Abstract
Background
Septic arthritis is a rare but devastating complication after anterior cruciate ligament reconstruction (ACLR). While early treatment can prevent significant graft complications, outcomes are often inferior to those in uncomplicated ACLR. Furthermore, whether to retain or remove the graft after infection remains debatable. Therefore, we sought to compare the outcomes of septic arthritis post ACLR with uncomplicated ACLR and evaluate graft retention versus removal in infected patients.
Methods
We conducted a systematic review and meta-analysis in which PubMed, Embase, and Cochrane Library databases were searched. Clinical studies were included if they compared patient-reported, clinician-reported, or radiographic outcomes (minimum follow-up of 12 months) between patients with post-ACLR septic arthritis and those with uncomplicated ACLR or that compared graft retention and removal in patients with post-ACLR septic arthritis.
Results
Thirteen studies were retrieved. Patients with post-ACLR septic arthritis reported inferior Lysholm Knee Scoring Scale scores (mean difference (MD) 7.53; 95% confidence interval (CI) 3.20–11.86; P = 0.0006), Tegner Activity Scale scores (MD, 1.42; 95% CI 1.07–1.76; P < .00001), and return to sports rates (53% versus 76%, respectively) to those of patients with uncomplicated ACLR. Patients with post-ACLR septic arthritis and those with uncomplicated ACLR did not differ in terms of the pooled estimate of various clinician-reported outcomes, such as the objective International Knee Documentation Committee score, anterior–posterior laxity, pivot shift, and Lachman test results. Furthermore, no significant difference was noted between the aforementioned patient groups regarding osteoarthritis (detected radiographically). Graft retention led to better patient- and clinician-reported outcomes than graft removal.
Conclusions
Despite similar clinician-reported outcomes and osteoarthritis rates, patients with post-ACLR septic arthritis reported worse outcomes than those with uncomplicated ACLR. Graft retention leads to improved patient- and clinician-reported outcomes compared with the outcomes of graft removal. Our findings may help develop realistic expectations and management strategies for this rare complication.
Supplementary Information
The online version contains supplementary material available at 10.1186/s43019-024-00248-z.
Keywords: Infection, Septic arthritis, Anterior cruciate ligament reconstruction, Patient-reported outcome, Clinician-reported outcome, Osteoarthritis, Graft retention, Graft removal
Introduction
Septic arthritis after anterior cruciate ligament (ACL) reconstruction (ACLR) is a rare but devastating complication, with an incidence rate of 0.14–1.8% [1]. Although prompt infection control can improve functional outcomes without graft laxity or retears, the outcomes of post-ACLR septic arthritis are often inferior to those of uncomplicated ACLR [2]. Poor knee function may substantially affect patients, preventing them from attaining their goals and ultimately leading to dissatisfaction.
Current management strategies involving early surgical debridement and concomitant intravenous antibiotic therapy minimize the severity of inflammation, thus preventing articular cartilage degradation [3–5]. However, whether successful infection eradication and graft recovery translates into clinical, patient-reported, and radiographic outcomes similar to those of patients with uncomplicated ACLRs at mid- to long-term follow-up remains debatable. Although some studies have reported inferior subjective and objective outcomes, such as functional knee scores, sports and activity levels, joint laxity, and radiographic osteoarthritis [6–9], others have reported similar outcomes [10]. Furthermore, outcomes may vary depending on graft retention or removal. Graft removal minimizes the risk of persistent infection, but ACL deficiency may increase the risk of additional meniscal and cartilage damage. By contrast, graft retention provides adequate stability but may lead to further damage and instability with persistent infection [11].
Very few studies have comprehensively compared post-ACLR septic arthritis and uncomplicated ACLR in terms of their outcomes. Small sample sizes limit the power of most studies, resulting in unreliable findings. As most affected patients are young and active athletes, understanding the prognosis of post-ACLR infection in this population is crucial. Because of the rarity of post-ACLR septic arthritis, clinical data may be required to optimize the clinical management and counseling of affected patients. Therefore, we conducted this systematic review and meta-analysis of the clinical, functional, and radiographic outcomes of septic arthritis developing at least 12 months after ACLR. Our objective was to examine whether there were differences in outcomes between patients with post-ACLR septic arthritis and those without complications. Additionally, we sought to determine whether outcomes varied between patients who retained their graft and those who required graft removal due to post-ACLR septic arthritis. We hypothesized that patients developing septic arthritis following ACLR would have both subjective and objective outcomes that were inferior compared with those without complications. Furthermore, we anticipated that graft removal in post-ACLR septic arthritis would result in worse outcomes.
Methods
Study design
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. This study was registered in the PROSPERO online public database (CRD42023390990).
Search strategy
To identify relevant studies, we searched the PubMed, Embase, and Cochrane Library databases from the inception of the databases up to January 2023. We used the following broad search terms: anterior cruciate ligament reconstruction AND (septic arthritis OR infection). Search terms were mapped to Medical Subject Headings terms where possible. All relevant references were checked for additional and unpublished citations. Afterward, all articles were combined into a single list, and duplicates were removed.
Selection criteria
We included studies comparing patients with post-ACLR septic arthritis with those without it in terms of outcomes. In addition, we included studies comparing the outcomes of graft retention with those of graft removal in patients with post-ACLR septic arthritis. Other inclusion criteria were as follows: availability of age and sex data of patients with post-ACLR septic arthritis and those with uncomplicated ACLR; follow-up period of at least 12 months, comparison of at least one outcome of interest between patients with post-ACLR septic arthritis and those with uncomplicated ACLR or between graft retention and graft removal in patients with post-ACLR septic arthritis, availability of data regarding treatment protocols for patients with post-ACLR septic arthritis, and publication in English-language peer-reviewed journals. The articles included in this review study met all of the aforementioned criteria. No restrictions were imposed for index ACLR type (primary or revision), graft choice, participant matching method, or cartilage or meniscus treatment method.
We reviewed the abstracts and excluded animal studies, commentaries or opinion pieces, review articles reporting data presented in already identified articles, and articles presenting primary data duplicated in another included article. In the case of duplicate data, we selected the articles with the most complete baseline information concerning the post-ACLR septic arthritis and uncomplicated ACLR groups and the graft retention and removal groups. After exclusion, a second reviewer reviewed the remaining studies for subsequent meta-analysis.
Data extraction
Data regarding patient characteristics, such as age, sex, and follow-up duration, were extracted to obtain an overview of the population. The diagnostic criteria for post-ACLR septic arthritis used in each study were also extracted. Surgical data, such as ACLR type (primary or revision), graft used for the index ACLR, prior knee procedures, and concomitant meniscal and cartilage surgery, were extracted (if reported) to compare the septic arthritis and uncomplicated ACLR groups. In addition, information regarding the management of septic arthritis was extracted. To compare the graft retention and removal groups, data regarding infection management, including time to presentation, Gächter stage, total number of irrigation and debridement (I&D), and graft reimplantation, were extracted (if reported). Two reviewers worked independently: one extracted the relevant data from the included studies, and another verified the extracted data. Any discrepancies between the two reviewers were resolved through consensus or by a third reviewer.
Methodological quality appraisal
We judged the quality of the included studies by assessing various aspects of study design that would likely introduce bias, such as variables prone to measurement bias, insufficient adjustment for confounding factors, and loss to follow-up for observational studies. To compare the septic arthritis and uncomplicated ACLR groups, we evaluated pre-exposure, at-exposure, post-exposure, and overall biases by using the Risk of Bias in Nonrandomized Studies of Exposures (ROBINS-E) tool [13]. To compare the graft retention and removal groups, we evaluated pre-intervention, at-intervention, post-intervention, and overall biases using the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool [14].
Outcomes
The main outcome measures were patient-reported outcomes, clinician-reported outcomes, and osteoarthritis risk. Patient-reported outcomes included Lysholm Knee Scoring Scale score, Tegner Activity Scale score, Knee Injury and Osteoarthritis Outcome Score (KOOS), subjective International Knee Documentation Committee (IKDC) score, and return to sports rate. Clinician-reported outcomes included objective IKDC score, KT-1000 score, pivot shift test result, and Lachman test result; the objective IKDC results were analyzed in terms of the number of knees classified as abnormal/severely abnormal (IKDC category C or D), and the pivot shift and Lachman test results were analyzed in terms of the number of patients with a grade of at least 1. Osteoarthritis was assessed on the basis of radiographic grading, and osteoarthritis was classified according to the grading used in each article. Data regarding the number of patients in each group with radiographic evidence of osteoarthritis were obtained.
Statistical analysis
Outcomes were pooled for meta-analysis by using RevMan (version 5.4.0) [14]. Continuous variables are presented as mean ± standard deviation (SD) values. If SD values were not reported, we contacted the corresponding authors and requested the statistical data. When authors could not be contacted, we calculated SD values using the available data according to a previously reported validated formula [15]. The mean difference (MD) and 95% confidence interval (CI) values were calculated for dichotomous variables. A random-effects model (DerSimonian and Laird) was used to compute the pooled estimates [16]. Cochrane Q tests and I2 statistics were used to evaluate the statistical heterogeneity and inconsistency among the effects of the included studies, respectively. Statistical significance was set at P < 0.05 for the Cochrane Q tests. Statistical heterogeneity was assessed using the I2 test, with I2 quantifying the proportion of the total outcome variability attributable to the variability among the studies. In addition, subgroup analyses were performed by pooling the estimates for similar patient subsets among studies, as appropriate.
Results
Included studies
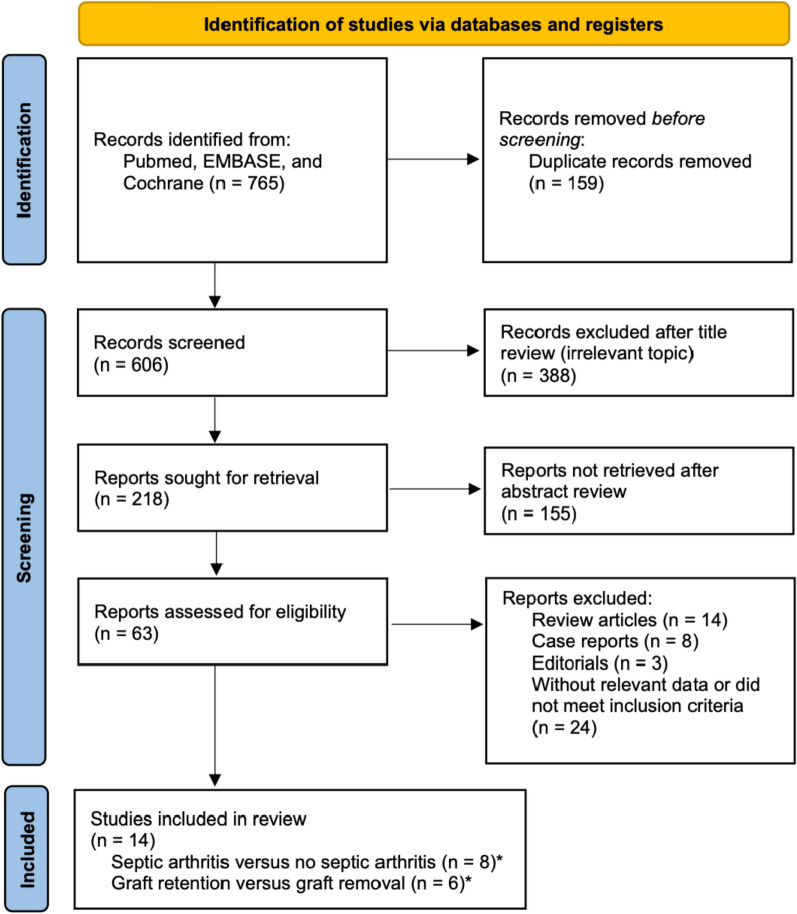
The searches yielded 2425 entries. After removing duplicates and excluding irrelevant articles, 183 were independently reviewed by two reviewers. Finally, 13 studies met the inclusion criteria and were selected for analysis (Fig. 1). Most of the studies (10 out of 13) reported the criteria of septic arthritis diagnosis, which included a combination of history, physical examination, and synovial fluid cultures (Supplementary Table 1).
Fig. 1.
PRISMA flowchart for article selection. *One study was included in both subgroups
Results of risk of bias assessment
The quality of evidence varied between the outcomes. High-quality evidence was rare (Supplementary Tables 2 and 3). Most studies were uncontrolled cohort studies or case series. If a single study was published in several outlets, we analyzed only the study with the most complete dataset to avoid duplication. Studies were divided into two categories, each corresponding to a research question of the present meta-analysis.
Demographic characteristics
Post-ACLR septic arthritis versus uncomplicated ACLR
Eight studies reported the clinical, functional, or radiographic outcomes of post-ACLR septic arthritis versus uncomplicated ACLR (Table 1) [7, 17–23]. A total of 6773 patients (septic arthritis group, 129; uncomplicated ACLR group, 6644) were included in this study. Patient demographics were generally similar between the septic arthritis and uncomplicated ACLR groups. The mean age was 29.0 ± 8.4 and 28.5 ± 10.8 years in the septic arthritis and uncomplicated ACLR groups, respectively (P = 0.59), and 72.1% and 71.5% of the respective groups were men (P = 0.44). The percentage of primary ACLR was also similar between the two groups (93.8% versus 94.2%; P = 0.43). However, the percentage of prior knee procedure (32.0% versus 7.3%; P < 0.05) and concomitant surgery (28.6% versus 46.7%; P < 0.05) was different between the two groups.
Table 1.
Characteristics of studies comparing patients with post-ACLR septic arthritis and those without it
| No. | Study [year] | Study design Country |
LOE | Study characteristics | No. of patients | Age (years), mean ± SD | Male, n (%) | Primary ACLR, n (%) | Graft type, n (%) | Prior knee procedure, n (%) | Concomitant surgery, n (%) | Follow-up period (months), mean ± SD (range) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPTB | Hamstring | Allograft | ||||||||||||
| 1 | Abdel-Aziz [17] |
Prospective Egypt |
II | Patients with ACLR with hamstring autograft; 2004–2011; CU |
S: 24 N: 24 |
S: 26 ± 5 N: 27 ± 4 |
S: 24 (100) N: 24 (100) |
NR |
S: 0 (0) N: 0 (0) |
S: 24 (100) N: 24 (100) |
S: 0 (0) N: 0 (0) |
S: 4 (17) N: NR |
NR |
S: 59 ± 21 (18–96) N: 55 ± 22 (18–96) |
| 2 | Bohu [18] |
Prospective France |
III | Patients with ACLR; 2012–2016; CdS |
S: 7 N: 1802 |
S: 36.4 ± 13.7 N: 29.1 ± 9.7 |
S: 6 (86) N: 1626 (90) |
S: 5 (71) N: 1627 (90) |
S: 2 (29) N: 172 (0.1) |
S: 5 (71) N: 1520 (84) |
S: 0 (0) N: 0 (0) |
S: 4 (57) N: 210 (12) |
694 (38)b 61 (3)c 376 (21)d 355 (20)e |
33.6 ± 14.4 (18.0–57.6) |
| 3 | Boström Windhamre [19] |
Retrospective Sweden |
III | Patients with primary ACLR; 2001–2009; CAC with complete rehabilitation |
S: 27 N: 27 |
S: 27 (16–43)* N: 28 (14–43)* |
S: 13 (48) N: 13 (48) |
S: 27 (100) N: 27 (100) |
S: 0 (0) N: 0 (0) |
S: 27 (100) N: 27 (100) |
S: 0 (0) N: 0 (0) |
S: 11 (41) N: 12 (44) |
S: 10 (37)f N: 9 (33)f |
S: 60 (13–108)* N: 66 (16–114)* |
| 4 | Brophy [20] |
Prospective United States |
IV | Patients with ACLR; 2002–2008; MOON |
S: 21 N: 3189 |
S: 25.8 ± 11.3 N: 26.6 ± 11.0 |
S: 12 (57) N: 1778 (56) |
S: 18 (86) N: 2982 (94) |
S: 4 (19) N: 1401 (44) |
S: 12 (57) N: 1093 (34) |
S: 5 (24) N: 695 (22) |
NR | NR |
S: 78 ± 3.6 N: 78 ± 4.8 |
| 5 | Calvo [21] |
Retrospective Chile |
IV | Patients with primary ACLR with hamstring autograft; 2000–2011; CA |
S: 7 N: 1557 |
S: 27.8 (14–51)* N: 28.3 (14–55)* |
S: 7 (100) N: 1281 (82) |
S: 7 (100) N: 1557 (100) |
S: 0 (0) N: 0 (0) |
S: 7 (100) N: 1557 (100) |
S: 0 (0) N: 0 (0) |
S: 0 (0) N: 20 (1) |
S: 3 (42)f N: 745 (48)a,f,g |
18–108† |
| 6 | Meglic [22] |
Prospective Slovenia |
II | Patients with primary ACLR; 2004–2014; UMCL with complete rehabilitation |
S: 18 N: 20 |
S: 31 ± 7 N: 33 ± 6 |
S: 11 (61) N: 12 (60) |
S: 18 (100) N: 20 (100) |
S: 11 (61) N: 12 (60) |
S: 7 (39) N: 8 (40) |
S: 0 (0) N: 0 (0) |
NR |
S: 2 (11)a N: 2 (10)a |
S: 48 ± 4 (42–56) N: 48 ± 4 (42–56) |
| 7 | Schollin-Borg [7] | Retrospective, Sweden | III | Patients with ACLR; 1996–1999; UUH |
S: 10 N: 10 |
S: 28.3 ± 5.5 N: 29.1 ± 5.7 |
S: 8 (80) N: 8 (80) |
NR |
S: 6 (60) N: 6 (60) |
S: 4 (40) N: 3 (30) |
S: 0 (0) N: 0 (0) |
S: 5 (50) N: 7 (70) |
S: 4 (40)f,g N: 2 (20)f |
36** |
| 8 | Torres-Claramunt [23] |
Retrospective, Spain |
IV | Patients with ACLR; 2006–2009; PdSM and ICATME with rehabilitation |
S: 15 N: 15 |
S: 33.5 ± 7.6 N: 34.7 ± 7.6 |
S: 12 (76) N: 10 (67) |
NR |
S: 4 (27) N: 5 (33) |
S: 11 (73) N: 10 (67) |
S: 0 (0) N: 0 (0) |
NR |
S: 3 (20)f N: NR |
S: 39.3 ± 13 N: 42.6 ± 7.5 |
*Mean (range), †Range, and **Mean
aMeniscal repair
bExtra-articular tenodesis (tensor fasciae latae)
cChondroplasty
dPartial medial meniscectomy and suturing
ePartial lateral meniscectomy and suturing
fMeniscectomy
gMeniscus microfracture
ACLR anterior cruciate ligament reconstruction, BPTB bone–patellar tendon–bone graft, CA Clinica Alemana, CAC Capio Artro Clinic, CdS Clinique du Sport, CU Cairo University, ICATME ICATME-Institut Universitari Dexeus, LOE level of evidence, MOON Multicenter Orthopaedic Outcomes Network knee group, N patients without post-ACLR septic arthritis, NR not reported, PdSM Parc de Salut Mar, S patients with septic arthritis, SD standard deviation, UMCL University Medical Centre Ljublijana, UUH Uppsala University Hospital
Graft retention versus graft removal in patients with post-ACLR septic arthritis
Six studies compared clinical or functional outcomes between the graft retention and removal groups (Supplementary Table 3) [1, 8, 11, 21, 24, 25]. After the removal of duplicates, 91 patients (graft retention group, 56; graft removal, 35) were included in this study. In general, patient demographics were similar between the two groups. The mean age was 28.8 ± 7.8 and 29.3 ± 9.7 years in the graft retention and removal groups, respectively (P = 0.80), and 89.6% and 78.3% of the respective groups were men (P = 0.20). The percentage of index primary ACLR (83.7% versus 76.2%; P = 0.46) and hamstring autograft were also similar between the two groups (69.6% versus 62.9%; P = 0.50). The mean follow-up period was 45.89 months.
Septic arthritis presentation and treatment protocol
The mean time to infection presentation and treatment is presented in Supplementary Table 5. In all eight studies, septic arthritis was managed with arthroscopic I&D and antibiotic treatment [7, 17–23]. The number of irrigation procedures ranged from 1 to 11. Graft retention (rate, 71–100%) was reported in all eight studies, whereas subsequent surgery (rate, 0–39%) was reported in five studies (Supplementary Table 5) [17, 18, 20, 22].
Patient-reported outcomes
Lysholm Knee Scoring Scale scores
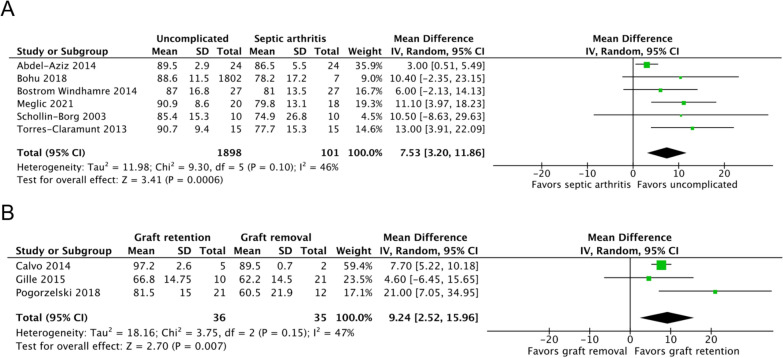
Six studies, including 1999 patients, reported the Lysholm Knee Scoring Scale scores of septic arthritis (n = 101) and uncomplicated ACLR (n = 1898) groups [7, 17–19, 22, 23]. The mean timepoint for assessment was at 35.36 months. The septic arthritis group had significantly lower scores than the uncomplicated ACLR group (MD, 7.53; 95% CI 3.20–11.86; P = 0.0006; I2, 46%; P = 0.10; Fig. 2A).
Fig. 2.
Lysholm Knee Scoring Scale scores of the (A) septic arthritis and uncomplicated ACLR groups and the (B) graft retention and removal groups. An inverse-variance random-effects model was used for meta-analysis. Mean differences are presented in terms of 95% confidence interval values
Three studies, including 71 patients, reported the Lysholm Knee Scoring Scale scores of the graft retention (n = 36) and removal (n = 35) groups [11, 21, 24]. The graft retention group reported significantly higher scores than did the graft removal group at a mean follow-up of 57.47 months (MD, 9.24; 95% CI 2.52–15.96; P = 0.007; I2, 47%; P = 0.15; Fig. 2B).
Tegner activity scale scores
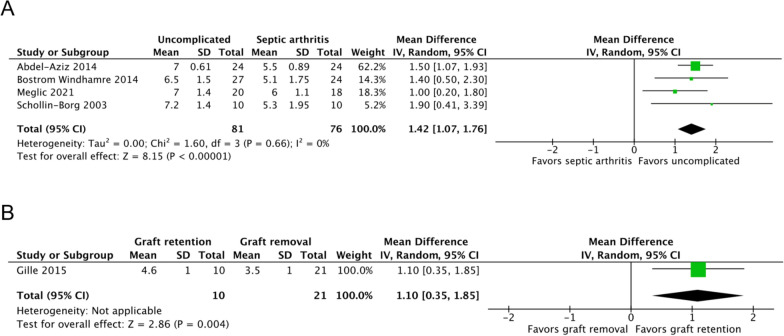
Four studies, including 157 patients, reported the Tegner Activity Scale scores of septic arthritis (n = 76) and uncomplicated ACLR (n = 81) groups [7, 17, 19, 22]. The septic arthritis group had significantly lower scores than did the uncomplicated ACLR group at a mean follow-up of 54.26 months (MD, 1.42; 95% CI 1.07–1.76; P < 0.00001; I2, 0%; P = 0.66; Fig. 3A).
Fig. 3.
Tegner Activity Scale scores of the (A) septic arthritis and uncomplicated ACLR groups and the (B) graft retention and removal groups. An inverse-variance random-effects model was used for meta-analysis. Mean differences are presented in terms of 95% confidence interval values
One study reported the Tegner Activity Scale scores of the graft retention (n = 10) and removal (n = 21) groups, respectively [24]. The graft retention group had higher scores than the graft removal group at 71 months of follow-up (MD, 1.10; 95% CI 0.35–1.85; P = 0.004).
KOOS
Five studies, including 4689 patients, reported the KOOS of septic arthritis (n = 83) and uncomplicated ACLR (n = 4606) groups [7, 17–20]. The overall KOOS varied significantly between the two groups at 62.04 months of follow-up (MD, 8.88; 95% CI 3.27–14.49; P = 0.002; I2, 96%; P ≤ 0.00001). As depicted in Supplementary Fig. 1, the pooled mean difference estimates were significant for the domains of symptoms (MD, 6.88; 95% CI 1.76–12.00; P = 0.008; I2, 61%; P = 0.05), pain (MD, 6.34; 95% CI 3.10–9.58; P = 0.0001; I2, 61%; P = 0.05), sports and recreation (MD, 9.37; 95% CI 2.11–16.64; P = 0.01; I2, 62%; P = 0.05), and quality of life (MD, 11.84; 95% CI 3.26–20.43; P = 0.007; I2, 73%; P = 0.01) at 62.14 months of follow-up. However, the results corresponding to the activities of daily living (ADL) were not significant (MD, 2.99; 95% CI −1.34–7.32; P = 0.18; I2, 77%; P = 0.004).
Subjective IKDC scores
Three studies, including 4597 patients, reported the subjective IKDC scores of septic arthritis (n = 37) and uncomplicated ACLR (4560) groups [18, 20, 23]. The septic arthritis group reported significantly lower subjective IKDC scores than did the uncomplicated group at 62.05 months of follow-up (MD, 10.45; 95% CI 2.00–18.90; P = 0.02; I2, 81%; P = 0.005; Supplementary Fig. 2A).
One study, including 33 patients, reported the subjective IKDC scores of the graft retention (n = 21) and removal (n = 12) groups [11]. The graft retention group had significantly higher scores than the graft removal group at 49.27 months of follow-up (MD, 21.00; 95% CI 7.05–34.95; P = 0.003; Supplementary Fig. 2B).
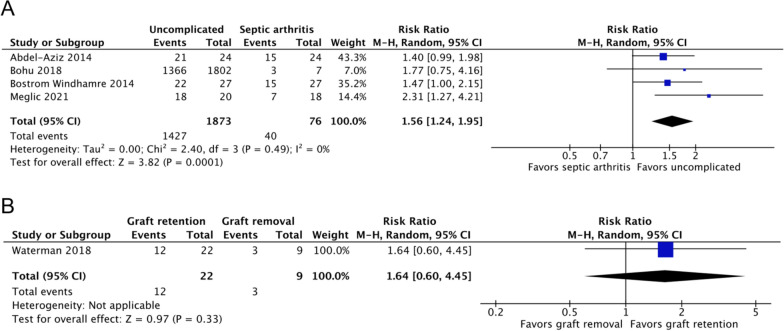
Return to sports rates
Four studies, including 1949 patients, reported the return to sports rates of septic arthritis (n = 76) and uncomplicated ACLR (n = 1873) groups [17–19, 22]. Compared with 76% of patients with uncomplicated ACLR, 53% of patients with post-ACLR septic arthritis returned to sports by the end of follow-up (risk ratio [RR], 1.56; 95% CI 1.24–1.95; P = 0.0001; I2, 0%; P = 0.49; Fig. 4A). The mean timepoint for assessment was at 35.27 months of follow-up.
Fig. 4.
Return to sports rates of the (A) septic arthritis and uncomplicated ACLR groups and the (B) graft retention and removal groups. A Mantel–Haenszel random-effects model was used for meta-analysis. Risk ratios are presented in terms of 95% confidence interval values
One study, including 31 patients, reported the return to sports rates of the graft retention (n = 22) and removal (n = 9) groups [1]. No significant difference was noted between the two groups at the mean of 29.6 months of follow-up (RR, 1.64; 95% CI 0.60–4.45; P = 0.33; Fig. 4B).
Clinician-reported outcomes
Objective IKDC scores
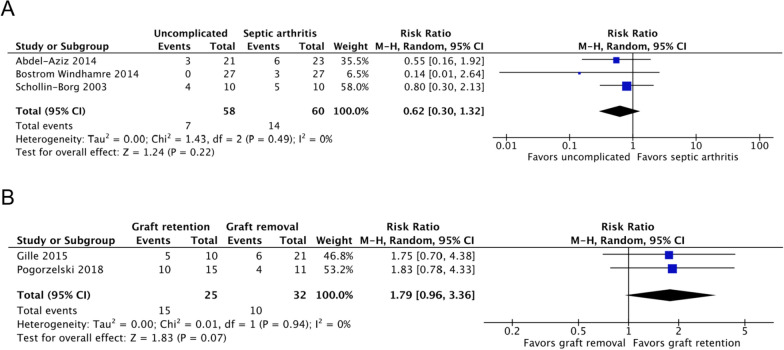
Three studies, including 118 patients, reported the objective IKDC scores of septic arthritis (n = 60) and uncomplicated ACLR (n = 58) groups [7, 17, 19]. No significant difference was observed between the two groups (RR, 0.62; 95% CI 0.30–1.32; P = 0.22; I2, 0%; P = 0.49; Fig. 5A). The mean timepoint for assessment was at 56.21 months of follow-up.
Fig. 5.
Objective IKDC scores of the (A) septic arthritis and uncomplicated ACLR groups and the (B) graft retention and removal groups. A Mantel–Haenszel random-effects model was used for meta-analysis. Risk ratios are presented in terms of 95% confidence interval values
Two studies, including 57 patients, reported the objective IKDC scores of the graft retention (n = 25) and graft removal (n = 32) groups [11, 24]. No significant difference was noted between the two groups at 57.47 months of follow-up (MD, 1.79, 95% CI 0.96–3.36; P = 0.07; I2, 0%; P = 0.94; Fig. 5B).
Anterior–posterior laxity side-to-side differences
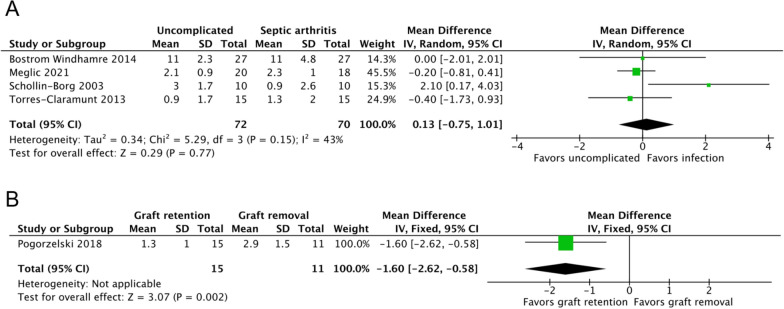
Four studies, including 142 patients, reported the mean KT-1000 scores of septic arthritis (n = 70) and uncomplicated ACLR (n = 72) groups [7, 19, 22, 23]. No significant difference was noted between the two groups (MD, 0.13; 95% CI 0.75–1.01; P = 0.77; I2, 43%; P = 0.15; Fig. 6A). The mean timepoint for assessment was at 50.52 months of follow-up.
Fig. 6.
KT-1000 scores of the (A) septic arthritis and uncomplicated ACLR groups and the (B) graft retention and removal groups. An inverse-variance random-effects model was used for meta-analysis. Mean differences are presented in terms of 95% confidence interval values
One study including 26 patients (graft retention group, 15; graft removal group, 11) reported significantly less laxity in the graft retention group compared with the graft removal group at 49.27 months of follow-up (MD, −1.60; 95% CI −2.62 to −0.58; P = 0.0002; Fig. 6B) [11].
Pivot shift and Lachman test results
Two studies, including 80 patients, reported the pivot shift and Lachman test results of septic arthritis (n = 37) and uncomplicated ACLR (n = 43) groups [17, 22]. No significant difference was observed in the risk of positive results between the two groups at the mean of 53.02 months of follow-up (pivot shift test: RR, 0.75; 95% CI 0.36–1.58; P = 0.45; I2, 0%; P = 0.83; Lachman test: RR, 0.63; 95% CI 0.24–1.62; P = 0.34; I2, 0%; P = 0.66; Supplementary Fig. 3A, B).
Radiographic osteoarthritis
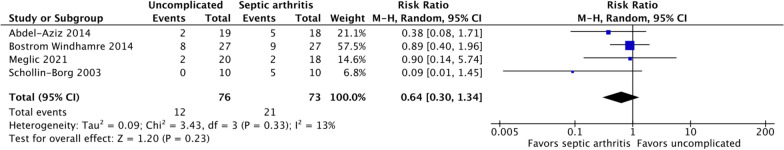
Radiographic evaluation for osteoarthritis was reported in four studies, including 149 patients (septic arthritis group, 73; uncomplicated ACLR group, 76) [7, 17, 19, 22]. Radiographic evidence of osteoarthritis was noted in 29% and 16% of all patients in septic arthritis and uncomplicated ACLR groups, respectively. No significant difference was observed in the risk of osteoarthritis between the two groups (RR, 0.64; 95% CI 0.30–1.34; P = 0.23; I2, 13%; P = 0.33; Fig. 7). The mean timepoint for radiographic assessment was 54.26 months of follow-up.
Fig. 7.
Radiographic osteoarthritis risks of the septic arthritis and uncomplicated ACLR groups. A Mantel–Haenszel random-effects model was used for meta-analysis. Risk ratios are presented in terms of 95% confidence interval values
Discussion
Our meta-analysis indicated that patients with post-ACLR septic arthritis reported poor outcomes at a follow-up of at least 12 months. However, the objective outcomes of post-ACLR septic arthritis, including clinician-reported outcomes and radiographic osteoarthritis, were not inferior to those of uncomplicated ACLR. Graft retention led to better patient- and clinician-reported outcomes than graft removal.
Because of the rarity of post-ACLR septic arthritis, large-scale studies of outcomes after treatment are limited in number; therefore, the synthesis of clinical data is crucial for obtaining evidence for clinicians to set realistic expectations regarding the clinical, functional, and radiographic outcomes of this complication. Compared with other reviews [10, 26–28], this study offered an in-depth review that evaluated the outcomes of patients who develop septic arthritis following ACLR and sought to determine whether graft retention or removal was the more effective treatment for these cases.
In this study, the subjective outcomes of post-ACLR septic arthritis were inferior to those of uncomplicated ACLR. Subjective knee functionality, measured based on the Lysholm Knee Scoring Scale and IKDC subjective scores, was significantly lower in the septic arthritis group than in the uncomplicated ACLR group. The average Lysholm Knee Scoring Scale score of the septic arthritis group was 7.5 points less than that of the uncomplicated ACLR group. Similarly, the average subjective IKDC score of the septic arthritis group was 10.45 points less than that of the uncomplicated ACLR group. To the best of our knowledge, no consensus has been achieved on the minimal clinically important differences (MCID) between the IKDC subjective scores; nevertheless, a 10-point difference may be clinically important, with MCID of 8.7 and 9.0, reported by Nwachukwu et al.’s studies, respectively [29, 30]. Scores corresponding to most KOOS dimensions were significantly lower in the septic arthritis group than in the uncomplicated ACLR group. Notably, KOOS and ADL were similar between the two groups, which is consistent with the trend noted in other studies—ADL is not strongly affected after ACLR [31–33]. In general, these findings suggest that, after septic arthritis, patients perceive their knee function to be inferior to that of patients with uncomplicated ACLR but adequate for ADL.
The objective outcomes of post-ACLR septic arthritis were similar to those of uncomplicated ACLR. The clinician-reported evaluation of knee status, which was assessed on the basis of objective IKDC scores, revealed no significant difference between the two groups in the risk of clinical failure. Both static and rotational joint laxity, assessed through the KT-1000 or Lachman and pivot shift test, were similar between the groups. These findings are quite similar to those reported in Torus-Claramunt et al.’s study, which also suggested that, if graft could be retained after the treatment of septic arthritis, the laxity obtained could be similar to that in patients who have not suffered an infection [23].
Counter to our expectation, the risk of osteoarthritis, as detected through radiography, was comparable between the two groups at a mean of 54.26 months of follow-up. Early I&D and antibiotic treatment in most studies may explain similar objective outcomes [34]. Because radiographs may not be as sensitive as magnetic resonance imaging (MRI) in distinguishing damage to cartilage surfaces and surrounding soft tissue, the early signs of osteoarthritis might have been missed [22, 35].
Despite similar knee laxity between the groups, the septic arthritis group had lower activity levels, as assessed using the Tegner Activity Scale, and return to sports rates compared with the uncomplicated group. The discrepancy between solid clinical outcome parameters and compromised activity and return to sports rates may be attributed to postoperative psychological dysfunction. Psychological factors, such as fear of reinjury and self-efficacy, have been reported as reasons for not returning to physical functioning [36–38]. Although this aspect has largely been highlighted in patients with uncomplicated ACLR, those with post-ACLR septic arthritis who undergo additional surgery and rehabilitation may perceive their condition as more severe than that of patients with uncomplicated ACLR; this perception may exacerbate their fear of returning to sports and activity [25]. Addressing these psychosocial factors may have implications for rehabilitation because they may influence the collaborative functional goals set by clinicians and patients.
Although consensus has been achieved on using early surgical intervention and intravenous antibiotics in patients with post-ACLR infection, whether to retain or remove the grafts remains debatable. Studies supporting graft removal have highlighted the increased risk of persistent infection, reoperation, and functional ACL deficiency with retention, whereas other studies have reported good clinical results after graft retention [2, 27, 39–42]. Graft retention appears to be associated with improved patient- and clinician-reported outcomes compared with the outcomes of graft removal. These results are consistent with those of a previous systematic review of 19 studies, including 203 patients, which found consistently better subjective and objective clinical outcomes reported by studies with higher rates of graft retention [10]. In general, these superior outcomes empirically confirm what one would intuitively expect: graft retention minimizes anatomic disruption and morbidity and rehabilitation from additional reconstructive surgery, all of which may affect clinical, functional, and patient-reported outcomes. However, it is important to note that it may not always be appropriate for a clinician to decide to retain a graft if a patient continues to show persistent infection with graft retention or if the graft shows significant structural damage. At that point, even if graft retention shows more favorable outcomes, clinical circumstances may require graft removal.
The present study has some limitations. First, throughout the review, the level of evidence was of a relatively low grade because few high-quality studies included in our review compared post-ACLR septic arthritis with uncomplicated ACLR or graft retention with removal in patients with post-ACLR septic arthritis; this limited our interpretation and conclusions. Second, although we attempted to match patients’ demographic characteristics, some confounding factors, including index ACLR type, graft type, prior or concomitant surgeries, and severity of infection, were not necessarily matched between the groups. Additionally, although limited range of motion is a significant complication of septic arthritis, we were unable to analyze this outcome variable owing to the diversely different presentation among the included studies. Finally, osteoarthritis was detected through plain radiography rather than by MRI, which could have detected the early signs of osteoarthritis. Furthermore, the number of patients in each cohort assessed for osteoarthritis was few, which may not be significant enough for the power of the study. Despite these limitations, the findings should remain of substantial interest to clinicians because they expand the evidence on the mid- to long-term outcomes of post-ACLR septic arthritis.
Conclusions
Despite similar clinician-reported outcomes and osteoarthritis rates, patients with post-ACLR septic arthritis reported worse outcomes than those with uncomplicated ACLR. Graft retention leads to improved patient- and clinician-reported outcomes compared with the outcomes of graft removal. Our findings may help develop realistic expectations and management strategies for this rare complication.
Supplementary Information
Acknowledgements
The authors are grateful to Wan Fang Hospital (Grant number 110-TMU-WFH-10 and 113-wf-swf-03) for supporting this research. This manuscript was English edited by Wallace Academic Editing.
Abbreviations
- ACL
Anterior cruciate ligament
- ACLR
Anterior cruciate ligament reconstruction
- PRISMA
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- I&D
Irrigation and debridement
- ROBINS-E
The Risk of Bias in Nonrandomized Studies of Exposures
- ROBINS-I
The Risk of Bias in Nonrandomized Studies of Interventions
- KOOS
Knee Injury and Osteoarthritis Outcome Score
- IKDC
International Knee Documentation Committee
- SD
Standard deviation
- CI
Confidence interval
- RR
Risk ratio
- ADL
Activities of daily living
- MRI
Magnetic resonance imaging
Author contributions
Y.J.K., Y.P.C., and S.W.H. conceived the project idea. A.P.L. and B.T.T.N. designed and executed the literature search. A.P.L., B.T.T.N., and Y.P.C. screened articles, extracted data, and completed risk of bias assessment. A.P.L. and S.Q.T. conducted the statistical analysis. A.P.L., B.T.T.N., and S.Q.T. wrote the original draft. Y.J.K., S.W.H., and Y.P.C. reviewed the draft and suggested revisions. All authors read and approved the final manuscript.
Funding
The author would like to thank Wan Fang Hospital (Grant number 110-TMU-WFH-10 and 113-wf-swf-03) for supporting this research.
Availability of data and materials
All data analyzed in this study are included in these published articles [1, 7, 8, 11, 17–25].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Waterman BR, Arroyo W, Cotter EJ, Zacchilli MA, Garcia EJ, Owens BD (2018) Septic arthritis after anterior cruciate ligament reconstruction: clinical and functional outcomes based on graft retention or removal. Orthop J Sports Med 6(3):2325967118758626. 10.1177/2325967118758626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAllister DR, Parker RD, Cooper AE, Recht MP, Abate J (1999) Outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med 27(5):562–70. 10.1177/03635465990270050301 [DOI] [PubMed] [Google Scholar]
- 3.Kim S-J, Postigo R, Koo S, Kim JH (2014) Infection after arthroscopic anterior cruciate ligament reconstruction. Orthopedics 37(7):477–84. 10.3928/01477447-20140626-06 [DOI] [PubMed] [Google Scholar]
- 4.Schuster P, Schulz M, Immendoerfer M, Mayer P, Schlumberger M, Richter J (2015) Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: evaluation of an arthroscopic graft-retaining treatment protocol. Am J Sports Med 43(12):3005–3012. 10.1177/0363546515603054 [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Lee YHD, Siebold R (2014) Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg, Sports Traumatol, Arthrosc 22(9):2136–44. 10.1007/s00167-013-2648-z [DOI] [PubMed] [Google Scholar]
- 6.Daniel Judd MAJ, Craig Bottoni LTC, David Kim CPT, Matthew Burke MAJ, Hooker S (2006) Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy 22(4):375–84. 10.1016/j.arthro.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 7.Schollin-Borg M, Michaëlsson K, Rahme H (2003) Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: a case control study. Arthroscopy 19(9):941–7. 10.1016/j.arthro.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 8.Schulz AP, Götze S, Schmidt HGK, Jürgens C, Faschingbauer M (2007) Septic arthritis of the knee after anterior cruciate ligament surgery: a stage-adapted treatment regimen. Am J Sports Med 35(7):1064–9. 10.1177/0363546507299744 [DOI] [PubMed] [Google Scholar]
- 9.Van Tongel A, Stuyck J, Bellemans J, Vandenneucker H (2009) Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, management and outcome. Arthroscopy 25(3):243–9. 10.1016/j.arthro.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Makhni EC, Steinhaus ME, Mehran N, Schulz BS, Ahmad CS (2015) Functional outcome and graft retention in patients with septic arthritis after anterior cruciate ligament reconstruction: a systematic review. Arthroscopy 31(7):1392–1401. 10.1016/j.arthro.2014.12.026 [DOI] [PubMed] [Google Scholar]
- 11.Pogorzelski J, Themessl A, Achtnich A, Fritz EM, Wörtler K, Imhoff AB et al (2018) Septic arthritis after anterior cruciate ligament reconstruction: how important is graft salvage? Am J Sports Med 46(10):2376–2383. 10.1177/0363546518782433 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 10.1371/journal.pmed.1000097 [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JPT, Morgan RL, Rooney AA, Taylor KW, Thayer KA, Silva RA et al (2024) A tool to assess risk of bias in non-randomized follow-up studies of exposure effects (ROBINS-E). Environ Int 186:108602. 10.1016/j.envint.2024.108602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds) (2019) Cochrane handbook for systematic reviews of interventions, 2nd edn. John Wiley & Sons, Chichester [Google Scholar]
- 15.Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5(1):13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Aziz A, Radwan YA, Rizk A (2014) Multiple arthroscopic debridement and graft retention in septic knee arthritis after ACL reconstruction: a prospective case-control study. Int Orthop 38(1):73–82. 10.1007/2Fs00264-013-2123-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohu Y, Klouche S, Herman S, de Pamphilis O, Gerometta A, Lefevre N (2019) professional athletes are not at a higher risk of infections after anterior cruciate ligament reconstruction: incidence of septic arthritis, additional costs, and clinical outcomes from the french prospective anterior cruciate ligament study (FAST) cohort. Am J Sports Med 47(1):104–111. 10.1177/0363546518810527 [DOI] [PubMed] [Google Scholar]
- 19.BoströmWindhamre H, Mikkelsen C, Forssblad M, Willberg L (2014) Postoperative septic arthritis after anterior cruciate ligament reconstruction: does it affect the outcome? A retrospective controlled study. Arthroscopy 30(9):1110–1119. 10.1016/j.arthro.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 20.Brophy RH, Huston LJ, Wright RW, Liu X, Amendola A, Andrish JT et al (2019) Patients treated with surgical irrigation and debridement for infection after ACL reconstruction have a high rate of subsequent knee surgery. J ISAKOS 4(2):73–78. 10.1136/jisakos-2018-000264 [Google Scholar]
- 21.Calvo R, Figueroa D, Anastasiadis Z, Vaisman A, Olid A, Gili F et al (2014) Septic arthritis in ACL reconstruction surgery with hamstring autografts. Eleven years of experience. Knee 21(3):717–720. 10.1016/j.knee.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 22.Meglic U, Salapura V, Zupanc O (2021) MRI findings of early osteoarthritis in patients who sustained septic arthritis of the knee after ACL reconstruction. Orthop J Sports Med. 10.1177/23259671211052519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres-Claramunt R, Pelfort X, Erquicia J, Gil-González S, Gelber PE, Puig L, Monllau JC et al (2013) Knee joint infection after ACL reconstruction: prevalence, management and functional outcomes. Knee Surg, Sports Traumatol, Arthrosc 21(12):2844–9. 10.1007/s00167-012-2264-3 [DOI] [PubMed] [Google Scholar]
- 24.Gille J, Gerlach U, Oheim R, Hintze T, Himpe B, Schultz AP (2015) Functional outcome of septic arthritis after anterior cruciate ligament surgery. Int Orthop 39(6):1195–201. 10.1007/s00264-014-2600-y [DOI] [PubMed] [Google Scholar]
- 25.Themessl A, Mayr F, Hatter K, Rupp MC, Pogorzelski J, Imhoff AA-O et al (2022) Patients return to sports and to work after successful treatment of septic arthritis following anterior cruciate ligament reconstruction. Knee Surg, Sports Traumatol, Arthrosc 30(6):1871–9. 10.1007/s00167-021-06819-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouzopoulos G, Fotopoulos VC, Tzurbakis M, Tzurbakis M (2009) Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg, Sports Traumatol, Arthrosc 17(9):1033–42. 10.1007/s00167-009-0793-1 [DOI] [PubMed] [Google Scholar]
- 27.Saper M, Stephenson K, Heisey M (2014) Arthroscopic irrigation and debridement in the treatment of septic arthritis after anterior cruciate ligament reconstruction. Arthroscopy 30(6):747–754. 10.1016/j.arthro.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 28.Scully WF, Fisher SG, Parada SA, Arrington ED (2013) Septic arthritis following anterior cruciate ligament reconstruction: a comprehensive review of the literature. J Surg Orthop Adv 22(2):127–33. 10.3113/jsoa.2013.0127 [DOI] [PubMed] [Google Scholar]
- 29.Nwachukwu BU, Sullivan SW, Rauck RC, James EW, Burger JA, Altchek DW et al (2021) Patient-reported outcomes and factors associated with achieving the minimal clinically important difference after ACL reconstruction: results at a mean 7.7-year follow-up. JBJS Open Access 6(4):e21.00056. 10.2106/jbjs.Oa.21.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nwachukwu BU, Chang B, Voleti PB, Berkanish P, Cohn MR, Altchek DW et al (2017) Preoperative short form health survey score is predictive of return to play and minimal clinically important difference at a minimum 2-year follow-up after anterior cruciate ligament reconstruction. Am J Sports Med 45(12):2784–2790. 10.1177/0363546517714472 [DOI] [PubMed] [Google Scholar]
- 31.Hill GN, O’Leary ST (2013) Anterior cruciate ligament reconstruction: the short-term recovery using the knee injury and osteoarthritis outcome score (KOOS). Knee Surg Sports Traumatol Arthrosc 21(8):1889–1894. 10.1007/s00167-012-2225-x [DOI] [PubMed] [Google Scholar]
- 32.Ingelsrud LH, Granan LP, Terwee CB, Engebretsen L, Roos EM (2015) Proportion of patients reporting acceptable symptoms or treatment failure and their associated KOOS values at 6 to 24 months after anterior cruciate ligament reconstruction: a study from the norwegian knee ligament registry. Am J Sports Med 43(8):1902–1907. 10.1177/0363546515584041 [DOI] [PubMed] [Google Scholar]
- 33.Samuelsson K, Magnussen RA, Alentorn-Geli E, Krupic F, Spindler KP, Johansson C et al (2017) Equivalent knee injury and osteoarthritis outcome scores 12 and 24 months after anterior cruciate ligament reconstruction: results from the Swedish national knee ligament register. Am J Sports Med 45(9):2085–2091. 10.1177/0363546517702871 [DOI] [PubMed] [Google Scholar]
- 34.Williams RJ, Laurencin CT 3rd, Warren RF, Speciale AC, Brause BD, Orien S (1997) Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Diagnosis and management. Am J Sports Med 25(2):261–7. 10.1177/036354659702500222 [DOI] [PubMed] [Google Scholar]
- 35.Lo Presti M, Costa GG, Grassi A, Cialdella S, Agrò G, Busacca M et al (2020) Graft-preserving arthroscopic debridement with hardware removal is effective for septic arthritis after anterior cruciate ligament reconstruction: a clinical, arthrometric, and magnetic resonance imaging evaluation. Am J Sports Med 48(8):1907–1915. 10.1177/0363546520924823 [DOI] [PubMed] [Google Scholar]
- 36.Baez SA-O, Hoch MC, Hoch JM (2020) Psychological factors are associated with return to pre-injury levels of sport and physical activity after ACL reconstruction. Knee Surg, Sports Traumatol, Arthrosc 28(2):495–501. 10.1007/s00167-019-05696-9 [DOI] [PubMed] [Google Scholar]
- 37.Burland JP, Toonstra JL, Howard JS (2019) Psychosocial barriers after anterior cruciate ligament reconstruction: a clinical review of factors influencing postoperative success. Sports Health 11(6):528–534. 10.1177/1941738119869333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonesson S, Kvist J, Ardern C, Österberg A, Silbernagel KG (2017) Psychological factors are important to return to pre-injury sport activity after anterior cruciate ligament reconstruction: expect and motivate to satisfy. Knee Surg Sports Traumatol Arthrosc 25(5):1375–1384. 10.1007/s00167-016-4294-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pola E, Logroscino G, De Santis V, Canducci F, Delcogliano A, Gasbarrini A (2003) Onset of Berger disease after Staphylococcus aureus infection: septic arthritis after anterior cruciate ligament reconstruction. Arthroscopy. 10.1053/jars.2003.50118 [DOI] [PubMed] [Google Scholar]
- 40.Schub DL, Schmitz LM, Sakamoto FA, Winalski CS, Parker RD (2012) Long-term outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med 40(12):2764–70. 10.1177/0363546512461903 [DOI] [PubMed] [Google Scholar]
- 41.Viola R, Marzano N, Vianello R (2000) An unusual epidemic of Staphylococcus-negative infections involving anterior cruciate ligament reconstruction with salvage of the graft and function. Arthroscopy 16(2):173–7. 10.1016/s0749-8063(00)90032-x [DOI] [PubMed] [Google Scholar]
- 42.Wang C, Ao Y, Wang J, Hu Y, Cui G, Yu J (2009) Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, presentation, treatment, and cause. Arthroscopy 25(3):243–9. 10.1016/j.arthro.2008.10.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed in this study are included in these published articles [1, 7, 8, 11, 17–25].