Abstract
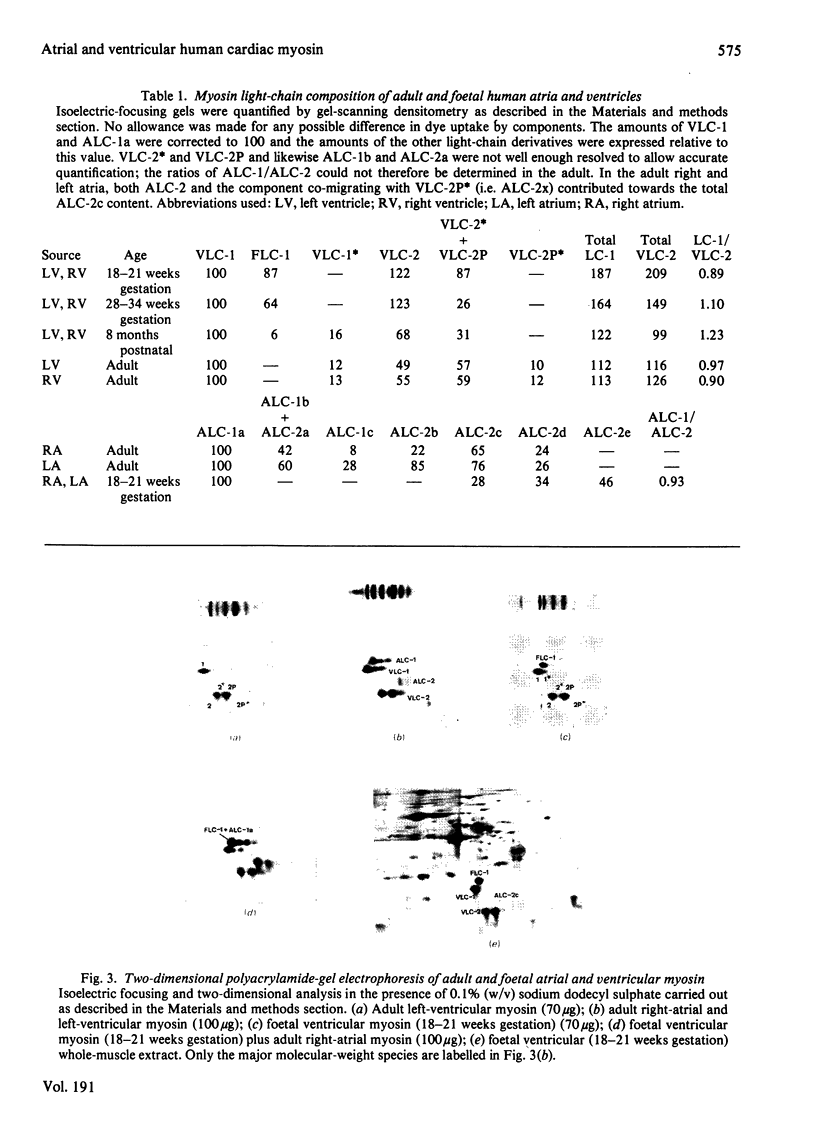
1. Myosin was isolated from human right- and left-atrial and -ventricular myocardium, and examined both in adult subjects and at different stages during pre- and post-natal development. 2. The myosin light-chain subunits in the atria and ventricles were different when characterized by isoelectric focusing and subsequent two-dimensional poly-acrylamide-gel electrophoresis. 3. No differences were observed between the light-chain subunits in the right and left ventricle at any stage of development. 4. The foetal ventricle contained a characteristic light chain that was a major component throughout the latter half of gestation. This foetal light chain, which disappeared in the postnatal period, could not be distinguished from adult atrial light chain 1 on two-dimensional electrophoresis. 5. Myosin in the adult atria, particularly the left, contained components similar to ventricular light-chain components. 6. The possible stimuli for the observed changes in myosin light-chain expression are discussed in relation to the known physiological changes occurring during development.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awan M. Z., Frearson N., Goldspink G., Waterson S. E. Biochemical efficiency of smooth muscle and different types of striated muscle. J Mechanochem Cell Motil. 1972 Dec;1(4):225–232. [PubMed] [Google Scholar]
- Buller A. J., Mommaerts W. F., Seraydarian K. Enzymic properties of myosin in fast and slow twitch muscles of the cat following cross-innervation. J Physiol. 1969 Dec;205(3):581–597. doi: 10.1113/jphysiol.1969.sp008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Close R. I. The transformation of myosin in cross-innervated rat muscles. J Physiol. 1971 Mar;213(2):455–474. doi: 10.1113/jphysiol.1971.sp009393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska R., Sosiński J., Drabikowski W. Changes in the composition of the myofibrillar fraction during development of the rabbit. FEBS Lett. 1977 Jul 15;79(2):295–300. doi: 10.1016/0014-5793(77)80806-5. [DOI] [PubMed] [Google Scholar]
- Diacono J. Suggestive evidence for the activation of an electrogenic sodium pump in stimulated rat atria: apparent discrepancy between the pump inhibition and the positive inotropic response induced by ouabain. J Mol Cell Cardiol. 1979 Jan;11(1):5–30. doi: 10.1016/0022-2828(79)90449-8. [DOI] [PubMed] [Google Scholar]
- Flink I. L., Rader J. H., Banerjee S. K., Morkin E. Atrial and ventricular cardiac myosins contain different heavy chain species. FEBS Lett. 1978 Oct 1;94(1):125–130. doi: 10.1016/0014-5793(78)80921-1. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Hobbs A. W. Fast and slow myosin in developing muscle fibres. Nature. 1978 Jul 6;274(5666):25–29. doi: 10.1038/274025a0. [DOI] [PubMed] [Google Scholar]
- HOLTZER H., MARSHALL J. M., Jr, FINCK H. An analysis of myogenesis by the use of fluorescent antimyosin. J Biophys Biochem Cytol. 1957 Sep 25;3(5):705–724. doi: 10.1083/jcb.3.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh J. F., Egerton L. J. Action of triiodothyronine on the synthesis of rat ventricular myosin isoenzymes. FEBS Lett. 1979 May 1;101(1):143–148. doi: 10.1016/0014-5793(79)81313-7. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., Yeoh G. P. Rabbit skeletal myosin isoenzymes from fetal, fast-twitch and slow-twitch muscles. Nature. 1979 Jul 26;280(5720):321–323. doi: 10.1038/280321a0. [DOI] [PubMed] [Google Scholar]
- Hoh J. Y., McGrath P. A., White R. I. Electrophoretic analysis of multiple forms of myosin in fast-twitch and slow-twitch muscles of the chick. Biochem J. 1976 Jul 1;157(1):87–95. doi: 10.1042/bj1570087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar G. Developmental changes of the primary structure and histidine methylation in rabbit skeletal muscle myosin. Nat New Biol. 1972 Dec 27;240(104):260–264. doi: 10.1038/newbio240260a0. [DOI] [PubMed] [Google Scholar]
- Libera L. D., Sartore S., Schiaffino S. Comparative analysis of chicken atrial and ventricular myosins. Biochim Biophys Acta. 1979 Dec 14;581(2):283–294. doi: 10.1016/0005-2795(79)90248-4. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- Long L., Fabian F., Mason D. T., Wikman-Coffelt J. A new cardiac myosin characterized from the canine atria. Biochem Biophys Res Commun. 1977 Jun 6;76(3):626–635. doi: 10.1016/0006-291x(77)91547-9. [DOI] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Malhotra A., Bhan A., Scheuer J. Biochemical characteristics of human cardiac myosin. J Mol Cell Cardiol. 1977 Jan;9(1):73–80. doi: 10.1016/0022-2828(77)90026-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelloni-Müller G., Ermini M., Jenny E. Changes in myosin light and heavy chain stoichiometry during development of rabbit fast, slow, and cardiac muscle. FEBS Lett. 1976 Nov;70(1):113–117. doi: 10.1016/0014-5793(76)80738-7. [DOI] [PubMed] [Google Scholar]
- Pelloni-muller G., Ermini M., Jenny E. Myosin light chains of developing fast and slow rabbit skeletal muscle. FEBS Lett. 1976 Aug 1;67(1):68–74. doi: 10.1016/0014-5793(76)80872-1. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein N. A., Pepe F. A., Holtzer H. Myosin types during the development of embryonic chicken fast and slow muscles. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4524–4527. doi: 10.1073/pnas.74.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S., Pierobon-Bormioli S., Schiaffino S. Immunohistochemical evidence for myosin polymorphism in the chicken heart. Nature. 1978 Jul 6;274(5666):82–83. doi: 10.1038/274082a0. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969 Dec;115(5):993–1004. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemankowski R. F., Dreizen P. Canine cardiac myosin with special referrence to pressure overload cardiac hypertrophy. I. Subunit composition. J Biol Chem. 1978 Dec 10;253(23):8648–8658. [PubMed] [Google Scholar]
- Sreter F. A., Elzinga M., Mabuchi K. The Ntau-methylhistidine content of myosin in stimulated and cross-reinnervated skeletal muscles of the rabbit. FEBS Lett. 1975 Sep 1;57(1):107–111. doi: 10.1016/0014-5793(75)80163-3. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Syrovy I., Delcayre C., Swynghedauw B. Comparison of ATPase activity and light subunits in myosins from left and right ventricles and atria in seven mammalian species. J Mol Cell Cardiol. 1979 Oct;11(10):1129–1135. doi: 10.1016/0022-2828(79)90398-5. [DOI] [PubMed] [Google Scholar]
- Syrový I., Gutmann E. Differentiation of myosin in soleus and extensor digitorum longus muscle in differnt animal species during development. Pflugers Arch. 1977 May 6;369(1):85–89. doi: 10.1007/BF00580815. [DOI] [PubMed] [Google Scholar]
- Trayer I. P., Harris C. I., Perry S. V. 3-Methyl histidine and adult and foetal forms of skeletal muscle myosin. Nature. 1968 Feb 3;217(5127):452–453. doi: 10.1038/217452a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Identification of a novel form of myosin light chain present in embryonic muscle tissue and cultured muscle cells. J Mol Biol. 1978 Dec 15;126(3):415–431. doi: 10.1016/0022-2836(78)90049-9. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Schwartz K., Bouveret P., Sell S. M., Gros F. Contractile protein isozymes in muscle development: identification of an embryonic form of myosin heavy chain. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5197–5201. doi: 10.1073/pnas.76.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman-Coffelt J., Fenner C., Smith A., Mason D. T. Comparative analyses of the kinetics and subunits of myosins from canine skeletal muscle and cardiac tissue. J Biol Chem. 1975 Feb 25;250(4):1257–1262. [PubMed] [Google Scholar]
- Wikman-Coffelt J., Zelis R., Fenner C., Mason D. T. Comparative purification of myocardial myosin and antigenic specificity of the two light chains. Prep Biochem. 1973;3(5):439–449. doi: 10.1080/00327487308061528. [DOI] [PubMed] [Google Scholar]