Abstract
Objective
Anti-IgLON5 disease is a rare autoimmune mediated disease. It is mainly featured by sleep-related disturbance, parkinsonism, chorea and limb ataxia. Previous studies had clarified its clinical manifestations and predisposing genes. However, as far as we know, anti-IgLON5 disease combined with paraneoplastic cerebellar degeneration (PCD) with the detection of anti-Sulfatide IgG antibody, masquerading as meningoencephalitis had not been reported before.
Case presentation
A 57-year-old Chinese female presented with walking unsteadily for 12 days and logagnosia for 2 days and was admitted to our hospital. She had a past history of breast cancer. Magnetic resonance imaging (MRI) revealed leptomeningeal enhancement (prominent in cerebellar hemisphere). Arterial spin labeling (ASL) perfusion showed hyperperfusion in the cerebellar hemisphere and interhemispheric fissure cistern. MRI and ASL indicated the diagnosis was meningoencephalitis. However, IgG anti-IgLON5 antibody was positive in both serum and cerebrospinal fluid. Therefore, the diagnosis was anti-IgLON5 disease. In addition, the patient combined with PCD due to positive anti-Yo-antibody in serum fluid .
Conclusions
Whereas sleep disturbance is the most common feature in patients with anti-IgLON5 disease, our case presented with walking unsteadily and logagnosia. Anti-IgLON5 disease combined with PCD with the detection of anti-Sulfatide IgG antibody, masquerading as meningoencephalitis is very rare. Therefore, if meningoencephalitis did not recover with conventional treatment, anti-IgLON5 disease and PCD should be considered as the differential diagnosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12883-024-03984-7.
Keywords: Anti-IgLON5 disease, Meningoencephalitis, Magnetic resonance imaging, Case report
Introduction
In 2014, Sabater et al. firstly described patients manifested with positive IgLON5 antibody, unique rapid eye movement and non-rapid eye movement sleep disorders, and tau deposition [1]. Currently, study had confirmed anti-IgLON5 disease is a rare chronic autoimmune-mediated tauopathy [2]. The clinical symptoms and severity of anti-IgLON5 disease vary widely individually, including sleep disturbance, gait disturbance, bulbar symptoms, disturbance of movement, and cognitive dysfunction [3]. Meanwhile, patients with anti-IgLON5 disease can display diverse magnetic resonance imaging (MRI) changes [4], including brain atrophy, T2 hyperintensity and white matter changes, etc [5]. However, as far as we know, patient with anti-IgLON5 disease combined with paraneoplastic cerebellar degeneration (PCD) whose MRI and arterial spin labeling (ASL) indicated the the diagnosis was meningoencephalitis is very rare until now. Here, we reported a patient diagnosed as anti-IgLON5 disease combined with PCD with the detection of anti-Sulfatide IgG antibody, masquerading as meningoencephalitis.
Case presentation
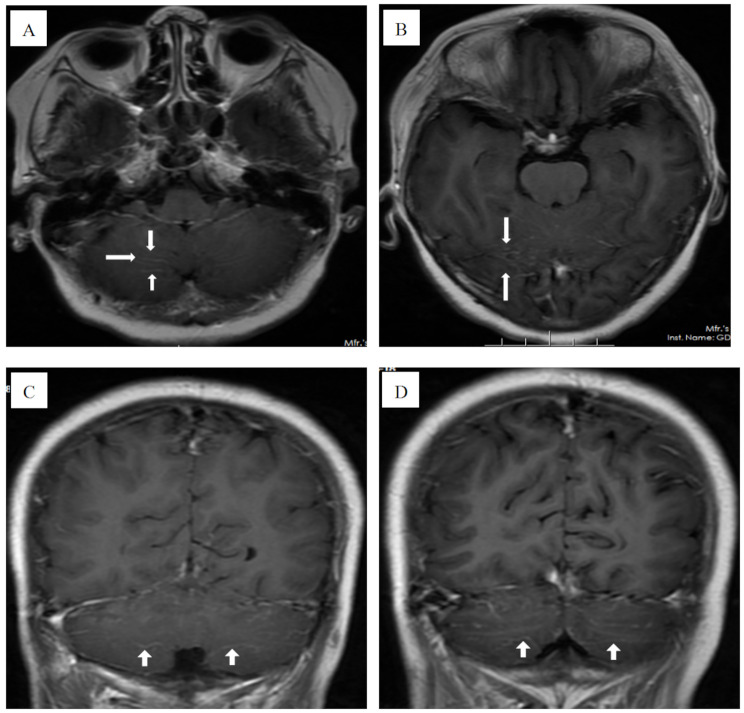
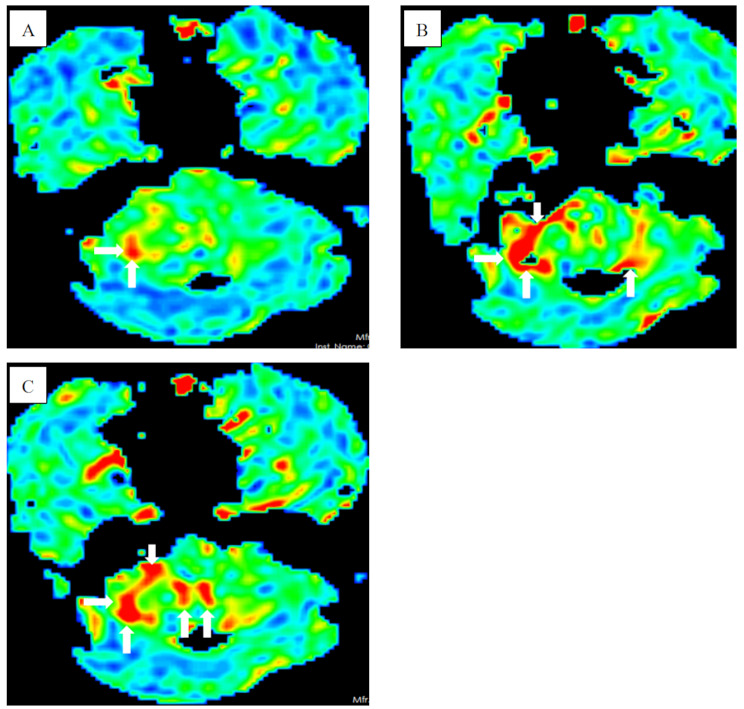
A 57-year-old Chinese female presented with walking unsteadily for 12 days and logagnosia for 2 days and was admitted to our hospital. She had a past history of breast cancer and had been cured after surgical treatment. Brain MRI revealed leptomeningeal enhancement (Fig. 1A, B, C, D) and ASL perfusion showed hyperperfusion in the cerebellar hemisphere and interhemispheric fissure cistern (Fig. 2A, B, C). Therefore, we speculated the diagnosis is meningoencephalitis. To further clarify the diagnosis, we arranged her to undergo polysomnography and electroencephalography. Polysomnogram revealed objective insomnia (severe), including difficult in falling asleep and maintaining sleep. A video electroencephalogram showed diffuse middle-amplitude slow waves and sharp waves during the awake period and no epileptic waves during both awake and sleep periods.
Fig. 1.
(A, B, C, D) Magnetic resonance imaging revealed leptomeningeal enhancement (white arrow)
Fig. 2.
(A, B, C) Arterial spin labeling perfusion showed hyperperfusion of the cerebellar hemisphere and interhemispheric fissure cistern (white arrow)
Due to the findings of brain MRI, ASL, polysomnogram and electroencephalogram could not explain the symptoms of walking unsteadily, logagnosia and sleep disorder.
A lumbar puncture was performed, and the intracranial pressure was normal (120 mmH2O). The cerebrospinal fluid analysis showed slightly increased protein levels of 0.74 g/L (reference value 0.15–0.45 g/L) and normal cell counts. An assay panel of autoimmune encephalitis, autoimmune peripheral neuropathy and paraneoplastic syndrome were implied. The assay panel of autoimmune encephalitis including autoantibodies against N-methyl-D-aspartate receptor, leucine-rich glioma inactivated 1,2 receptors, leucine-rich glioma inactivating protein 1 receptor,γ-aminobutyric acid type B receptor, contact protein association with protein 2 receptor, IgLON5, Glycine receptor 1, dopamine receptor 2 and Glutamamic acid decarboxylase 2 (serum and cerebrospinal fluid, cell based assay, CBA). The assay panel of autoimmune peripheral neuropathy including autoantibodies against GM 1,2,3,4, GD1a,1b, GD2,3, GT1a,1b and Sulfatide (cerebrospinal fluid, western blotting, WB). The assay panel of paraneoplastic syndrome including autoantibodies against Hu, Yo, Ri, contactin response mediator protein 5, amphiphysin, Ma1, Ma2, SRY-box transcription factor 1, delta/notch-like epidermal growth factor-related receptor, Zic family member 4, protein kinase C gamma, Recoverin, and Titin antibodies (serum, western blotting, WB). All autoantibodies were implied by Guangzhou kingmed center for clinical laboratory (Guangzhou, Guangdong Province, China). The patient was positive for IgG anti-IgLON5 antibody (1:100 in serum fluid and 1:10 in cerebrospinal fluid). Meanwhile, anti-Yo-IgG antibody was positive in serum fluid and anti-Sulfatide IgG antibody was positive in cerebrospinal fluid.
Therefore, the final diagnosis of anti-IgLON5 disease combined with PCD was made. The patient was treated with plasma exchange and methylprednisolone pulse therapy. After 2 weeks of immunotherapy, the patient showed no significant improvement in clinical symptoms and was subsequently discharged. The patient died during the following-up period.
Discussion
Anti-IgLON5 disease is a complex disease with heterogeneous clinical manifestations between different patients. There is no clear diagnosis-detection criteria for the disease. The detection of anti-IgLON5 antibody in serum fluid and cerebrospinal fluid is still a recognized diagnostic basis [6, 7]. Therefore, there is no doubt that this patient had a definite diagnosis of anti-IgLON5 disease. However, if patients onset with non-specific symptoms or combined with other neurological diseases, the diagnostic difficulty will greatly increased.
Previous studies cimfirmed anti-IgLON5 disease may complicated with rectal adenocarcinoma and breast cancer [2, 8]. As far as we know, the relationship between tumor and the production of anti-IgLON5 IgG antibody as well as the anti-IgLON5 disease remains unknown. Therefore, we suggested tumor screening should be considered in patients with anti-IgLON5 disease to investigate the relationship between tumor and this disease.
This patient had a history of breast cancer and her anti-Yo IgG-antibody was positive in serum fluid and anti-Sulfatide IgG antibody was positive in cerebrospinal fluid. Among patients with anti-Yo-antibodies, 75% have a pelvic gynecologic malignancy, and 10-15% have breast cancer [9–11]. We speculated anti-Yo-IgG antibody in this patient was related to the history of breast cancer.
PCD is associated with fewer than 1% of cancers and is strongly related to breast and gynecolozgic malignancies [12]. Anti-Yo-antibody is the antibody that most frequently identified with PCD [12]. Hence, if the diagnosis was suspected to be PCD in patient with anti-Yo-antibody, systemic tumor screening should also be performed. A consensus paper revealed anti-Yo-antibody can be used as a biomarker of PCD, but it has no direct pathogenicity [13]. In addition, almost 100% of anti-Yo-antibody positive patients diagnosed with PCD [14]. Meanwhile, most of them only had simple cerebellar symptoms, such as acute or subacute onset of significant cerebellar ataxia, dysarthria and spontaneous nystagmus [14]. In our case, we speculated anti-IgLON5 disease is responsible for objective insomnia (severe) and PCD is responsible for walking unsteadily and logagnosia. Of course, it is worth noting that anti-IgLON5 disease can also manifest ataxia [15]. Therefore, we speculated this patient should be diagnised as anti-IgLON5 disease combined with PCD.
In addition, anti-Yo-antibody was measured by only one method (WB), there is some possibility of false positive or false negative. Therefore, this patient should also be considered as a case of cerebellar enhancement accompanied by anti-IgLON5 disease and anti-Sulfatide IgG antibody.
In one study, 12.5% (9/72) patients had distinct lesions on MRI, including brain atrophy [3], T2 hyperintensity [16]and white matter changes [17], etc. However, as far as we know, leptomeningeal enhancement (MRI indicated) and hyperperfusion of the cerebellar hemisphere and interhemispheric fissure cistern (ASL indicated) had not be reported in this situation before. Unfortunately, we can not explain the reasons for the above phenomenon. Meanwhile, whether anti-IgLON5 disease is associated with anti-Sulfatide IgG antibody is also not clear.
Conclusion
Herein, we described an unusual anti-IgLON5 disease combined with PCD with the detection anti-Sulfatide IgG antibody, masquerading as cephalomeningitis. We proposed tumor screening should be considered in patients with anti-IgLON5 disease to explore the relationship between tumors and this disease. Anti-IgLON5 disease may be combined with other complex neurological diseases, the overlap of symptoms between diseases will greatly increase the diagnosis difficulty.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank the guardian for her cooperation.
Author contributions
SD-Z: data collection, analysis and manuscript writing. ZY-B, XM-T, JX, CM: data collection and manuscript writing. SS-Z: manuscript revision and interpretation of data. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The Institutional Review Board of the Guangdong Sanjiu Brain Hosptial approved the study.
Consent for publication
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sabater L, Gaig C, Gelpi E, Bataller L, Lewerenz J, Torres-Vega E, et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Zhang L, Wang Y. Anti-IgLON5 disease complicated with rectal adenocarcinoma: a case report. BMC Neurol. 2023;23(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaig C, Graus F, Compta Y, Högl B, Bataller L, Brüggemann N, et al. Clinical manifestations of the anti-IgLON5 disease. Neurology. 2017;88(18):1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagliapietra M, Frasson E, Cardellini D, Mariotto S, Ferrari S, Zanusso G, et al. Hypothalamic-Bulbar MRI Hyperintensity in Anti-IgLON5 Disease with serum-restricted antibodies: a case report and systematic review of literature. J Alzheimers Dis. 2021;79(2):683–91. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YH, Ni Y, Gao YN, Shen DD, He L, Yin D, et al. Anti-IgLON5 disease: a novel topic beyond neuroimmunology. Neural Regen Res. 2023;18(5):1017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaig C, Ercilla G, Daura X, Ezquerra M, Fernández-Santiago R, Palou E, Sabater L, et al. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grüter T, Möllers FE, Tietz A, Dargvainiene J, Melzer N, Heidbreder A, et al. Clinical, serological and genetic predictors of response to immunotherapy in anti-IgLON5 disease. Brain. 2023;146(2):600–11. [DOI] [PubMed] [Google Scholar]
- 8.Honorat JA, Komorowski L, Josephs KA, Fechner K, St Louis EK, Hinson SR, et al. IgLON5 antibody: neurological accompaniments and outcomes in 20 patients. Neurol Neuroimmunol Neuroinflamm. 2017;4(5):e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojas-Marcos I, Rousseau A, Keime-Guibert F, Reñé R, Cartalat-Carel S, Delattre JY, et al. Spectrum of paraneoplastic neurologic disorders in women with breast and gynecologic cancer. Med (Baltim). 2003;82(3):216–23. [DOI] [PubMed] [Google Scholar]
- 10.Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58(1):96–107. [DOI] [PubMed] [Google Scholar]
- 11.Kumari VA, Gupta P, Srivastava MV, Kumar L, Kriplani A, Bhatla N. Paraneoplastic cerebellar degeneration as the first evidence of malignancy: a case report. J Obstet Gynaecol Res. 2014;40(5):1463–5. [DOI] [PubMed] [Google Scholar]
- 12.Le May M, Dent S. Anti-yo antibody-mediated paraneoplastic cerebellar degeneration associated with cognitive affective syndrome in a patient with breast cancer: a case report and literature review. Curr Oncol. 2018;25(6):e585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, et al. Consensus Paper: Neuroimmune mechanisms of Cerebellar Ataxias. Cerebellum. 2016;15(2):213–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan HZ, Ren HT, Peng B, Ni J, Li LB, Lu Q, et al. Anti-yo antibody-associated paraneoplastic cerebellar degeneration: a report of 6 patients. Chin J Neurol. 2018;48(2):89–93. [Google Scholar]
- 15.Ono Y, Tadokoro K, Yunoki T, Yamashita T, Sato D, Sato H, et al. Anti-IgLON5 disease as a differential diagnosis of multiple system atrophy. Parkinsonism Relat Disord. 2024;124:106992. [DOI] [PubMed] [Google Scholar]
- 16.Macher S, Zimprich F, De Simoni D, Höftberger R, Rommer PS. Management of Autoimmune Encephalitis: an Observational Monocentric Study of 38 patients. Front Immunol. 2018;9:2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macher S, Milenkovic I, Zrzavy T, Höftberger R, Seidel S, Berger-Sieczkowski E, et al. Ocular Motor abnormalities in Anti-IgLON5 Disease. Front Immunol. 2021;12:753856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.