Abstract
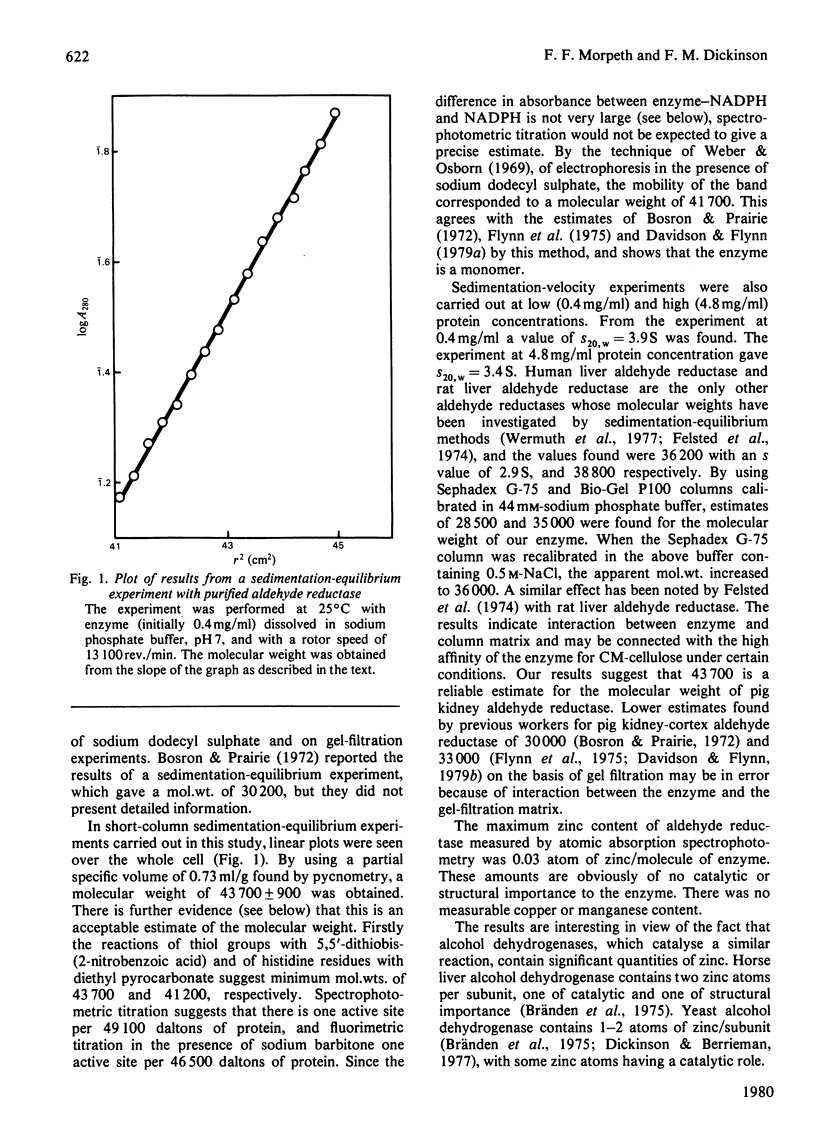
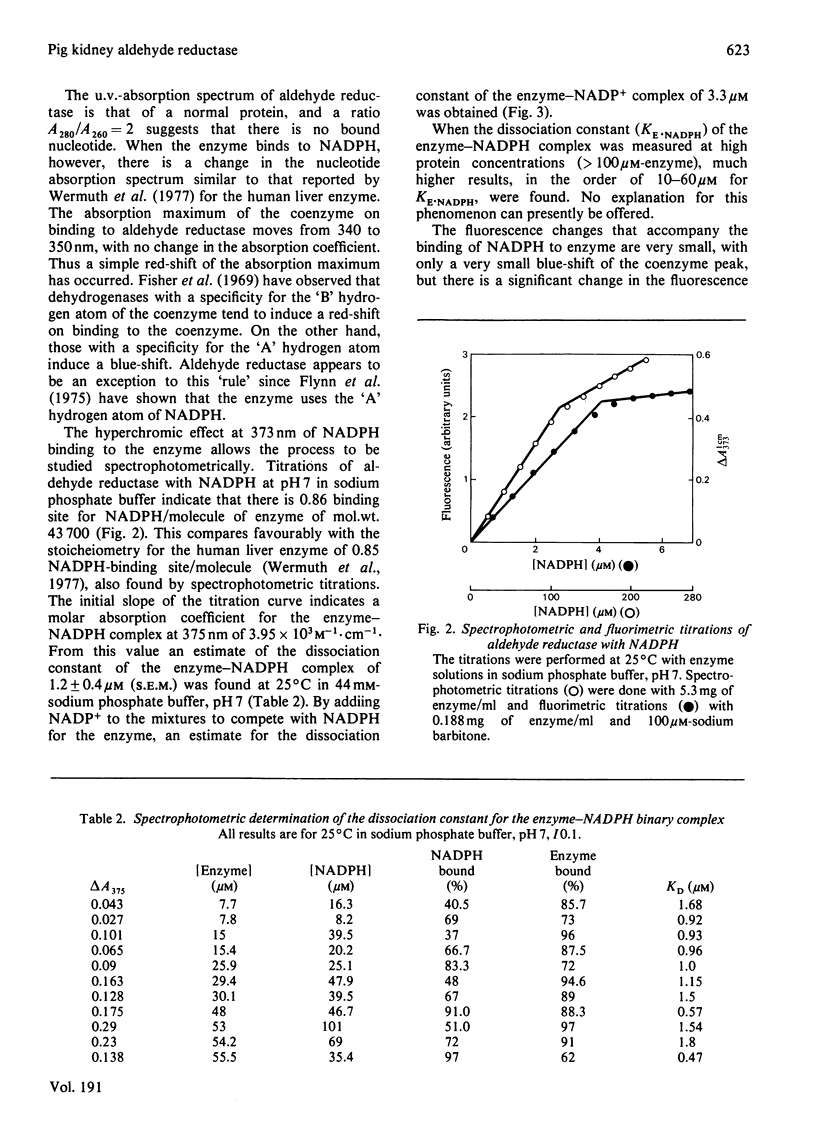
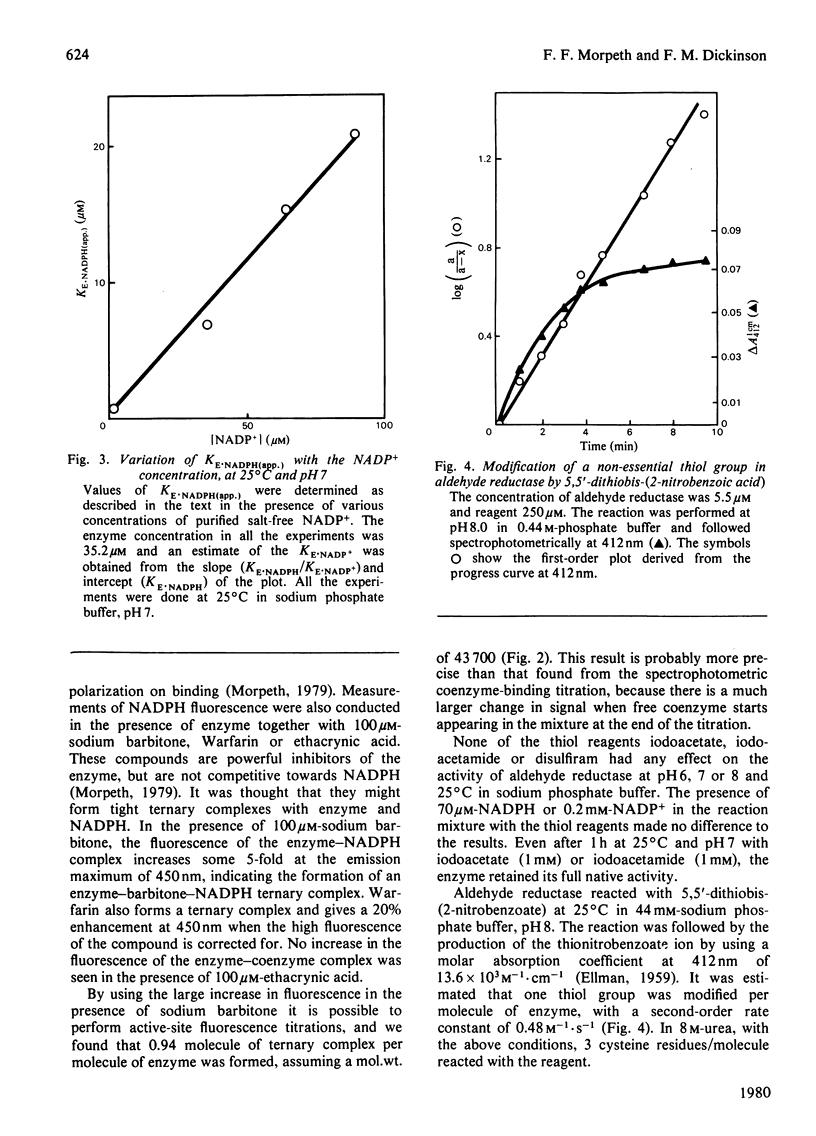
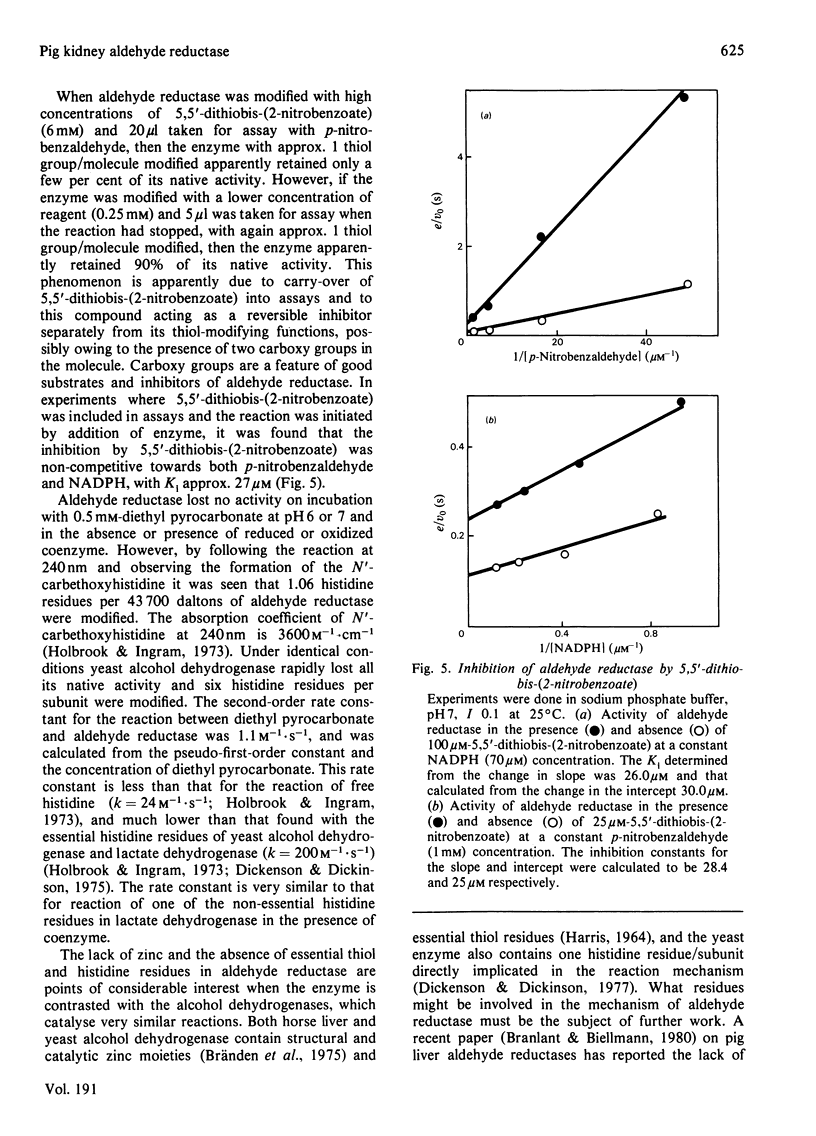
Aldehyde reductase was purified from pig kidney cortex to homogeneity by a new procedure. The molecular weight of the enzyme was estimated by sedimentation equilibrium to be 43 700 and by gel electrophoresis in the presence of sodium dodecyl sulphate to be 41 700. The enzyme is clearly a monomer. The enzyme preparation contained no significant quantities of zinc, manganese or copper and had no essential histidine or thiol groups. Changes in the absorption and fluorescence spectra of NADPH were observed on formation of the enzyme-NADPH complexes. Large changes in the fluorescence spectra were also observed in the presence of sodium barbitone or Warfarin. These changes were used as the basis of active-site titrations, which showed that the enzyme had one active site per molecule. The dissociation constants of NADPH and NADP+ from binary complexes with the enzyme were estimated in spectrophotometric titrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosron W. F., Prairie R. L. Triphosphopyridine nucleotide-linked aldehyde reductase. I. Purification and properties of the enzyme from pig kidney cortex. J Biol Chem. 1972 Jul 25;247(14):4480–4485. [PubMed] [Google Scholar]
- Branlant G., Biellmann J. F. Purification and some properties of aldehyde reductases from pig liver. Eur J Biochem. 1980 Apr;105(3):611–621. doi: 10.1111/j.1432-1033.1980.tb04539.x. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Davidson W. S., Flynn T. G. A functional arginine residue in NADPH-dependent aldehyde reductase from pig kidney. J Biol Chem. 1979 May 25;254(10):3724–3729. [PubMed] [Google Scholar]
- Davidson W. S., Flynn T. G. Kinetics and mechanism of action of aldehyde reductase from pig kidney. Biochem J. 1979 Feb 1;177(2):595–601. doi: 10.1042/bj1770595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. A study of the ionic properties of the essential histidine residue of yeast alcohol dehydrogenase in complexes of the enzyme with its coenzymes and substrates. Biochem J. 1977 Jan 1;161(1):73–82. doi: 10.1042/bj1610073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson C. J., Dickinson F. M. The role of an essential histidine residue of yeast alcohol dehydrogenase. Eur J Biochem. 1975 Apr 1;52(3):595–603. doi: 10.1111/j.1432-1033.1975.tb04031.x. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Berrieman S. The reactions of 1,10-phenanthroline with yeast alcohol dehydrogenase. Biochem J. 1977 Oct 1;167(1):237–244. doi: 10.1042/bj1670237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Berrieman S. The separation of sheep liver cytoplasmic and mitochondrial aldehyde dehydrogenases by isoelectric focusing, and observations on the purity of preparations of the cytoplasmic enzyme, and their sensitivity towards inhibition by disulfiram. Biochem J. 1979 Jun 1;179(3):709–712. doi: 10.1042/bj1790709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson F. M., Engel P. C. The preparation of pure salt-free nicotinamide coenzymes. Anal Biochem. 1977 Oct;82(2):523–531. doi: 10.1016/0003-2697(77)90191-9. [DOI] [PubMed] [Google Scholar]
- Dickinson F. M., Monger G. P. A study of the kinetics and mechanism of yeast alcohol dehydrogenase with a variety of substrates. Biochem J. 1973 Feb;131(2):261–270. doi: 10.1042/bj1310261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Felsted R. L., Gee M., Bachur N. R. Rat liver daunorubicin reductase. An aldo-keto reductase. J Biol Chem. 1974 Jun 25;249(12):3672–3679. [PubMed] [Google Scholar]
- Fisher H. F., Adija D. L., Cross D. G. Dehydrogenae-reduced coenzyme difference spectra, their resolution and relationship to the stereospecificity of hydrogen transfer. Biochemistry. 1969 Nov;8(11):4424–4431. doi: 10.1021/bi00839a030. [DOI] [PubMed] [Google Scholar]
- Flynn T. G., Shires J., Walton D. J. Properties of the nicotinamide adenine dinucleotide phosphate-dependent aldehyde reductase from pig kidney. Amino acid composition, reactivity of cysteinyl residues, and stereochemistry of D-glyceraldehyde reduction. J Biol Chem. 1975 Apr 25;250(8):2933–2940. [PubMed] [Google Scholar]
- HARRIS I. STRUCTURE AND CATALYTIC ACTIVITY OF ALCOHOL DEHYDROGENASES. Nature. 1964 Jul 4;203:30–34. doi: 10.1038/203030a0. [DOI] [PubMed] [Google Scholar]
- Kawalek J. C., Gilbertson J. R. Partial purification of the NADPH-dependent aldehyde reductase from bovine cardiac muscle. Arch Biochem Biophys. 1976 Apr;173(2):649–657. doi: 10.1016/0003-9861(76)90302-7. [DOI] [PubMed] [Google Scholar]
- Macfarlane N., Mathews B., Dalziel K. The purification and properties of NADP-dependent isocitrate dehydrogenase from ox-heart mitochondria. Eur J Biochem. 1977 Apr 15;74(3):553–559. doi: 10.1111/j.1432-1033.1977.tb11424.x. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Touster Resolution and partial characterization of two aldehyde reductases of mammalian liver. J Biol Chem. 1977 Apr 25;252(8):2545–2550. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wermuth B., Münch J. D., von Wartburg J. P. Purification and properties of NADPH-dependent aldehyde reductase from human liver. J Biol Chem. 1977 Jun 10;252(11):3821–3828. [PubMed] [Google Scholar]
- Whittle S. R., Turner A. J. Effects of the anticonvulsant sodium valproate on gamma-aminobutyrate and aldehyde metabolism in ox brain. J Neurochem. 1978 Dec;31(6):1453–1459. doi: 10.1111/j.1471-4159.1978.tb06572.x. [DOI] [PubMed] [Google Scholar]


