278. [DOI] [PubMed] [Google Scholar]
- Bettiol A, et al. , 2021. The first 1000 days of life: traffic-related air pollution and development of wheezing and asthma in childhood. A systematic review of birth cohort studies. Environ. Health 20, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolte G, et al. , 2021. Integrating sex/gender into environmental health research: development of a conceptual framework. Int. J. Environ. Res. Public Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, et al. , 2018. Prenatal nitrate air pollution exposure and reduced child lung function: timing and fetal sex effects. Environ. Res 167, 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, et al. , 2015. Timing and duration of traffic-related air pollution exposure and the risk for childhood wheeze and asthma. Am. J. Respir. Crit. Care Med 192, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, et al. , 2014. Commentary: does air pollution confound studies of temperature? Epidemiology 25, 242–245. [DOI] [PubMed] [Google Scholar]
- Buckley JP, Richardson DB, 2012. Seasonal modification of the association between temperature and adult emergency department visits for asthma: a case-crossover study. Environ. Health 11, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri PH, 1984. Fetal and postnatal development of the lung. Annu. Rev. Physiol 46, 617–628. [DOI] [PubMed] [Google Scholar]
- Burris HH, et al. , 2013. Association between birth weight and DNA methylation of IGF2, glucocorticoid receptor and repetitive elements LINE-1 and Alu. Epigenomics 5, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, et al. , 2020. Prenatal, early-life, and childhood exposure to air pollution and lung function: the ALSPAC cohort. Am. J. Respir. Crit. Care Med 202, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro H, et al. , 1978. A simplified method for diagnosis of gestational age in the newborn infant. J. Pediatr 93, 120–122. [DOI] [PubMed] [Google Scholar]
- Carey MA, et al. , 2007a. It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18, 308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, et al. , 2007b. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am. J. Physiol. Lung Cell. Mol. Physiol 293, L272–L278. [DOI] [PubMed] [Google Scholar]
- Carlson JM, et al. , 2023. Critical windows of susceptibility for the effects of prenatal exposure to heat and heat variability on gestational growth. Environ. Res 216, 114607. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. , 2023. Identifying the critical windows and joint effects of temperature and PM(2.5) exposure on small for gestational age. Environ. Int 173, 107832. [DOI] [PubMed] [Google Scholar]
- Cisneros R, et al. , 2021. Nitrogen dioxide and asthma emergency department visits in California, USA during cold season (November to February) of 2005 to 2015: a time-stratified case-crossover analysis. Sci. Total Environ 754, 142089. [DOI] [PubMed] [Google Scholar]
- Clark NA, et al. , 2010. Effect of early life exposure to air pollution on development of childhood asthma. Environ. Health Perspect 118, 284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G, et al. , 2010. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J. Investig. Allergol. Clin. Immunol 20, 95–102. [PubMed] [Google Scholar]
- Daouda M, et al. , 2024. Prenatal household air pollution exposure and childhood blood pressure in rural Ghana. Environ. Health Perspect 132, 37006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, et al. , 2020. High and low temperatures aggravate airway inflammation of asthma: Evidence in a mouse model. Environ. Pollut 256, 113433. [DOI] [PubMed] [Google Scholar]
- Ducharme FM, et al. , 2014. Diagnosis, management, and prognosis of preschool wheeze. Lancet 383, 1593–1604. [DOI] [PubMed] [Google Scholar]
- Fandiño-Del-Rio M, et al. , 2022. Phthalate biomarkers and associations with respiratory symptoms and healthcare utilization among low-income urban children with asthma. Environ. Res 212, 113239. [DOI] [PubMed] [Google Scholar]
- Fuertes E, et al. , 2013. A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. PeerJ 1, e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffin JM, et al. , 2014. Perinatal and early childhood environmental factors influencing allergic asthma immunopathogenesis. Int. Immunopharmacol 22, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, 2014. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat. Med 33, 881–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, et al. , 2010. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am. J. Respir. Crit. Care Med 181, 596–603. [DOI] [PubMed] [Google Scholar]
- Gehring U, et al. , 2015. Exposure to air pollution and development of asthma and rhinoconjunctivitis throughout childhood and adolescence: a population-based birth cohort study. Lancet Respir. Med 3, 933–942. [DOI] [PubMed] [Google Scholar]
- Glencross DA, et al. , 2020. Air pollution and its effects on the immune system. Free Radic. Biol. Med 151, 56–68. [DOI] [PubMed] [Google Scholar]
- Granés L, et al. , 2024. Early life cold and heat exposure impacts white matter development in children. Nat. Clim. Chang 1–7. [Google Scholar]
- Gref A, et al. , 2017. Genome-wide interaction analysis of air pollution exposure and childhood asthma with functional follow-up. Am. J. Respir. Crit. Care Med 195, 1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert A, et al. , 2023a. Prenatal and childhood exposure to ambient air pollution and cognitive function in school-age children: examining sensitive windows and sex-specific associations. Environ. Res 235, 116557. [DOI] [PubMed] [Google Scholar]
- Guilbert A, et al. , 2023b. Association of prenatal and postnatal exposures to warm or cold air temperatures with lung function in young infants. JAMA Netw. Open 6, e233376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Avila I, et al. , 2021. A spatiotemporal reconstruction of daily ambient temperature using satellite data in the Megalopolis of Central Mexico from 2003 to 2019. Int. J. Climatol 41, 4095–4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Avila I, et al. , 2022. Prediction of daily mean and one-hour maximum PM (2.5) concentrations and applications in Central Mexico using satellite-based machine-learning models. J. Eposure Sci. Environ. Epidemiol 32, 917–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlehurst MF, et al. , 2024. Associations of prenatal ambient air pollution exposures with asthma in middle childhood. Int. J. Hyg. Environ. Health 258, 114333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, et al. , 2019. The association of early-life exposure to air pollution with lung function at ~17.5 years in the “Children of 1997” Hong Kong Chinese Birth Cohort. Environ. Int 123, 444–450. [DOI] [PubMed] [Google Scholar]
- He MZ, et al. , 2023. Predicting fine-scale daily NO(2) over Mexico City using an ensemble modeling approach. Atmos. Pollut. Res 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, et al. , 2007. Early childhood lower respiratory illness and air pollution. Environ. Health Perspect 115, 1510–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HH, et al. , 2015. Prenatal particulate air pollution and asthma onset in urban children. identifying sensitive windows and sex differences. Am. J. Respir. Crit. Care Med 192, 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HL, et al. , 2024. Sensitive development windows of prenatal air pollution and cognitive functioning in preschool age Mexican children. Environ. Epidemiol 8, e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, et al. , 2020. Season-stratified effects of meteorological factors on childhood asthma in Shanghai. China. Environ. Res 191, 110115. [DOI] [PubMed] [Google Scholar]
- Hu Y, et al. , 2022. Evaluation of climate change adaptation measures for childhood asthma: a systematic review of epidemiological evidence. Sci. Total Environ 839, 156291. [DOI] [PubMed] [Google Scholar]
- Hua L, et al. , 2023. Outdoor air pollution exposure and the risk of asthma and wheezing in the offspring. Environ. Sci. Pollut. Res. Int 30, 14165–14189. [DOI] [PubMed] [Google Scholar]
- Ibrahim MF, et al. , 2021. Association between ambient air pollution and childhood respiratory diseases in low-and middle-income Asian countries: a systematic review. Atmos. Environ 256, 118422. [Google Scholar]
- Jakpor O, et al. , 2020. Term birthweight and critical windows of prenatal exposure to average meteorological conditions and meteorological variability. Environ. Int 142, 105847. [DOI] [PubMed] [Google Scholar]
- Ji H, et al. , 2016a. Air pollution, epigenetics, and asthma. Allergy Asthma Clin. Immunol 12, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, et al. , 2016b. Air pollution, epigenetics, and asthma. Allergy Asthma Clin. Immunol 12, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR, et al. , 2019. Fine particulate matter exposure during pregnancy and infancy and incident asthma. J. Allergy Clin. Immunol 143, 2254–2262.e5. [DOI] [PubMed] [Google Scholar]
- Just AC, et al. , 2015. Using high-resolution satellite aerosol optical depth to estimate daily PM2. 5 geographical distribution in Mexico City. Environ. Sci. Tech 49, 8576–8584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajekar R, 2007. Environmental factors and developmental outcomes in the lung. Pharmacol. Ther 114, 129–145. [DOI] [PubMed] [Google Scholar]
- Kato A, et al. , 2007. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol 179, 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khreis H, et al. , 2017. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ. Int 100, 1–31. [DOI] [PubMed] [Google Scholar]
- Lavigne É, et al. , 2018. Effect modification of perinatal exposure to air pollution and childhood asthma incidence. Eur. Respir. J 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, et al. , 2019. Spatiotemporal variations in ambient ultrafine particles and the incidence of childhood asthma. Am. J. Respir. Crit. Care Med 199, 1487–1495. [DOI] [PubMed] [Google Scholar]
- Lee A, et al. , 2018. Prenatal fine particulate exposure and early childhood asthma: effect of maternal stress and fetal sex. J. Allergy Clin. Immunol 141, 1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon Hsu H-H, et al. , 2015. Prenatal particulate air pollution and asthma onset in urban children. Identifying sensitive windows and sex differences. Am. J. Respir. Crit. Care Med 192, 1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptzin DR, et al. , 2015. Sex and the lung: observations, hypotheses, and future directions. Pediatr. Pulmonol 50, 1159–1169. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. , 2016. Impact of meteorological factors on lower respiratory tract infections in children. J. Int. Med. Res 44, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, et al. , 2022. Interaction effect of prenatal and postnatal exposure to ambient air pollution and temperature on childhood asthma. Environ. Int 167, 107456. [DOI] [PubMed] [Google Scholar]
- Lu C, et al. , 2023. Interaction of high temperature and NO(2) exposure on asthma risk: in vivo experimental evidence of inflammation and oxidative stress. Sci. Total Environ 869, 161760. [DOI] [PubMed] [Google Scholar]
- Madsen C, et al. , 2017. Pregnancy exposure to air pollution and early childhood respiratory health in the Norwegian Mother and Child Cohort Study (MoBa). BMJ Open 7, e015796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallol J, et al. , 2010. Regional variation in asthma symptom prevalence in Latin American children. J. Asthma 47, 644–650. [DOI] [PubMed] [Google Scholar]
- Martens DS, et al. , 2019. Early biological aging and fetal exposure to high and low ambient temperature: a birth cohort study. Environ. Health Perspect 127, 117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FD, 2016. Early-life origins of chronic obstructive pulmonary disease. N. Engl. J. Med 375, 871–878. [DOI] [PubMed] [Google Scholar]
- Mata Fernández C, et al. , 2005. Validation of the Spanish version of the Phase III ISAAC questionnaire on asthma. J. Investig. Allergol. Clin. Immunol 15, 201–210. [PubMed] [Google Scholar]
- McGuinn LA, et al. , 2020. Fine particulate matter exposure and lipid levels among children in Mexico city. Environ. Epidemiol 4, e088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Marty MA, 2010. Impact of environmental chemicals on lung development. Environ. Health Perspect 118, 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern V, et al. , 2008. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am. J. Respir. Crit. Care Med 177, 1331–1337. [DOI] [PubMed] [Google Scholar]
- Nordling E, et al. , 2008. Traffic-related air pollution and childhood respiratory symptoms, function and allergies. Epidemiology 19, 401–408. [DOI] [PubMed] [Google Scholar]
- Nurmagambetov T, et al. , 2018. The economic burden of asthma in the United States, 2008–2013. Ann. Am. Thorac. Soc 15, 348–356. [DOI] [PubMed] [Google Scholar]
- Parker JD, et al. , 2009. Air pollution and childhood respiratory allergies in the United States. Environ. Health Perspect 117, 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden DB, 2005. The epidemiology and genetics of asthma risk associated with air pollution. J. Allergy Clin. Immunol 115, 213–219. [DOI] [PubMed] [Google Scholar]
- Pedersen M, et al. , 2023. Early-life exposure to ambient air pollution from multiple sources and asthma incidence in children: a nationwide birth cohort study from Denmark. Environ. Health Perspect 131, 57003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politis MD, et al. , 2024. Recent ambient temperature and fine particulate matter (PM (2.5)) exposure is associated with urinary kidney injury biomarkers in children. Sci. Total Environ 907, 168119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, et al. , 2015. Greater temperature variation within a day associated with increased emergency hospital admissions for asthma. Sci. Total Environ 505, 508–513. [DOI] [PubMed] [Google Scholar]
- Rancìere F, et al. , 2017. Early exposure to traffic-related air pollution, respiratory symptoms at 4 years of age, and potential effect modification by parental allergy, stressful family events, and sex: a prospective follow-up study of the PARIS birth cohort. Environ. Health Perspect 125, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinmuth-Selzle K, et al. , 2017. Air pollution and climate change effects on allergies in the anthropocene: abundance, interaction, and modification of allergens and adjuvants. Environ. Sci. Tech 51, 4119–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice MB, et al. , 2016. Lifetime exposure to ambient pollution and lung function in children. Am. J. Respir. Crit. Care Med 193, 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MA, 2008. The effect of air pollution on asthma and allergy. Curr. Allergy Asthma Rep 8, 139–146. [DOI] [PubMed] [Google Scholar]
- Rosa MJ, et al. , 2019. Association between prenatal particulate air pollution exposure and telomere length in cord blood: Effect modification by fetal sex. Environ. Res 172, 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, 2007. Health effects of ambient air pollution in children. Paediatr. Respir. Rev 8, 275–280. [DOI] [PubMed] [Google Scholar]
- Sampath V, et al. , 2023. Mechanisms of climate change and related air pollution on the immune system leading to allergic disease and asthma. Semin. Immunol 67, 101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, et al. , 2022. Global, regional, and national prevalence of asthma in 2019: a systematic analysis and modelling study. J. Glob. Health 12, 04052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria-Contreras DC, et al. , 2020. Patterns of weight change one year after delivery are associated with cardiometabolic risk factors at six years postpartum in mexican women. Nutrients 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks J, et al. , 2013. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir. Med 1, 728–742. [DOI] [PubMed] [Google Scholar]
- Stolwijk AM, et al. , 1999. Studying seasonality by using sine and cosine functions in regression analysis. J. Epidemiol. Community Health 53, 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian F, et al. , 2024. Mutual associations of exposure to ambient air pollutants in the first 1000 Days of Life with asthma/wheezing in children: prospective cohort study in Guangzhou, China. JMIR Public Health Surveill. 10, e52456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillaut H, et al. , 2023. Prenatal exposure to perfluoroalkyl substances and child behavior at age 12: a PELAGIE mother-child cohort study. Environ. Health Perspect 131, 117009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torday JS, et al. , 1981. Sex differences in fetal lung maturation. Am. Rev. Respir. Dis 123, 205–208. [DOI] [PubMed] [Google Scholar]
- Tuazon JA, et al. , 2022. Emerging insights into the impact of air pollution on immune-mediated asthma pathogenesis. Curr. Allergy Asthma Rep 22, 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A, et al. , 2017. Potential for bias when estimating critical windows for air pollution in children’s health. Am. J. Epidemiol 186, 1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, 2010. Perinatal stress and early life programming of lung structure and function. Biol. Psychol 84, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, et al. , 2024. High ambient temperature may increase the risk of anemia in pregnancy: identifying susceptible exposure windows. Sci. Total Environ 926, 172059. [DOI] [PubMed] [Google Scholar]
- Xu Z, et al. , 2012. Impact of ambient temperature on children’s health: a systematic review. Environ. Res 117, 120–131. [DOI] [PubMed] [Google Scholar]
- Xu Z, et al. , 2013. Diurnal temperature range and childhood asthma: a time-series study. Environ. Health 12, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, et al. , 2023. High temperature exacerbates ozone-induced airway inflammation: implication of airway microbiota and metabolites. Sci. Total Environ 903, 166795. [DOI] [PubMed] [Google Scholar]
- Yang IV, et al. , 2017. The environment, epigenome, and asthma. J. Allergy Clin. Immunol 140, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitshak-Sade M, et al. , 2021. The effect of prenatal temperature and PM(2.5) exposure on birthweight: weekly windows of exposure throughout the pregnancy. Environ. Int 155, 106588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, et al. , 2024. Early-life exposure to air pollution and childhood asthma cumulative incidence in the ECHO CREW consortium. JAMA Netw. Open 7, e240535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. , 2021. Early-life exposure to submicron particulate air pollution in relation to asthma development in Chinese preschool children. J. Allergy Clin. Immunol 148, 771–782.e12. [DOI] [PubMed] [Google Scholar]
- Zhao Q, et al. , 2021. Air pollution during infancy and lung function development into adolescence: The GINIplus/LISA birth cohorts study. Environ. Int 146, 106195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.


