Abstract
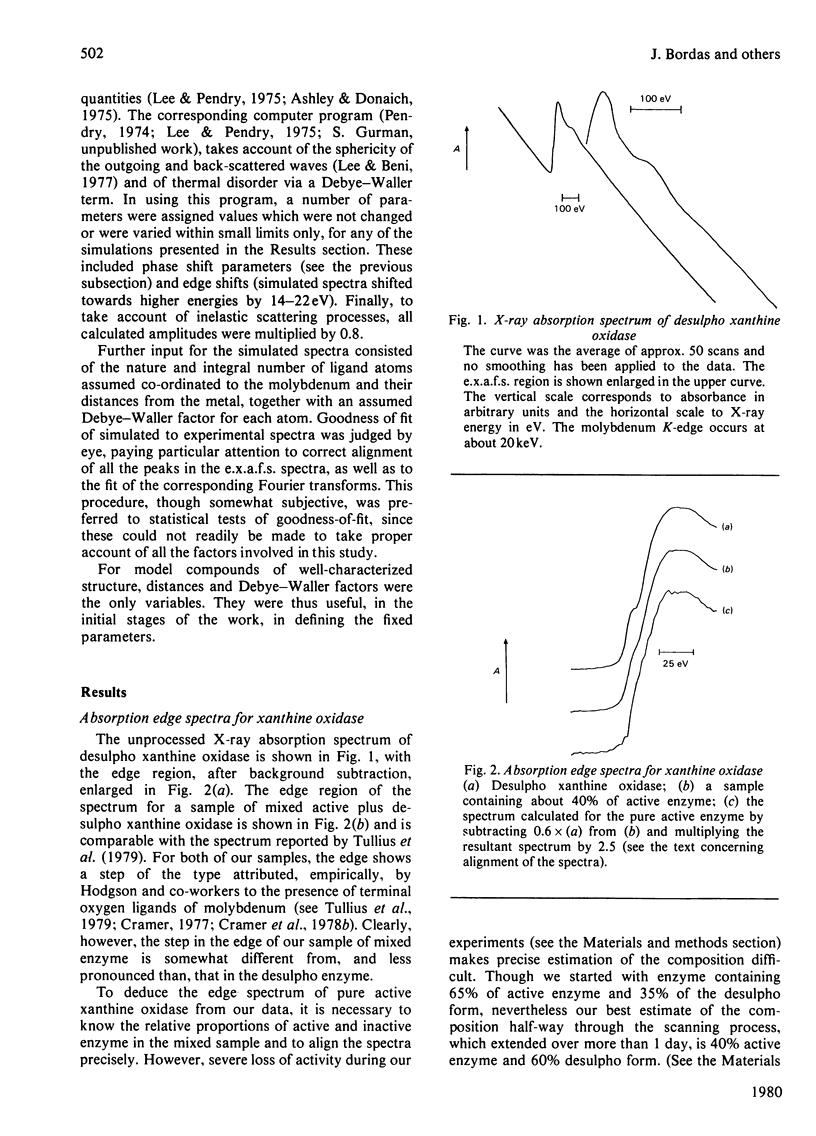
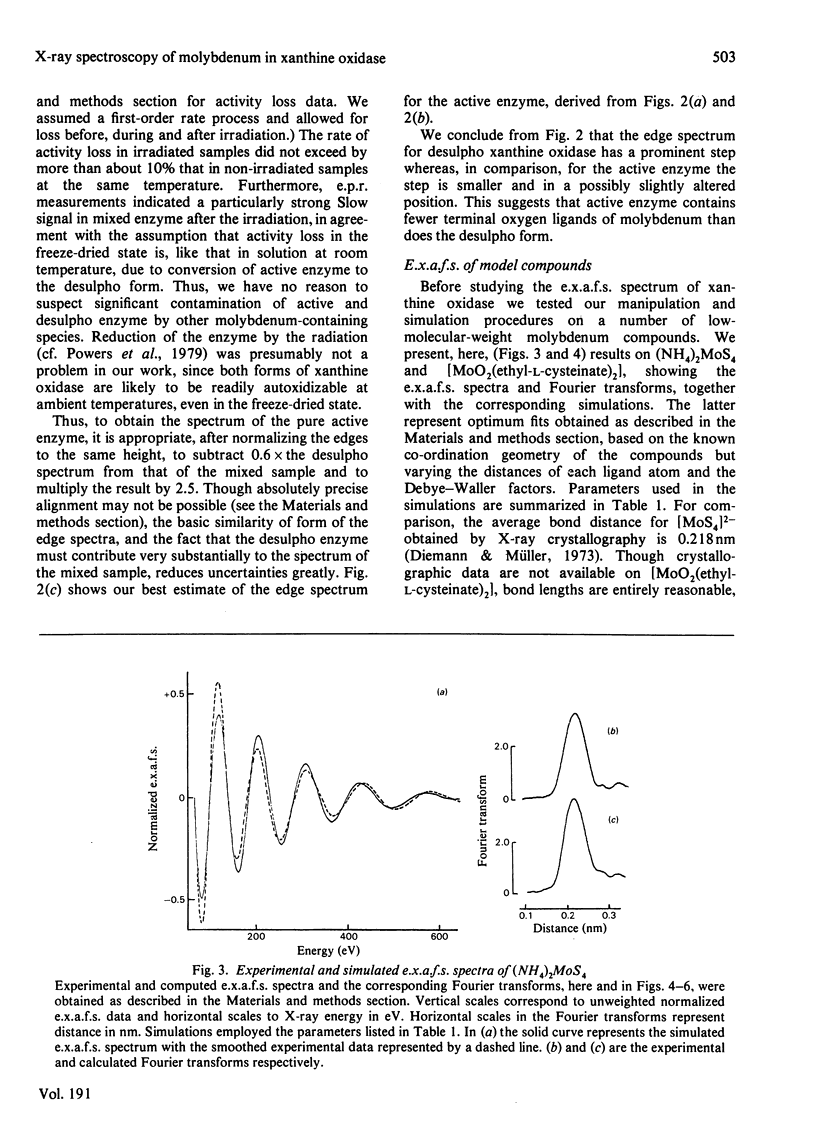
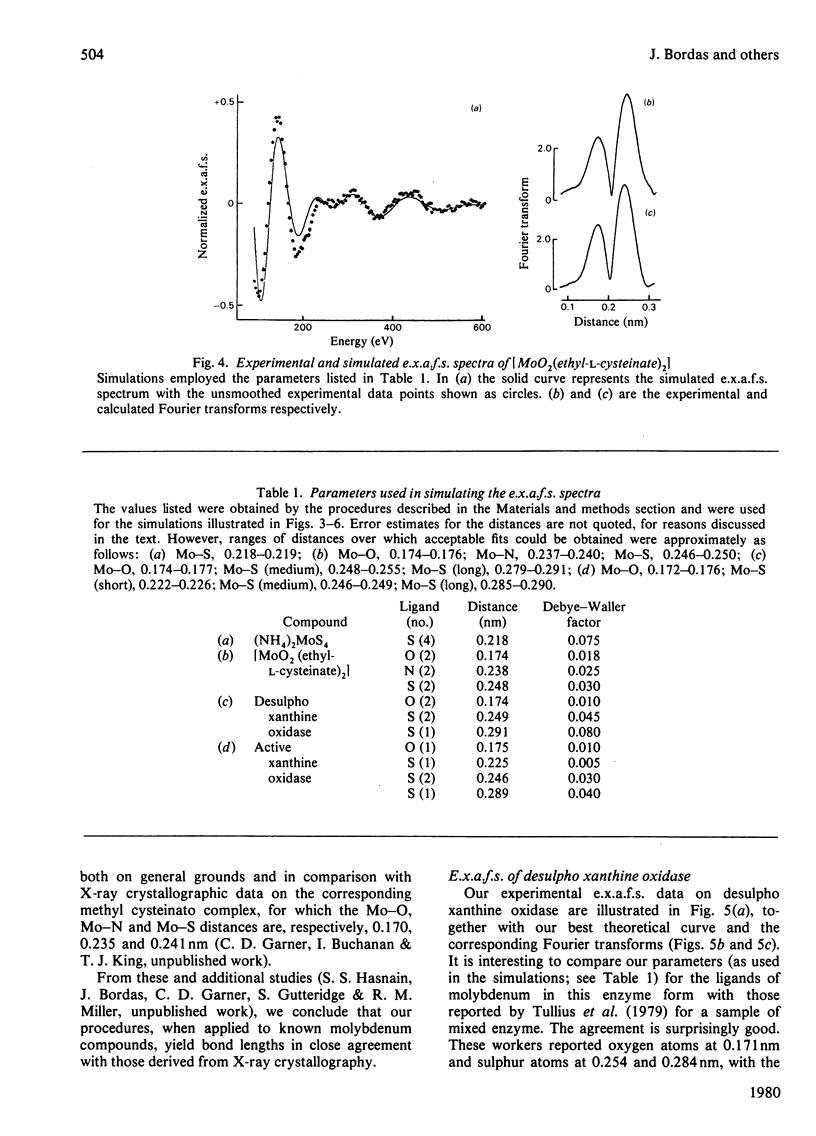
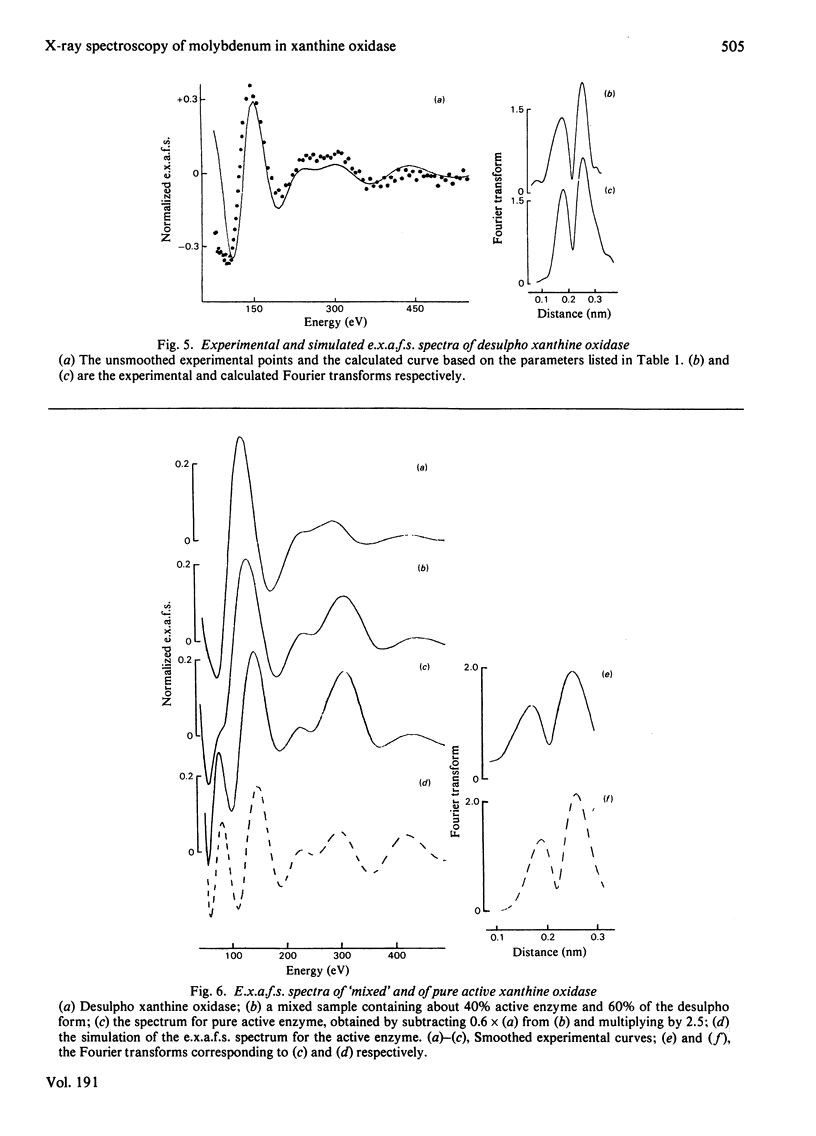
X-ray absorption spectra have been recorded for the molybdenum K-edge region of xanthine oxidase. Both the absorption edge and the extended fine structure (e.x.a.f.s.) regions were investigated. Spectra were obtained for samples of the desulpho enzyme as well as for mixtures of this with the active enzyme. The spectrum of the pure active form was then obtained by difference. The desulpho enzyme shows a pronounced step in the absorption edge, of a type previously associated terminal oxygen ligands. In the active enzyme this step has decreased markedly. Satisfactory simulations of the e.x.a.f.s. spectrum of the desulpho enzyme could be obtained by assuming the molybdenum to be bonded to two terminal oxygen atoms (Mo = O about .175 nm), two sulphur atoms (presumably from cysteine residues, Mo-S about .0250 nm) and one sulphur atom (presumably from a methionine residue, Mo-S about 0.290 nm). E.x.a.f.s. of the active enzyme differed appreciably from this. In keeping with earlier proposals [Gutteridge, Tanner & Bray (1978) Biochem. J. 175, 887-897], the spectrum of the active enzyme could be simulated if a sulphur atom at about 0.225 nm (i.e. presumably a terminal sulphur atom) replaced one of the terminal oxygen atoms of the desulpho from, with small changes in the other bond distances. Validity of the interpretative procedures, which involved phase shift and amplitude calculations ab initio, was demonstrated by using low molecular weight compounds of known structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betcher-Lange S. L., Coughlan M. P., Rajagopalan K. V. Syncatalytic modification of chicken liver xanthine dehydrogenase by hydrogen peroxide. The nature of the reaction. J Biol Chem. 1979 Sep 25;254(18):8825–8829. [PubMed] [Google Scholar]
- Bordas J., Bray R. C., Garner C. D., Gutteridge S., Hasnain S. S. EXAFS studies of the molybdenum center of xanthine oxidase. J Inorg Biochem. 1979 Oct;11(2):181–186. doi: 10.1016/s0162-0134(00)80182-8. [DOI] [PubMed] [Google Scholar]
- Bray R. C. The reactions and the structures of molybdenum centers in enzymes. Adv Enzymol Relat Areas Mol Biol. 1980;51:107–165. doi: 10.1002/9780470122969.ch3. [DOI] [PubMed] [Google Scholar]
- Cammack R., Barber M. J., Bray R. C. Oxidation-reduction potentials of molybdenum, flavin and iron-sulphur centres in milk xanthine oxidase. Biochem J. 1976 Aug 1;157(2):469–478. doi: 10.1042/bj1570469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. I., Gamble R. C. X-ray absorption spectroscopy of metalloproteins. Methods Enzymol. 1978;54:323–345. doi: 10.1016/s0076-6879(78)54022-6. [DOI] [PubMed] [Google Scholar]
- Coughlan M. P. On the origin of the cyanolysable sulphur in molybdenum iron/sulphur flavin hydroxylases. FEBS Lett. 1977 Sep 1;81(1):1–6. doi: 10.1016/0014-5793(77)80914-9. [DOI] [PubMed] [Google Scholar]
- Coughlan M. P., Rajagopalan K. V., Handler P. The role of molybdenum in xanthine oxidase and related enzymes. Reactivity with cyanide, arsenite, and methanol. J Biol Chem. 1969 May 25;244(10):2658–2663. [PubMed] [Google Scholar]
- Edmondson D., Massey V., Palmer G., Beacham L. M., 3rd, Elion G. B. The resolution of active and inactive xanthine oxidase by affinity chromatography. J Biol Chem. 1972 Mar 10;247(5):1597–1604. [PubMed] [Google Scholar]
- Gutteridge S., Bray R. C. Oxygen-17 splitting of the very rapid molybdenum(V) e.p.r. signal from xanthine oxidase. Rate of exchange with water of the coupled oxygen atom. Biochem J. 1980 Sep 1;189(3):615–623. doi: 10.1042/bj1890615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. Comparison of the molybdenum centres of native and desulpho xanthine oxidase. The nature of the cyanide-labile sulphur atom and the nature of the proton-accepting group. Biochem J. 1978 Dec 1;175(3):887–897. doi: 10.1042/bj1750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- McGartoll M. A., Pick F. M., Swann J. C., Bray R. C. Properties of xanthine oxidase preparations dependent on the proportions of active and inactivated enzyme. Biochim Biophys Acta. 1970 Sep 16;212(3):523–526. doi: 10.1016/0005-2744(70)90264-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. C., Pygall C. F. Reactions of molybdenum-sulphur compounds with cyanide: chemical evolution and deactivation of molybdoenzymes. J Inorg Biochem. 1979 Aug;11(1):25–29. doi: 10.1016/s0162-0134(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Powers L., Blumberg W. E., Chance B., Barlow C. H., Leigh J. S., Jr, Smith J., Yonetani T., Vik S., Peisach J. The nature of the copper atoms of cytochrome c oxidase as studied by optical and x-ray absorption edge spectroscopy. Biochim Biophys Acta. 1979 Jun 5;546(3):520–538. doi: 10.1016/0005-2728(79)90085-9. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Eisenberger P., Kincaid B. M. X-ray absorption spectroscopy of biological molecules. Annu Rev Biophys Bioeng. 1978;7:559–578. doi: 10.1146/annurev.bb.07.060178.003015. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I. Proposed molecular mechanism for the action of molybedenum in enzymes: coupled proton and electron transfer. Proc Natl Acad Sci U S A. 1973 Apr;70(4):988–992. doi: 10.1073/pnas.70.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]


