Abstract
Rising temperatures can affect stomatal and nonstomatal control over photosynthesis, through stomatal closure in response to increasing vapor pressure deficit (VPD), and biochemical limitations, respectively. To explore the independent effects of temperature and VPD, we conducted leaf-level temperature-response measurements while controlling VPD on three tropical tree species. Photosynthesis and stomatal conductance consistently decreased with increasing VPD, whereas photosynthesis typically responded weakly to changes in temperature when a stable VPD was maintained during measurements, resulting in wide parabolic temperature-response curves. We have shown that the negative effect of temperature on photosynthesis in tropical forests across ecologically important temperature ranges does not stem from direct warming effects on biochemical processes but from the indirect effect of warming, through changes in VPD. Understanding the acclimation potential of tropical trees to elevated VPD will be critical to anticipate the consequences of global warming for tropical forests.
Keywords: climate change, photosynthesis, stomatal conductance, temperature response, tropical forest, vapor pressure deficit
Highlights
Vapor pressure deficit limits tropical tree photosynthesis
Photosynthesis and stomatal conductance decrease as vapor pressure deficit rises
Warming impacts photosynthesis via vapor pressure deficit in tropical trees
Introduction
Tropical forests are responsible for one-third of the global terrestrial primary production (Beer et al. 2010) but are currently experiencing reduced growth rates and increased mortality due to atmospheric and climate change (Sullivan et al. 2020, Gora and Esquivel-Muelbert 2021). The gross primary productivity of tropical forests results from a delicate balance of large fluxes of CO2 exchanged between the tropical biosphere and the atmosphere, where both fluxes are strongly affected by environmental factors such as sunlight, temperature, and humidity. Rising temperatures and their effects on tropical forest growth and gross primary productivity have interested tropical plant scientists for decades (e.g., Clark et al. 2003, 2010, 2013; Corlett 2011, Pau et al. 2018, Sullivan et al. 2020). In recent years, vapor pressure deficit (VPD) has emerged as a potentially decisive environmental variable that may significantly affect the physiology of tropical forests (Rowland et al. 2015, Slot and Winter 2017b, Smith et al. 2020). A better understanding of the mechanisms underlying photosynthetic responses to increasing VPD is critical for improving our ability to predict the future of tropical forests.
The saturating vapor pressure of the atmosphere increases exponentially with temperature, so without an increase in atmospheric water vapor content, VPD, the difference between the saturation pressure and actual vapor pressure, increases with increasing temperature. Hence as global temperatures rise, the VPD increases (Barkhordarian et al. 2019). High VPD induces stomatal closure, minimizing water loss but reducing CO2 uptake and leaf cooling. High VPD associated with climate change has been discussed as a major contributor to recently observed drought-induced plant mortality in several studies (Breshears et al. 2013, Eamus et al. 2013, Stovall et al. 2019, Grossiord et al. 2020, Hammond et al. 2022). Furthermore, by limiting cell turgor pressure, high VPD has been recently implicated in reducing stem growth in tropical forest trees (Peters et al. 2023).
Separating the direct effects of elevated temperatures on photosynthetic CO2 uptake from indirect effects through changes in VPD is challenging due to the strong correlation between both factors in natural environments (Grossiord et al. 2020, Mills et al. 2024). Comparison of Gross Ecosystem Productivity between low VPD conditions at the Biosphere 2 experimental facility in Arizona with forests in Brazil and Mexico in which VPD increased with temperature suggested that stomatal responses to VPD drove the decrease in stand-level photosynthetic carbon uptake (Smith et al. 2020). Likewise, statistical separation of VPD and temperature effects on photosynthesis in tropical forest canopies showed that stomatal sensitivity to rising VPD lowered the estimated temperature optimum by ~4°C (Slot et al. 2024). These studies suggest that across leaves and plants, those that experience higher temperatures have lower stomatal conductance and lower rates of photosynthesis. What remains unknown, however, is how consistent this pattern is across and within species when individual leaves undergo gradual warming.
To explore the independent short-term effects of VPD and temperature on photosynthetic CO2 uptake of tropical trees, we experimentally controlled VPD across ranges of temperatures during leaf-level photosynthesis measurements on three tropical tree species in central Panama, using the enhanced humidity control feature of the LI-6800 photosynthesis system (LI-COR, Lincoln, NE, USA). We hypothesized that VPD consistently drives the apparent temperature response of photosynthesis in tropical forest trees.
Materials and methods
Plant material
Three tree species were examined in Panama City, Panama (8.9824°N, 79.5199°W), characterized by a seasonally dry climate with mean annual temperature (MAT) of 25.9°C, mean annual precipitation (MAP) of ~1,900 mm, and a distinct 4-month dry season (Paton 2020): Persea americana Mill., Plumeria rubra L. (white and pink varieties), and Terminalia catappa L. In the following, species will be referred to only by their genus name. The species were selected based on their proximity to one another in the same soil and microclimate, and the accessibility of sun-exposed branches of reproductively mature trees. We prioritized the number of temperature and VPD conditions measured across multiple species, over replication at the species level. Measurements were taken in situ on free-growing (i.e., not potted) trees from March to May 2022.
VPD-controlled temperature response measurements
All measurements were taken with an LI-6800 portable photosynthesis system (Li-Cor, Lincoln, NE, USA) with a CO2 mixer controlling the incoming (reference) CO2 concentration at approximately ambient concentration (410 μmol mol–1). For each species, we first measured 1–3 light-response curves to determine a saturating and non-photoinhibitory level of photosynthetically active radiation (PAR). Based on these light-response curves, we set the PAR level for all three species to 1,500 μmol(quantum) m–2 s–1, provided by 90% red and 10% blue LED lights in the leaf gas-exchange chamber.
To determine light-saturated net photosynthesis (PNmax) over a range of different temperatures while maintaining a constant VPD, we used the humidifying column of the LI-6800 with water-saturated Stuttgarter Masse, which enabled the maintenance of a low VPD at high temperature. There are, however, limits to the temperature range over which a given VPD can be maintained, due to the risk of condensation at low temperatures, and an insufficient capacity of the humidifying column to maintain low VPD at high temperatures. To extend the humidity control, the airflow rate was reduced from 600 μmol s–1 to a minimum of 200 μmol s–1 at high temperatures, when necessary. See Fig. 1S (supplement) for the target VPD levels and associated leaf temperature (Tleaf) ranges, and the data selected for further analysis. At each target VPD level, PNmax was measured at a series of temperatures sequentially on the same leaf. At each temperature, we waited for stabilization of stomatal conductance (gs) and PNmax before recording values, typically after 5–15 min, but occasionally exceeding 20 min.
We determined the effect of temperature on the maximum rate of RuBP carboxylation (Vcmax) and maximum rate of RuBP regeneration (Jmax) by measuring CO2 response (P–Ci) curves at ambient (~31°C) and high (~38°C) temperatures. Photosynthesis was first measured at 410 μmol(CO2) mol–1, after which values were recorded at 13 additional CO2 concentrations, first below ambient CO2 concentrations, after which the curve was completed through step-wise increases above ambient levels to a maximum of 1,800 μmol mol–1. For each species (variety), two curves were measured at each temperature. Vcmax and Jmax were estimated from the relationship between PNmax and substomatal CO2 concentration (Ci) using the fitaci function from the Plantecophys package in R (Duursma 2015). The chloroplast CO2 concentration is lower than Ci because of mesophyll resistance to CO2 transport, and as a result, Vcmax derived from P–Ci curves is underestimated (Niinemets et al. 2009). Reliable mesophyll conductance measurements are, however, challenging at best (Leverett and Kromdijk 2024), and doing so in the field was not feasible in this project.
Data analyses
Even with extended humidity control and reduced flow rates, it was not always possible to maintain VPD within a narrow range of the target as temperature increased. We restricted the analyses to cases where VPD was maintained within 0.5 kPa of the target (see Fig. 1S for the VPD–Tleaf relations, and the selected data).
For each target VPD, a temperature-response curve was fitted using Eq. 1 (Gunderson et al. 2010) to estimate Topt (optimum temperature) and Popt (photosynthetic rate at the optimum temperature) for each replicate leaf, as:
| (1) |
where b is a constant that is proportional to the width of the curve. The curves were fitted with nonlinear least squares regression analyses, using the ‘nls_multstart’ function in the ‘nls.multstart’ package (Padfield and Matheson 2018) in R (R Development Core Team 2021). To test for stomatal limitation of photosynthesis, we plotted photosynthesis against the Ci/Ca ratio at each target VPD.
We further calculated the stomatal slope parameter g1 by Medlyn et al. (2011) as the slope of gs vs. , where Ca is the CO2 concentration of the atmosphere surrounding the leaf. The intercept, g0, was also recorded. g1 represents the carbon cost of water supply and is inversely related to water-use efficiency. As such, VPD sensitivity might scale with g1, and g1 might predict species differences in VPD effects on photosynthesis.
The relative limitations placed on CO2 uptake by the stomatal diffusion (Ls) and the biochemical capacity (Lb) were estimated from the P–Ci response curves according to the approach of Grassi and Magnani (2005):
| (2) |
| (3) |
where gtc is the total conductance to CO2, gsc is the stomatal conductance to CO2, and ∂PN/∂Ci is the slope of the P–Ci curves calculated over a range of 50–100 ppm CO2 as suggested by Tomás et al. (2013).
All data were analyzed using R version 4.1.2 (R Development Core Team 2021).
Results
Leaf level measurements with VPD control
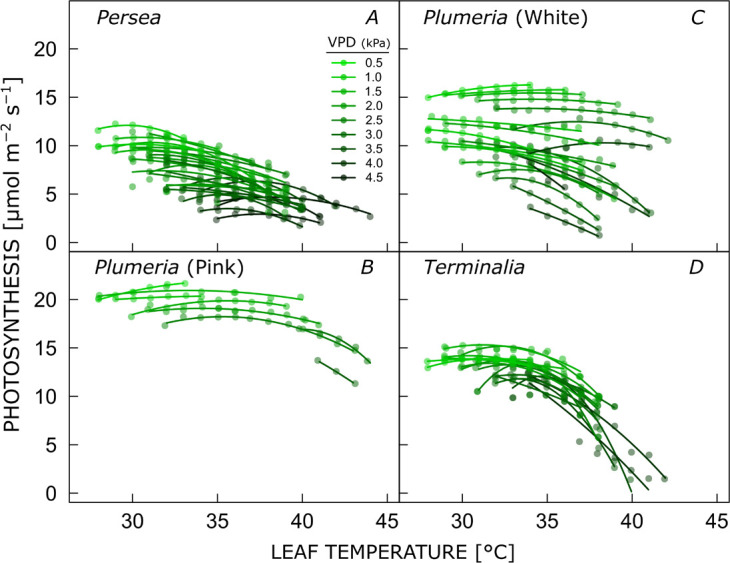
When VPD was controlled experimentally in the cuvette of the LI-6800 (i.e., when different levels of VPD were maintained over certain temperature ranges), leaf-level temperature response curves of PN were typically shallow and PN only approached zero at very high temperatures when VPD was also high, e.g., at 38°C in Plumeria (white) and 40°C in Terminalia, both at 4.0 kPa VPD (Fig. 1). On average, photosynthesis could be maintained at ≥80% of the maximum rate across a 14°C range from 25.2 to 39.2°C; for measurements <3.0 kPa VPD the range was even wider, going from 23.4 to 39.6°C.
Fig. 1. Leaf-level temperature responses of photosynthesis at different controlled levels of vapor pressure deficit (VPD) for three species, including two varieties of Plumeria rubra. Temperature-response curves are fitted at the leaf level using Eq. 1.
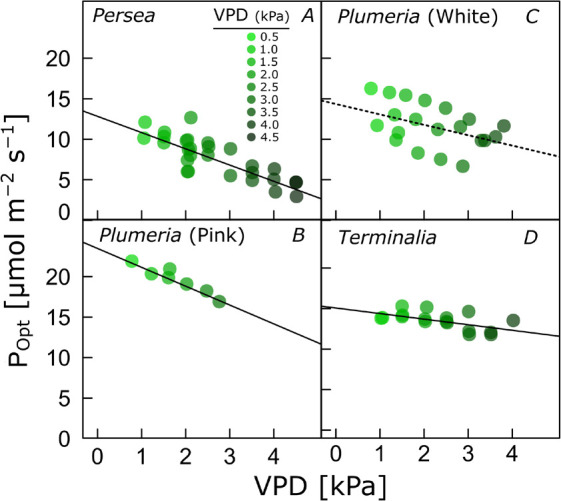
Popt decreased significantly with increasing VPD for all species, by 2.0, 2.3, 1.3, and 0.7 μmol m–2 s–1 kPa–1 for Persea, Plumeria (pink), Plumeria (white), and Terminalia, respectively (when only including curves for which Popt could be reliably estimated with P<0.05) (Fig. 2). While the rate of photosynthesis decreased with increasing VPD, there was a tendency for Topt to increase, by on average 1.8°C per kPa VPD (Fig. 2S, supplement). The increase in Topt was significant with P<0.05 in Persea.
Fig. 2. Photosynthetic CO2 uptake at optimum temperature (Popt) plotted against the vapor pressure deficit (VPD) at which the parameters were determined, for three species, including two varieties of Plumeria rubra, for which leaf-level temperature response curves at different VPD were measured. Solid and dotted lines indicate linear regressions with P<0.05 and P>0.05, respectively.
Photosynthesis correlated positively with the Ci/Ca ratio at almost all VPD levels in Plumeria (white) and Terminalia, without a systematic change in the slope (Fig. 3S, supplement), indicating that decreases in photosynthesis at any given VPD level were associated with increasing stomatal limitations. The strong correlation between PN and Ci/Ca across all VPD levels with the lowest Ci/Ca ratios was observed at the highest VPD in both species. In contrast, in Persea, there was a small positive relationship at most VPD levels consistent with stomatal limitations, but while photosynthesis decreased with increasing VPD, there was no parallel decrease in Ci/Ca. Finally, in Plumeria (pink), no consistent patterns were found, possibly due to a smaller sample size for this species. The stomatal slope parameter g1, proportional to the marginal water cost of carbon gain, varied considerably among species, ranging from 2.1 kPa0.5 in Persea to 7.4 kPa0.5 in Plumeria (white) (Table 1).
Table 1. Biochemical parameters measured at local ambient (31°C) and elevated (38°C) temperatures, and water-use parameters of the species for which photosynthesis was measured with VPD control. Maximum rates of RuBP carboxylation (Vcmax) and RuBP regeneration (Jmax) ± SEM (n = 2), the mean temperature during the measurements, their ratio, the relative limitation to PN by stomatal diffusion (Ls) and biochemical capacity (Lb), and the stomatal slope parameter, g1.
| Species | Tleaf [°C] | Vcmax [μmol m–2 s–1] | Jmax [μmol m–2 s–1] | Jmax/Vcmax | Ls [%] | Lb [%] | g1 [kPa0.5] |
| Persea americana | 31.0 | 69 ± 3 | 73 ± 6 | 1.07 | 53.6 | 45.7 | 2.1 |
| 38.0 | 162 ± 70 | 45 ± 10 | 0.38 | 57.6 | 41.8 | ||
| Plumeria rubra | 31.0 | 105 ± 9 | 118 ± 1 | 1.13 | 29.7 | 68.7 | 4.9 |
| (pink variety) | 38.0 | 192 ± 8 | 157 ± 27 | 0.82 | 38.0 | 60.8 | |
| Plumeria rubra | 31.0 | 112 ± 4 | 125 ± 7 | 1.12 | 31.2 | 67.3 | 7.4 |
| (white variety) | 38.0 | 189 ± 18 | 148 ± 6 | 0.78 | 38.8 | 60.0 | |
| Terminalia catappa | 31.0 | 80 ± 3 | 106 ± 1 | 1.32 | 31.9 | 66.9 | 4.2 |
| 38.0 | 153 ± 7 | 126 ± 8 | 0.83 | 44.0 | 55.0 |
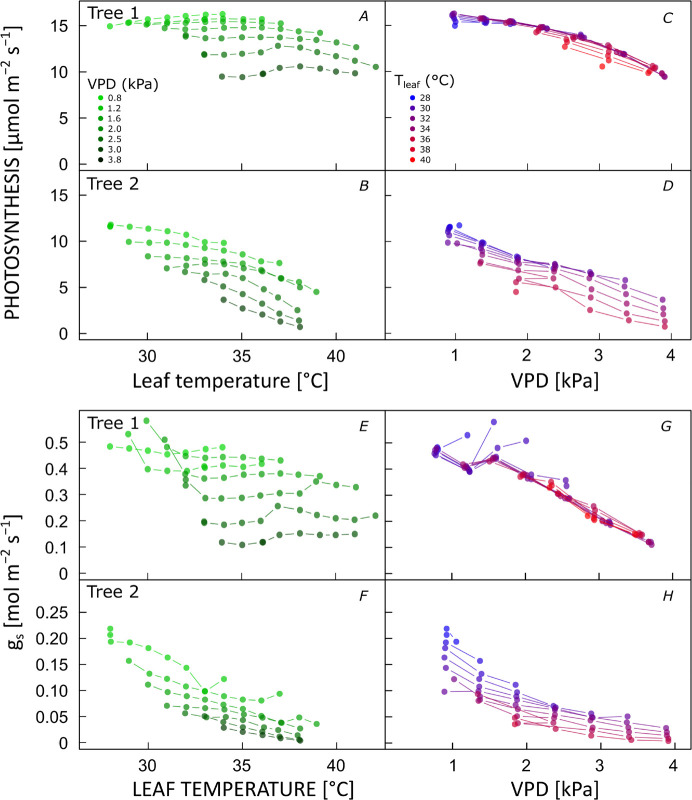
Temperature and VPD responses were not consistent among individuals of the same species (Fig. 3), despite growing close to one another, in the same soil, hydraulic, and light conditions. For example, whereas Plumeria (white) tree 1 was able to maintain high PN and gs with increasing temperature when VPD was controlled, in tree 2, PN and gs decreased with increasing temperature despite maintaining a stable VPD. The VPD response of PN and gs was almost completely independent of temperature in tree 1. Only at the very highest temperatures, a moderate reduction in the elevation of the curve (Fig. 3C,G) was observed. In tree 2, the slopes of the VPD responses were independent of temperature, but the elevation of the curves decreased with increasing temperature across the entire temperature range (Fig. 3D,H). These patterns represented the extremes across all trees measured (Fig. 3; Fig. 4S, supplement).
Fig. 3. Temperature and vapor pressure deficit (VPD) responses of photosynthesis (A–D) and stomatal conductance (gs) (E–H) of two different Plumeria rubra (white) trees.
Biochemical parameters
Between 31 and 38°C, Vcmax increased in all species by an average of 94%, from an average of 91 to 174 μmol m–2 s–1, respectively (Table 1). Jmax of Persea decreased by 39% between 31 and 38°C, while Jmax of the remaining species increased by, on average, 24%, from an average of 116 μmol m–2 s–1 at 31°C to 144 μmol m–2 s–1 at 38°C. The Jmax/Vcmax ratio at 38°C was lower than at 31°C in all species, by on average 40%.
Relative limitations to photosynthesis by CO2 diffusion
The relative limitation to photosynthesis by stomatal diffusion (Ls) increased in all species by an average of 24% (range: 7.5–37.9%) between 31 and 38°C while the limitation by biochemical capacity (Lb) decreased by an average of 12% (8.5–17.8%; Table 1). At both temperatures, the Lb values were consistently higher than the Ls values for each species except Persea, where the reverse was the case.
Discussion
We have presented experimental support for our hypothesis that the closure of stomata in response to rising vapor pressure deficit (VPD) is the primary driver of the short-term temperature response of photosynthesis of tropical trees. When controlling VPD, photosynthesis exhibited relatively weak responses to temperature, especially below ~35°C, although exact patterns varied among species and individual trees. Across ecologically-relevant temperature ranges the photosynthetic response of sun-exposed tropical forest trees to temperature is thus predominantly governed by the response of stomatal conductance to VPD. Here we showed that temperature manipulation at the leaf level yields this same pattern as observed from pooling measurements across canopy leaves (Slot et al. 2024).
Temperature responses and cumulative heat effects
The extended humidity control of the LI-6800 enabled us to measure photosynthesis across a wide enough temperature range to estimate Topt and Popt at each target VPD. The decrease in Popt with increasing VPD was consistent with our hypothesis, but the increasing Topt was contrary to expectations (e.g., Kumarathunge et al. 2020, Slot et al. 2024). However, firstly, when studying PN–temperature relationships under controlled VPD conditions, temperature-response curves were relatively flat in the temperature range around Topt, so Topt becomes a somewhat notional concept, with P frequently at ≥80% of Popt across the entire measurement range. Secondly, the Topt increase was observed when restricting the analysis to Topt values that could be estimated with confidence; Topt values outside the measured temperature range were not included, because their estimates were poorly constrained. With increasing VPD, the measured temperature ranges shifted up. For example, in Persea, photosynthesis was measured between 28 and 34°C when VPD was 1.0 kPa, but between 35 and 44°C when VPD was 4.5 kPa (see Fig. 1S), and the Topt values within those ranges also increased with increasing VPD (see Fig. 2S). Thus, despite the extended humidity control of the LI-6800, the VPD control approach has its limits, as the risk of condensation and lack of humidity set limits to the minimum and maximum temperatures achievable at a given target VPD, respectively. Even with experimental control of VPD, temperature, and VPD thus still covaried across the full dataset, i.e., our dataset did not include measurements at high temperature and low VPD or low temperature and high VPD (Fig. 1S).
Despite VPD control, increasing temperature frequently decreased photosynthesis, resulting in negative temperature-response slopes at most VPD levels. The decrease in photosynthesis at a given VPD tended to be accompanied by decreasing Ci/Ca, suggesting that photosynthesis was limited by stomatal conductance. The effect of temperature on stomatal conductance independent of VPD has been less explored (Grossiord et al. 2020, Mills et al. 2024), and the mechanism is still a subject of debate (Buckley 2019). Some authors report increasing stomatal conductance with increasing temperature at a constant VPD (Fredeen and Sage 1999, Mott and Peak 2010, Urban et al. 2017, Mills et al. 2024). Sadok et al. (2021) suggest that such an increase in stomatal conductance due to an increase in temperature might be associated with increased hydraulic conductivity as the viscosity of water decreases, and because the transmembrane water movement enabled by aquaporins increases with increasing temperature. In contrast, and in line with some of the temperature responses in the current study, Eamus et al. (2008) reported that at a constant VPD of 2.1 kPa, stomatal conductance of Eucalyptus haemastoma leaves declined as temperature increased from 18 to 38°C. They argue that increased cuticular transpiration at high temperatures causes a reduction in water supply to guard cells, thereby decreasing guard cell turgor and stomatal aperture.
Cuticular conductance varies widely among species (Schuster et al. 2017, Duursma et al. 2019), and so does its temperature sensitivity (Riederer 2006, Bueno et al. 2019, Slot et al. 2021). However, the stomatal response to temperature also varies within species (Fig. 3). Intraspecific variation in cuticle conductance has been linked to growth conditions (Bueno et al. 2020) and the presence of leaf endophytes (Arnold and Engelbrecht 2007), neither of which are expected to vary enough among neighboring trees to cause significant differences in cuticular water loss in the current study. Besides potential genetic differences, which can be substantial within species (Alonso-Blanco et al. 2009), it thus remains unclear what explains the stomatal temperature response at fixed VPD and its variation across and within species.
Differences in temperature responses of photosynthesis and stomatal conductance may also be affected by differences in mesophyll conductance and its temperature response (e.g., von Caemmerer and Evans 2015). There is some evidence for intraspecific variation in mesophyll conductance, but only across a species distribution range (Peguero-Pina et al. 2017), and not between neighboring individuals. To evaluate the relative importance of inter and intraspecific variation in controls over the temperature response of photosynthesis, greater species-level replication is required.
The temperature-response curves of photosynthesis shown in Fig. 1 might be influenced by cumulative heat effects. Under natural conditions, leaf temperatures are highly dynamic (e.g., Vogel 2009, Fauset et al. 2018) and even forest canopy leaves in the tropics are unlikely to experience sustained high temperatures. For example, five-minute averages of leaf temperature monitored at a semi-deciduous forest in Panama rarely exceed 35°C (Rey-Sánchez et al. 2016). In contrast, in our experiments, individual leaves were sequentially exposed to a series of increasing high temperatures, including four or more temperatures at ≥35°C, and measurements were not taken until gs and P had stabilized at each target temperature. Increased heat exposure duration could increase cuticular water loss, and/or lead to changes in the expression of aquaporins and heat shock proteins (e.g., Araújo et al. 2019). This cumulative heat exposure might have contributed to VPD-independent changes in stomatal conductance and photosynthesis during leaf-level temperature responses. Given the dynamic nature of leaf temperatures within forest canopies, parameters estimated from exposing leaves to a series of increasing high temperatures might need validation to confirm the behavior of canopy leaves.
Persea americana was the only species for which Jmax was lower at 38°C than at 31°C, suggesting that reduced electron transport rate capacity may have limited photosynthesis at high temperatures, consistent with a recent model by Scafaro et al. (2023), in which Rubisco activation state and the electron transport capacity were identified as the key drivers of the decrease in photosynthesis above Topt. In this species, growing alongside the other species in the same soil and microclimate, measurements at high VPD levels were not associated with greater stomatal limitation and a decrease in Ci/Ca. The decrease in photosynthesis with VPD being independent of Ci/Ca might reflect the higher temperature ranges across which high VPD values could be maintained (Fig. 1S), and the associated decrease of electron transport capacity in this species.
Long-term effects of rising temperature and VPD
Our study investigated the effect of VPD on the short-term temperature response of photosynthesis. In response to ongoing climate change, over time, forest trees may exhibit acclimation or adaptation responses to rising temperature and VPD that differ from the short-term response (Berry and Björkman 1980, Hikosaka et al. 2006, Kumarathunge et al. 2019, Crous et al. 2022). The differential response between the long- and short-term has been shown to vary between tropical (see Slot and Winter 2017c) and temperate forests (see Marchin et al. 2016 and Schönbeck et al. 2022). While experiments have been conducted on the acclimation of temperate forest tree species to warming with VPD manipulation (Marchin et al. 2016, Dusenge et al. 2021, Schönbeck et al. 2022), similar experiments are rare in the tropics (Middleby et al. 2024). Growing tropical forest species under a range of temperature and VPD conditions is clearly warranted.
Concluding remarks
Photosynthetic carbon uptake by tropical forests is a critical regulator of the earth's climate, especially in the context of anthropogenic climate change (Malhi et al. 2008). The stabilizing influence of tropical forests is threatened by rising temperatures and associated increases in VPD (Tan et al. 2017, Smith et al. 2020). Model predictions could be fine-tuned with improved mechanistic understanding of the independent roles of temperature and VPD in affecting the photosynthetic CO2 uptake of tropical trees. It is well established that photosynthesis decreases above a temperature optimum that corresponds roughly to local mean temperatures (Slot and Winter 2017a, Tan et al. 2017, Huang et al. 2019, Kumarathunge et al. 2019), but the processes responsible for this decrease are not yet entirely clear (Slot and Winter 2016, 2017b; Scafaro et al. 2023). We showed here that independent of temperature, VPD adversely affects the photosynthesis of tropical trees. Conversely, photosynthesis exhibited relatively weak responses to temperature when controlling VPD, especially at moderate temperatures.
High VPD can result in water-deficit stress in plants, and lead to decreased vegetation productivity through reduced stomatal conductance and thus photosynthesis (Yuan et al. 2019, Gharun et al. 2020, Grossiord et al. 2020, Schönbeck et al. 2022). It is currently unknown to what extent the photosynthesis of tropical vegetation can acclimate to changes in VPD, so more experimental data on the long-term effects of high VPD and temperature on tropical tree photosynthesis are needed.
Acknowledgments
This research was supported by the Smithsonian Tropical Research Institute. CEE was the recipient of a Smithsonian Institution postdoctoral fellowship.
Abbreviations
- C a
CO2 concentration of the atmosphere surrounding the leaf
- C i
intercellular CO2 concentration
- g s
stomatal conductance
- g sc
stomatal conductance to CO2
- g tc
total conductance to CO2
- J max
maximum rate of RuBP regeneration
- L b
relative limitation to PN by biochemical capacity
- L s
relative limitation to PN by stomatal diffusion
- P N
net photosynthesis
- P Nmax
light-saturated net photosynthetic rate
- P opt
photosynthetic rate at the optimum temperature
- T leaf
leaf temperature
- T opt
optimum temperature
- V cmax
maximum rate of RuBP carboxylation
- VPD
vapor pressure deficit
Supplementary Materials
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Alonso-Blanco C., Aarts M.G.M., Bentsink L. et al. : What has natural variation taught us about plant development, physiology, and adaptation? – Plant Cell 21: 1877-1896, 2009. 10.1105/tpc.109.068114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo M., Ferreira de Oliveira J.M.P., Santos C. et al. : Responses of olive plants exposed to different irrigation treatments in combination with heat shock: physiological and molecular mechanisms during exposure and recovery. – Planta 249: 1583-1598, 2019. 10.1007/s00425-019-03109-2 [DOI] [PubMed] [Google Scholar]
- Arnold A.E., Engelbrecht B.M.J.: Fungal endophytes nearly double minimum leaf conductance in seedlings of a neotropical tree species. – J. Trop. Ecol. 23: 369-372, 2007. 10.1017/S0266467407004038 [DOI] [Google Scholar]
- Barkhordarian A., Saatchi S.S., Behrangi A. et al. : A recent systematic increase in vapor pressure deficit over tropical South America. – Sci. Rep.-UK 9: 15331, 2019. 10.1038/s41598-019-51857-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer C., Reichstein M., Tomelleri E. et al. : Terrestrial gross carbon dioxide uptake: global distribution and covariation with climate. – Science 329: 834-838, 2010. 10.1126/science.1184984 [DOI] [PubMed] [Google Scholar]
- Berry J., Björkman O.: Photosynthetic response and adaptation to temperature in higher plants. – Annu. Rev. Plant Physiol. 31: 491-543, 1980. 10.1146/annurev.pp.31.060180.002423 [DOI] [Google Scholar]
- Breshears D.D., Adams H.D., Eamus D. et al. : The critical amplifying role of increasing atmospheric moisture demand on tree mortality and associated regional die-off. – Front. Plant Sci. 4: 266, 2013. 10.3389/fpls.2013.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley T.N.: How do stomata respond to water status? – New Phytol. 224: 21-36, 2019. 10.1111/nph.15899 [DOI] [PubMed] [Google Scholar]
- Bueno A., Alfarhan A., Arand K. et al. : Effects of temperature on the cuticular transpiration barrier of two desert plants with water-spender and water-saver strategies. – J. Exp. Bot. 70: 1613-1625, 2019. 10.1093/jxb/erz018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno A., Sancho-Knapik D., Gil-Pelegrín E. et al. : Cuticular wax coverage and its transpiration barrier properties in Quercus coccifera L. leaves: does the environment matter? – Tree Physiol. 40: 827-840, 2020. 10.1093/treephys/tpz110 [DOI] [PubMed] [Google Scholar]
- Clark D.A., Clark D.B., Oberbauer S.F.: Field-quantified responses of tropical rainforest aboveground productivity to increasing CO2 and climatic stress, 1997–2009. – J. Geophys. Res.: Biogeo. 118: 783-794, 2013. 10.1002/jgrg.20067 [DOI] [Google Scholar]
- Clark D.A., Piper S.C., Keeling C.D., Clark D.B.: Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. – PNAS 100: 5852-5857, 2003. 10.1073/pnas.0935903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D.B., Clark D.A., Oberbauer S.F.: Annual wood production in a tropical rain forest in NE Costa Rica linked to climatic variation but not to increasing CO2. – Glob. Change Biol. 16: 747-759, 2010. 10.1111/j.1365-2486.2009.02004.x [DOI] [Google Scholar]
- Corlett R.T.: Impacts of warming on tropical lowland rainforests. – Trends Ecol. Evol. 26: 606-613, 2011. 10.1016/j.tree.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Crous K.Y., Uddling J., De Kauwe M.G.: Temperature responses of photosynthesis and respiration in evergreen trees from boreal to tropical latitudes. – New Phytol. 234: 353-374, 2022. 10.1111/nph.17951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusenge M.E., Wittemann M., Mujawamariya M. et al. : Limited thermal acclimation of photosynthesis in tropical montane tree species. – Glob. Change Biol. 27: 4860-4878, 2021. 10.1111/gcb.15790 [DOI] [PubMed] [Google Scholar]
- Duursma R.A.: Plantecophys – an R package for analysing and modelling leaf gas exchange data. – PLoS ONE 10: e0143346, 2015. 10.1371/journal.pone.0143346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duursma R.A., Blackman C.J., Lopez R. et al. : On the minimum leaf conductance: its role in models of plant water use, and ecological and environmental controls. – New Phytol. 221: 693-705, 2019. 10.1111/nph.15395 [DOI] [PubMed] [Google Scholar]
- Eamus D., Boulain N., Cleverly J., Breshears D.D.: Global change-type drought-induced tree mortality: vapor pressure deficit is more important than temperature per se in causing decline in tree health. – Ecol. Evol. 3: 2711-2729, 2013. 10.1002/ece3.664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamus D., Taylor D.T., Macinnis-Ng C.M.O. et al. : Comparing model predictions and experimental data for the response of stomatal conductance and guard cell turgor to manipulations of cuticular conductance, leaf-to-air vapour pressure difference and temperature: feedback mechanisms are able to account for all observations. – Plant Cell Environ. 31: 269-277, 2008. 10.1111/j.1365-3040.2007.01771.x [DOI] [PubMed] [Google Scholar]
- Fauset S., Freitas H.C., Galbraith D.R. et al. : Differences in leaf thermoregulation and water use strategies between three co-occurring Atlantic forest tree species. – Plant Cell Environ. 41: 1618-1631, 2018. 10.1111/pce.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredeen A.L., Sage R.F.: Temperature and humidity effects on branchlet gas exchange in white spruce: an explanation for the increase in transpiration with branchlet temperature. – Trees-Struct. Funct. 14: 161-168, 1999. 10.1007/s004680050220 [DOI] [Google Scholar]
- Gharun M., Hörtnagl L., Paul-Limoges E. et al. : Physiological response of Swiss ecosystems to 2018 drought across plant types and elevation. – Philos. T. Roy. Soc. B 375: 20190521, 2020. 10.1098/rstb.2019.0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gora E.M., Esquivel-Muelbert A.: Implications of size-dependent tree mortality for tropical forest carbon dynamics. – Nat. Plants 7: 384-391, 2021. 10.1038/s41477-021-00879-0 [DOI] [PubMed] [Google Scholar]
- Grassi G., Magnani F.: Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. – Plant Cell Environ. 28: 834-849, 2005. 10.1111/j.1365-3040.2005.01333.x [DOI] [Google Scholar]
- Grossiord C., Buckley T.N., Cernusak L.A. et al. : Plant responses to rising vapor pressure deficit. – New Phytol. 226: 1550-1566, 2020. 10.1111/nph.16485 [DOI] [PubMed] [Google Scholar]
- Gunderson C.A., O'Hara K.H., Campion C.M. et al. : Thermal plasticity of photosynthesis: the role of acclimation in forest responses to a warming climate. – Glob. Change Biol. 16: 2272-2286, 2010. 10.1111/j.1365-2486.2009.02090.x [DOI] [Google Scholar]
- Hammond W.M., Williams A.P., Abatzoglou J.T. et al. : Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests. – Nat. Commun. 13: 1761, 2022. 10.1038/s41467-022-29289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K., Ishikawa K., Borjigidai A. et al. : Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. – J. Exp. Bot. 57: 291-302, 2006. 10.1093/jxb/erj049 [DOI] [PubMed] [Google Scholar]
- Huang M., Piao S., Ciais P. et al. : Air temperature optima of vegetation productivity across global biomes. – Nat. Ecol. Evol. 3: 772-779, 2019. 10.1038/s41559-019-0838-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarathunge D.P., Drake J.E., Tjoelker M.G. et al. : The temperature optima for tree seedling photosynthesis and growth depend on water inputs. – Glob. Change Biol. 26: 2544-2560, 2020. 10.1111/gcb.14975 [DOI] [PubMed] [Google Scholar]
- Kumarathunge D.P., Medlyn B.E., Drake J.E. et al. : Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. – New Phytol. 222: 768-784, 2019. 10.1111/nph.15668 [DOI] [PubMed] [Google Scholar]
- Leverett A., Kromdijk J.: The long and tortuous path towards improving photosynthesis by engineering elevated mesophyll conductance. – Plant Cell Environ. 47: 3411-3427, 2024. 10.1111/pce.14940 [DOI] [PubMed] [Google Scholar]
- Malhi Y., Roberts J.T., Betts R.A. et al. : Climate change, deforestation, and the fate of the Amazon. – Science 319: 169-172, 2008. 10.1126/science.1146961 [DOI] [PubMed] [Google Scholar]
- Marchin R.M., Broadhead A.A., Bostic L.E. et al. : Stomatal acclimation to vapour pressure deficit doubles transpiration of small tree seedlings with warming. – Plant Cell Environ. 39: 2221-2234, 2016. 10.1111/pce.12790 [DOI] [PubMed] [Google Scholar]
- Medlyn B.E., Duursma R.A., Eamus D. et al. : Reconciling the optimal and empirical approaches to modelling stomatal conductance. – Glob. Change Biol. 17: 2134-2144, 2011. 10.1111/j.1365-2486.2010.02375.x [DOI] [Google Scholar]
- Middleby K.B., Cheesman A.W., Cernusak L.A.: Impacts of elevated temperature and vapour pressure deficit on leaf gas exchange and plant growth across six tropical rainforest tree species. – New Phytol. 243: 648-661, 2024. 10.1111/nph.19822 [DOI] [PubMed] [Google Scholar]
- Mills C., Bartlett M.K., Buckley T.N.: The poorly-explored stomatal response to temperature at constant evaporative demand. – Plant Cell Environ. 47: 3428-3446, 2024. 10.1111/pce.14911 [DOI] [PubMed] [Google Scholar]
- Mott K.A., Peak D.: Stomatal responses to humidity and temperature in darkness. – Plant Cell Environ. 33: 1084-1090, 2010. 10.1111/j.1365-3040.2010.02129.x [DOI] [PubMed] [Google Scholar]
- Niinemets Ü., Díaz-Espejo A., Flexas J. et al. : Importance of mesophyll diffusion conductance in estimation of plant photosynthesis in the field. – J. Exp. Bot. 60: 2271-2282, 2009. 10.1093/jxb/erp063 [DOI] [PubMed] [Google Scholar]
- Padfield D., Matheson G.: Package ‘nls.multstart’. Robust non-linear regression using AIC scores, 2018. Available at: https://cran.r-project.org/package=nls.multstart.
- Paton S.: Yearly Reports_Parque Natural Metropolitano Crane. Smithsonian Tropical Research Institute, Dataset, 2020. 10.25573/data.11799348.v5 [DOI] [Google Scholar]
- Pau S., Detto M., Kim Y., Still C.J.: Tropical forest temperature thresholds for gross primary productivity. – Ecosphere 9: e02311, 2018. 10.1002/ecs2.2311 [DOI] [Google Scholar]
- Peguero-Pina J.J., Aranda I., Cano F.J. et al. : The role of mesophyll conductance in oak photosynthesis: among- and within-species variability. – In: Gil-Pelegrín E., Peguero-Pina J., Sancho-Knapik D. (ed.): Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L. Tree Physiology. Vol. 7. Pp. 303-325. Springer, Cham: 2017. 10.1007/978-3-319-69099-5_9 [DOI] [Google Scholar]
- Peters R.L., Kaewmano A., Fu P.-L: et al. : High vapour pressure deficit enhances turgor limitation of stem growth in an Asian tropical rainforest tree. – Plant Cell Environ. 46: 2747-2762, 2023. 10.1111/pce.14661 [DOI] [PubMed] [Google Scholar]
- R Development Core Team: R: A language and environment for statistical computing. Version 4.1.2. R Foundation for Statistical Computing, Vienna 2021. Available at: https://www.r-project.org/.
- Rey-Sánchez A.C., Slot M., Posada J.M., Kitajima K.: Spatial and seasonal variation in leaf temperature within the canopy of a tropical forest. – Clim. Res. 71: 75-89, 2016. 10.3354/cr01427 [DOI] [Google Scholar]
- Riederer M.: Thermodynamics of the water permeability of plant cuticles: characterization of the polar pathway. – J. Exp. Bot. 57: 2937-2942, 2006. 10.1093/jxb/erl053 [DOI] [PubMed] [Google Scholar]
- Rowland L., Harper A., Christoffersen B.O. et al. : Modelling climate change responses in tropical forests: similar productivity estimates across five models, but different mechanisms and responses. – Geosci. Model Dev. 8: 1097-1110, 2015. 10.5194/gmd-8-1097-2015 [DOI] [Google Scholar]
- Sadok W., Lopez J.R., Smith K.P.: Transpiration increases under high-temperature stress: Potential mechanisms, trade-offs and prospects for crop resilience in a warming world. – Plant Cell Environ. 44: 2102-2116, 2021. 10.1111/pce.13970 [DOI] [PubMed] [Google Scholar]
- Scafaro A.P., Posch B.C., Evans J.R. et al. : Rubisco deactivation and chloroplast electron transport rates co-limit photosynthesis above optimal leaf temperature in terrestrial plants. – Nature Commun. 14: 2820, 2023. 10.1038/s414647477-023-38496-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönbeck L.C., Schuler P., Lehmann M.M. et al. : Increasing temperature and vapour pressure deficit lead to hydraulic damages in the absence of soil drought. – Plant Cell Environ. 45: 3275-3289, 2022. 10.1111/pce.14425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A.-C., Burghardt M., Riederer M.: The ecophysiology of leaf cuticular transpiration: are cuticular water permeabilities adapted to ecological conditions? – J. Exp. Bot. 68: 5271-5279, 2017. 10.1093/jxb/erx321 [DOI] [PubMed] [Google Scholar]
- Slot M., Nardwattanawong T., Hernández G.G. et al. : Large differences in leaf cuticle conductance and its temperature response among 24 tropical tree species from across a rainfall gradient. – New Phytol. 232: 1618-1631, 2021. 10.1111/nph.17626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot M., Rifai S.W., Eze C.E., Winter K.: The stomatal response to vapor pressure deficit drives the apparent temperature response of photosynthesis in tropical forests. – New Phytol., 2024. 10.1111/nph.19806 [DOI] [PubMed] [Google Scholar]
- Slot M., Winter K.: The effects of rising temperature on the ecophysiology of tropical forest trees. – In: Goldstein G., Santiago L.S. (ed.): Tropical Tree Physiology. Tree Physiology. Vol. 6. Pp. 385-412. Springer, Cham: 2016. 10.1007/978-3-319-27422-5_18 [DOI] [Google Scholar]
- Slot M., Winter K.: In situ temperature response of photosynthesis of 42 tree and liana species in the canopy of two Panamanian lowland tropical forests with contrasting rainfall regimes. – New Phytol. 214: 1103-1117, 2017a. 10.1111/nph.14469 [DOI] [PubMed] [Google Scholar]
- Slot M., Winter K.: In situ temperature relationships of biochemical and stomatal controls of photosynthesis in four lowland tropical tree species. – Plant Cell Environ. 40: 3055-3068, 2017b. 10.1111/pce.13071 [DOI] [PubMed] [Google Scholar]
- Slot M., Winter K.: Photosynthetic acclimation to warming in tropical forest tree seedlings. – J. Exp. Bot. 68: 2275-2284, 2017c. 10.1093/jxb/erx071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.N., Taylor T.C., van Haren J. et al. : Empirical evidence for resilience of tropical forest photosynthesis in a warmer world. – Nat. Plants 6: 1225-1230, 2020. 10.1038/s41477-020-00780-2 [DOI] [PubMed] [Google Scholar]
- Stovall A.E.L., Shugart H., Yang X.: Tree height explains mortality risk during an intense drought. – Nat. Commun. 10: 4385, 2019. 10.1038/s41467-019-12380-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M.J.P., Lewis S.L., Affum-Baffoe K. et al. : Long-term thermal sensitivity of Earth’s tropical forests. – Science 368: 869-874, 2020. 10.1126/science.aaw7578 [DOI] [PubMed] [Google Scholar]
- Tan Z.-H., Zeng J., Zhang Y.-J. et al. : Optimum air temperature for tropical forest photosynthesis: mechanisms involved and implications for climate warming. – Environ. Res. Lett. 12: 054022, 2017. 10.1088/1748-9326/aa6f97 [DOI] [Google Scholar]
- Tomás M., Flexas J., Copolovici L. et al. : Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. – J. Exp. Bot. 64: 2269-2281, 2013. 10.1093/jxb/ert086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J., Ingwers M., McGuire M.A., Teskey R.O.: Stomatal conductance increases with rising temperature. – Plant Signal. Behav. 12: e1356534, 2017. 10.1080/15592324.2017.1356534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S.: Leaves in the lowest and highest winds: temperature, force and shape. – New Phytol. 183: 13-26, 2009. 10.1111/j.1469-8137.2009.02854.x [DOI] [PubMed] [Google Scholar]
- von Caemmerer S., Evans J.R.: Temperature responses of mesophyll conductance differ greatly between species. – Plant Cell Environ. 38: 629-637, 2015. 10.1111/pce.12449 [DOI] [PubMed] [Google Scholar]
- Yuan W., Zheng Y., Piao S. et al. : Increased atmospheric vapor pressure deficit reduces global vegetation growth. – Sci. Adv. 5: eaax1396, 2019. 10.1126/sciadv.aax1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.