Abstract
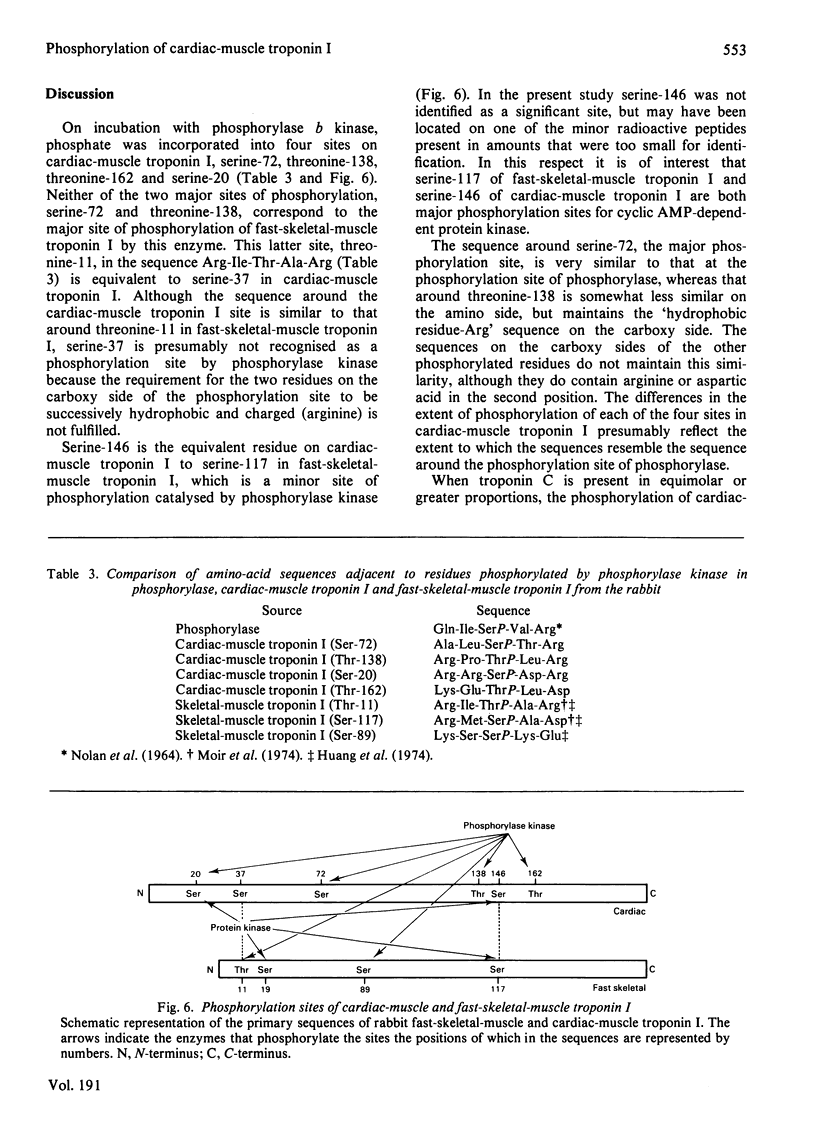
1. Incubation of rabbit cardiac-muscle troponin I with phosphorylase b kinase leads to the incorporation of .07-1.2 mol of Pi/mol. 2. The major site of phosphorylation is a serine residue at position 72. 3. Lesser amounts of phosphate are incorporated into threonine-138, threonine-162 and serine 20. 4. Serine-20 is the only site that contains a significant amount of phosphate before incubation with phosphorylase b kinase. 5. Unlike the situation with serine-20, the extent of phosphorylation of serine-72 and threonine-138 in the perfused rabbit heart does not change when the heart is exposed to adrenaline (4 microM).
Full text
PDF
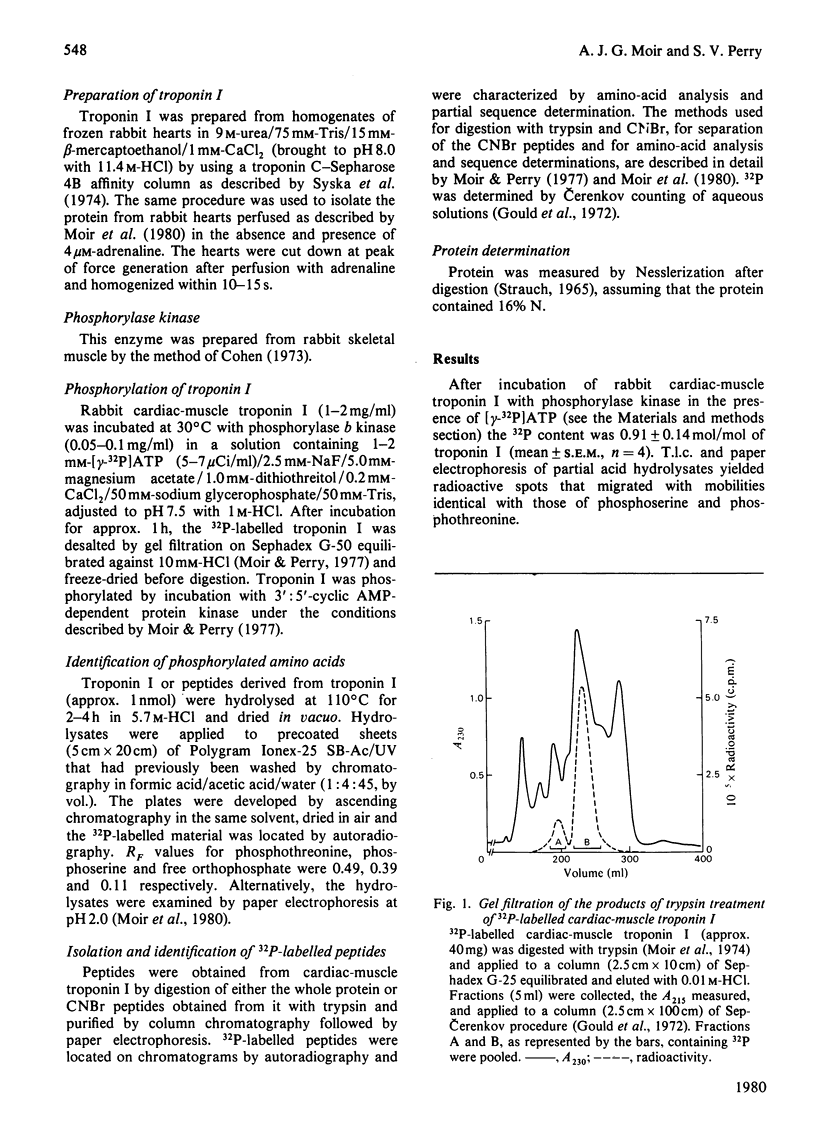
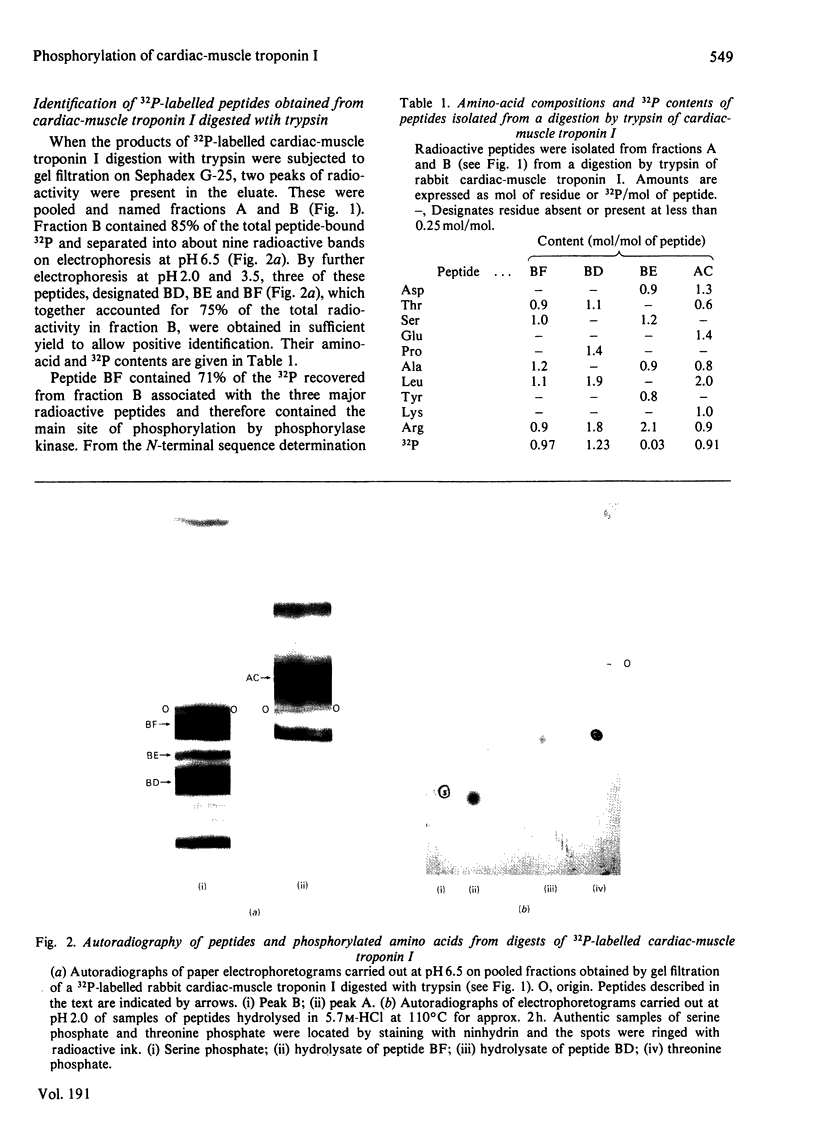
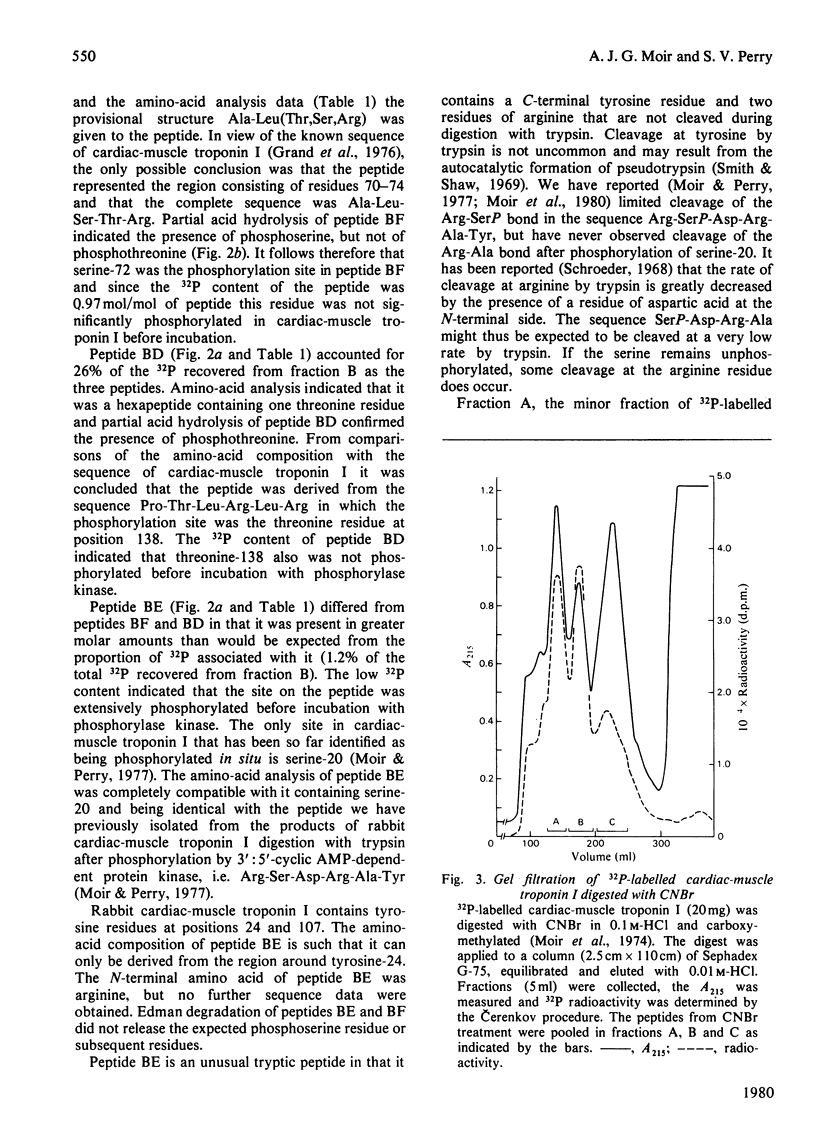

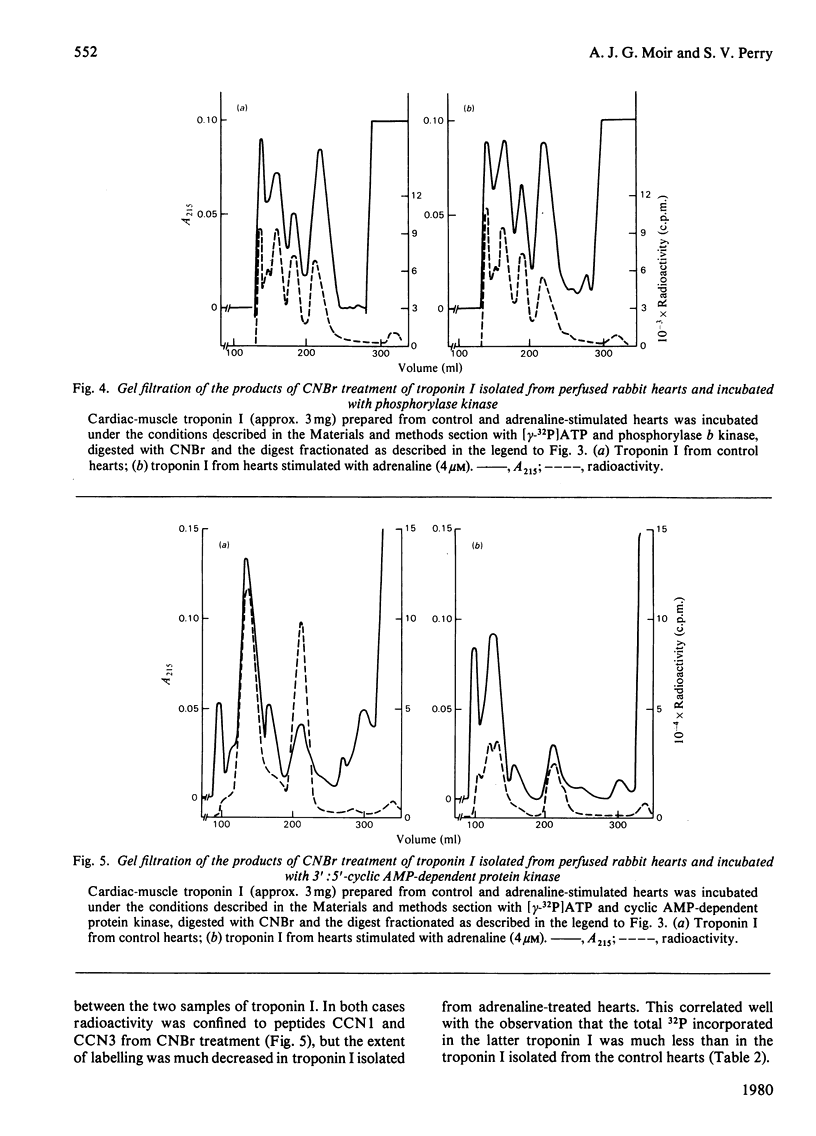

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce C. A., Barra H. S., Rodriguez J. A., Caputto R. Tentative identification of the amino acid that binds tyrosine as a single unit into a soluble brain protein. FEBS Lett. 1975 Jan 15;50(1):5–7. doi: 10.1016/0014-5793(75)81027-1. [DOI] [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Cole H. A., Perry S. V. The phosphorylation of troponin I from cardiac muscle. Biochem J. 1975 Sep;149(3):525–533. doi: 10.1042/bj1490525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J. Phosphorylation of the inhibitory subunit of troponin in perfused hearts of mice deficient in phosphorylase kinase. Evidence for the phosphorylation of troponin by adenosine 3':5'-phosphate-dependent protein kinase in vivo. Biochem J. 1977 Nov 15;168(2):307–310. doi: 10.1042/bj1680307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould J. M., Cather R., Winget G. D. Advantages of the use of Cerenkov vounting for determination of P 32 in photophosphorylation research. Anal Biochem. 1972 Dec;50(2):540–548. doi: 10.1016/0003-2697(72)90064-4. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Wilkinson J. M. The amino acid sequence of rabbit cardiac troponin I. Biochem J. 1976 Dec 1;159(3):633–641. doi: 10.1042/bj1590633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. S., Bylund D. B., Stull J. T., Krebs E. G. The amino acid sequences of the phosphorylated sites in troponin-I from rabbit skeletal muscle. FEBS Lett. 1974 Jun 15;42(3):249–252. doi: 10.1016/0014-5793(74)80738-6. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Role of the contractile proteins and sarcoplasmic reticulum in the response of the heart to catecholamines: an historical review. Adv Cyclic Nucleotide Res. 1979;11:303–343. [PubMed] [Google Scholar]
- Moir A. J., Perry S. V. The sites of phosphorylation of rabbit cardiac troponin I by adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Effect of interaction with troponin C. Biochem J. 1977 Nov 1;167(2):333–343. doi: 10.1042/bj1670333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. J., Solaro R. J., Perry S. V. The site of phosphorylation of troponin I in the perfused rabbit heart. The effect of adrenaline. Biochem J. 1980 Feb 1;185(2):505–513. doi: 10.1042/bj1850505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A. J., Wilkinson J. M., Perry S. V. The phosphorylation sites of troponin I from white skeletal muscle of the rabbit. FEBS Lett. 1974 Jun 15;42(3):253–256. doi: 10.1016/0014-5793(74)80739-8. [DOI] [PubMed] [Google Scholar]
- NOLAN C., NOVOA W. B., KREBS E. G., FISCHER E. H. FURTHER STUDIES ON THE SITE PHOSPHORYLATED IN THE PHOSPHORYLASE B TO A REACTION. Biochemistry. 1964 Apr;3:542–551. doi: 10.1021/bi00892a013. [DOI] [PubMed] [Google Scholar]
- Perry S. V. The regulation of contractile activity in muscle. Biochem Soc Trans. 1979 Aug;7(4):593–617. doi: 10.1042/bst0070593. [DOI] [PubMed] [Google Scholar]
- Smith R. L., Shaw E. Pseudotrypsin. A modified bovine trypsin produced by limited autodigestion. J Biol Chem. 1969 Sep 10;244(17):4704–4712. [PubMed] [Google Scholar]
- Solaro R. J., Moir A. J., Perry S. V. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Syska H., Perry S. V., Trayer I. P. A new method of preparation of troponin I (inhibitory protein) using affinity chromatography. Evidence for three different forms of troponin I in striated muscle. FEBS Lett. 1974 Apr 1;40(2):253–257. doi: 10.1016/0014-5793(74)80238-3. [DOI] [PubMed] [Google Scholar]
- Syska H., Wilkinson J. M., Grand R. J., Perry S. V. The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem J. 1976 Feb 1;153(2):375–387. doi: 10.1042/bj1530375. [DOI] [PMC free article] [PubMed] [Google Scholar]



