Abstract
The recent determination of the entire antigenic structure of sperm-whale myoglobin with rabbit and goat antisera has permitted the examination of whether the antigenic structure recognized by antibodies depends on the species in which the antisera are raised. Also, by knowledge of the antigenic structure, the molecular factors that determine and influence antigenicity can be better understood in terms of the effects of amino acid substitutions occurring in the antigenic sites and in the environmental residues of the sites. In the present work, the myoglobins from finback whale, killer whale, horse, chimpanzee, sheep, goat, bovine, echidna, viscacha, rabbit, dog, cape fox, mouse and chicken were examined for their ability to cross-react with antisera to sperm-whale myoglobin. By immunoadsorbent titration studies with radioiodinated antibodies, each of these myoglobins was able to bind antibodies to sperm-whale myoglobin raised in goat, rabbit, chicken, cat, pig and outbred mouse. It was found that the extent of cross-reaction of a given myoglobin was not dependent on the species in which the antisera were raised. This indicated that the antibody response to sperm-whale myoglobin (i.e. its antigenic structure) is independent of the species in which the antisera are raised and is not directed to regions of sequence differences between the injected myoglobin and the myoglobin of the immunized host. Indeed, in each antiserum from a given species examined, that antiserum reacted with the myoglobin of that species. The extent of this auto-reactivity for a given myoglobin was comparable with the general extent of cross-reactivity shown by that myoglobin with antisera raised in other species. The cross-reactivities and auto-reactivities (both of which are of similar extents for a given myoglobin) can be reasonably rationalized in terms of the effects of amino acid substitutions within the antigenic sites and within the residues close to these sites. These findings confirm that the antigenicity of the sites is inherent in their three-dimensional locations.
Full text
PDF


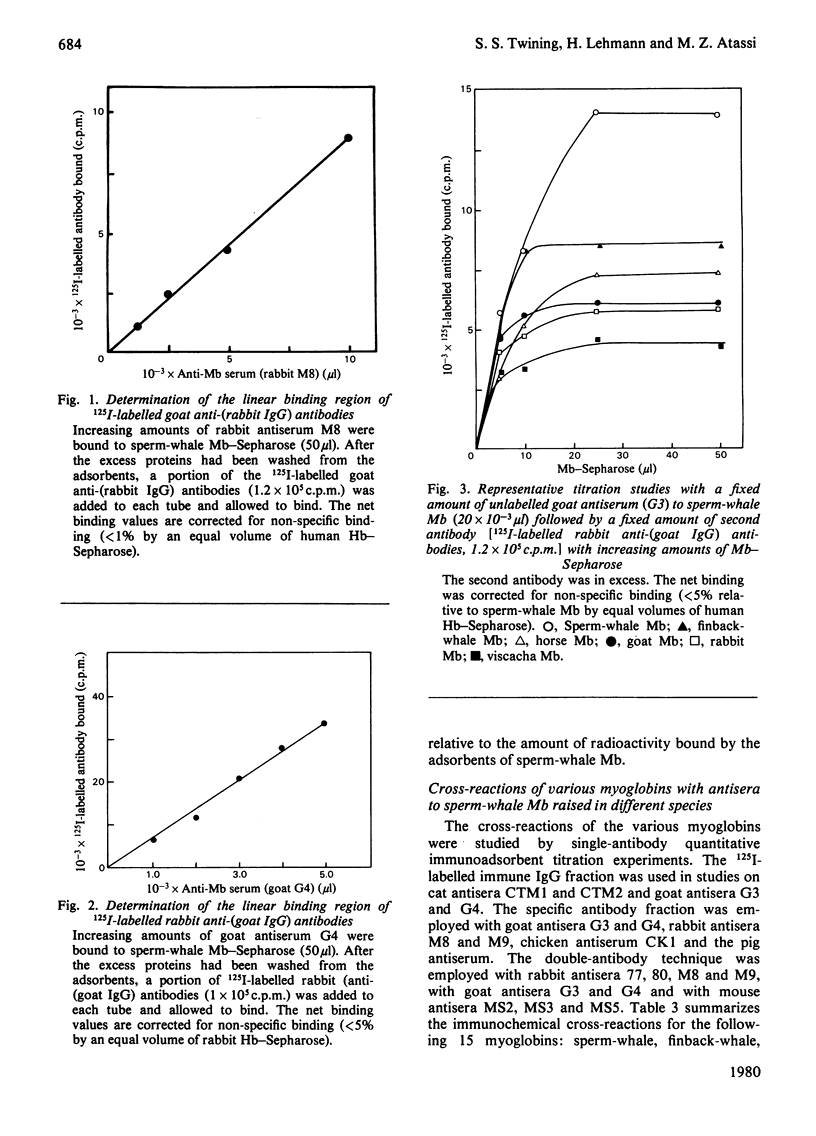
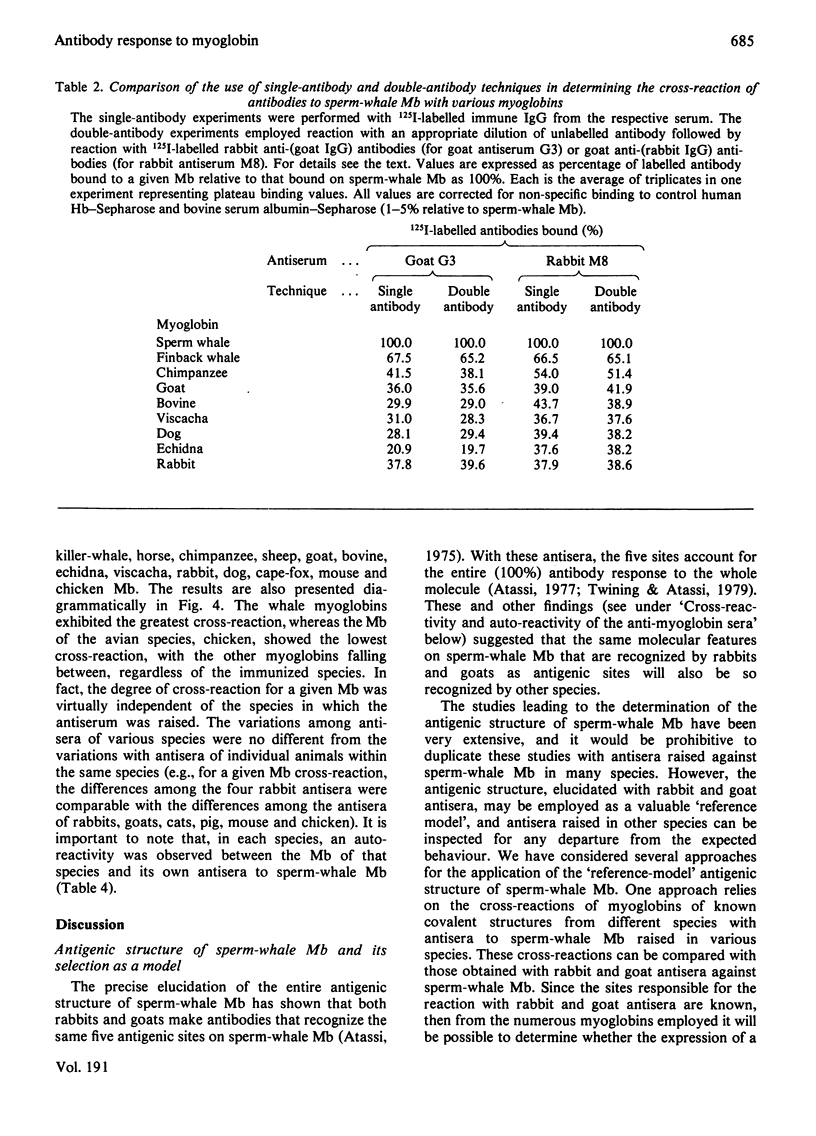
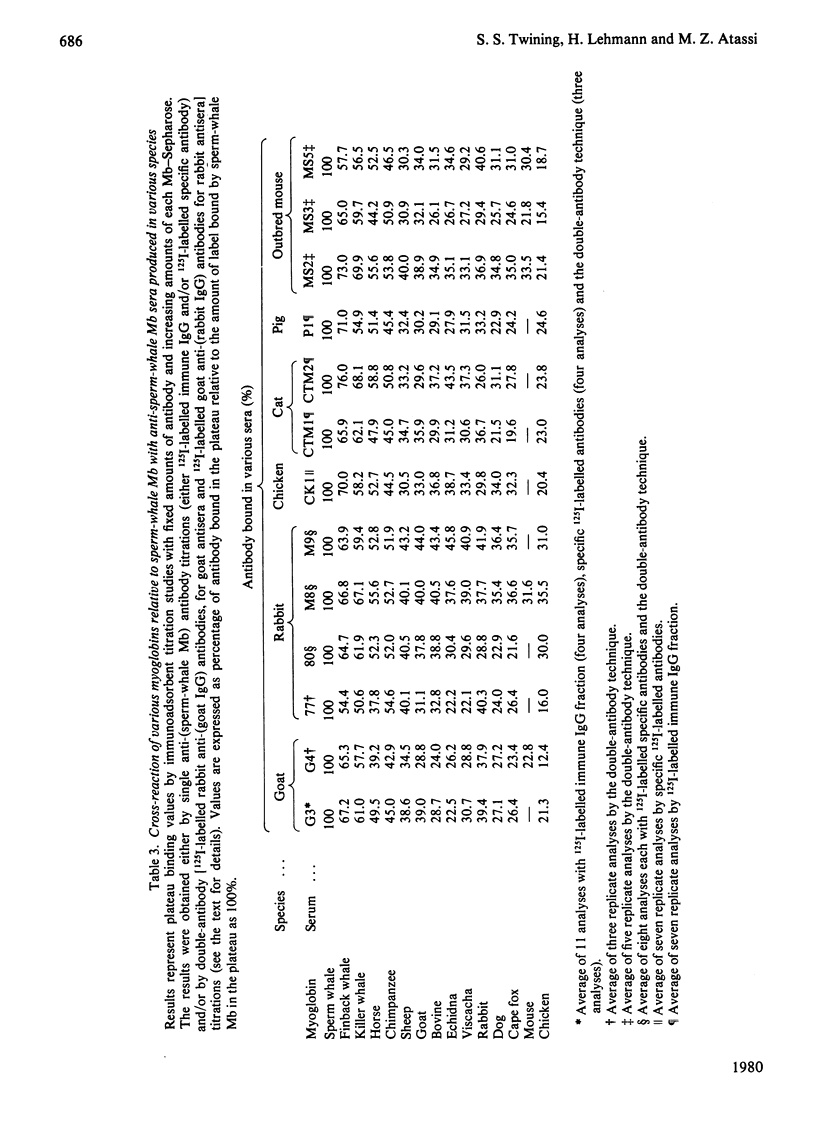
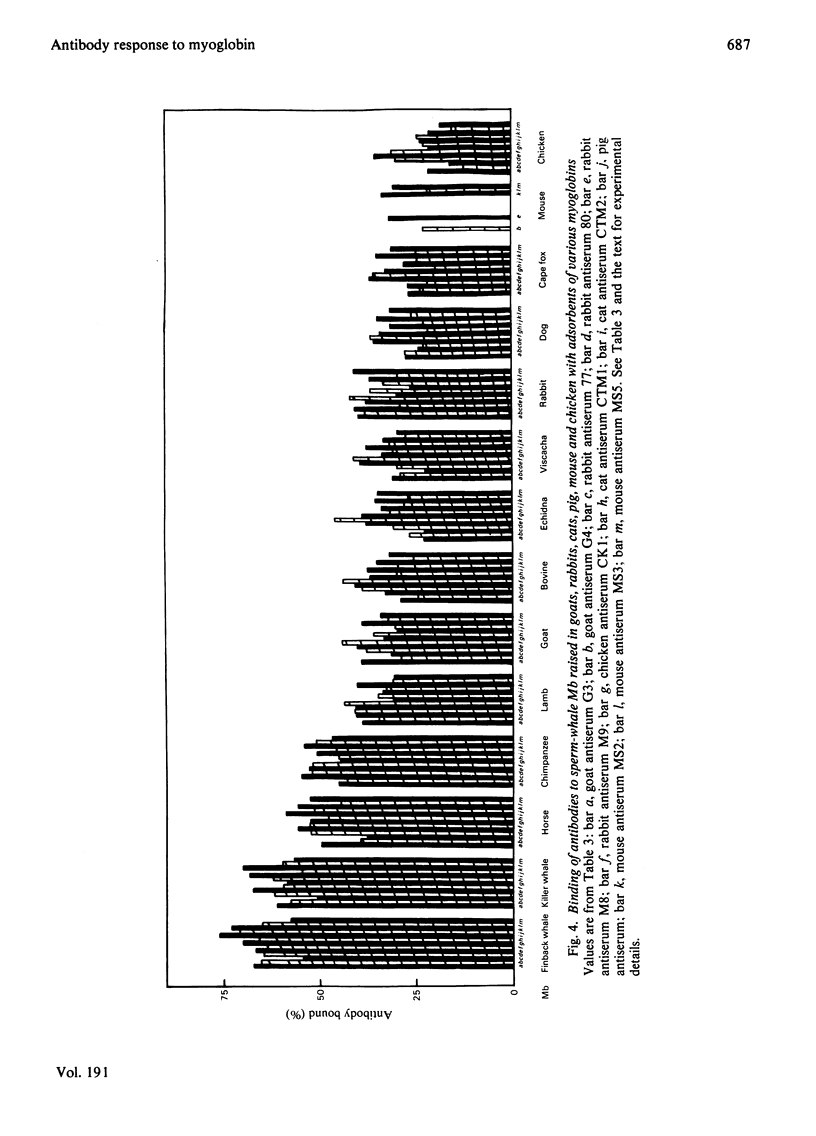






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATASSI M. Z. PROPERTIES OF COMPONENTS OF MYOGLOBIN OF THE SPERM WHALE. Nature. 1964 May 2;202:496–498. doi: 10.1038/202496a0. [DOI] [PubMed] [Google Scholar]
- Andres S. F., Atassi M. Z. Conformational studies on modified proteins and peptides. Artificial myoglobins prepared with modified and metalloporphyrins. Biochemistry. 1970 May 26;9(11):2268–2275. doi: 10.1021/bi00813a007. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Antigenic structure of myoglobin: the complete immunochemical anatomy of a protein and conclusions relating to antigenic structures of proteins. Immunochemistry. 1975 May;12(5):423–438. doi: 10.1016/0019-2791(75)90010-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Chemical studies on haemoglobins A1 and A0. Biochem J. 1964 Oct;93(1):189–197. doi: 10.1042/bj0930189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Immunochemistry of sperm whale myoglobin. VI. Preparation and conformational analysis of eight mammalian myoglobins. Biochim Biophys Acta. 1970 Dec 22;221(3):612–622. doi: 10.1016/0005-2795(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Immunochemistry of sperm-whale myoglobins prepared with various modified porphyrins and metalloporphyrins. Biochem J. 1967 Apr;103(1):29–35. doi: 10.1042/bj1030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Kazim A. L. Distance calculation of residues neighbouring to lysozyme antigenic sites. Site-neighbouring residues whose evolutionary substitution can modify the characteristics and binding energy of the sites. Biochem J. 1980 Apr 1;187(1):163–172. doi: 10.1042/bj1870163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Periodate oxidation of sperm-whale myoglobin and the role of the methionine residues in the antigen-antibody reaction. Biochem J. 1967 Feb;102(2):478–487. doi: 10.1042/bj1020478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z. Precise determination of the entire antigenic structure of lysozyme: molecular features of protein antigenic structures and potential of "surface-simulation" synthesis--a powerful new concept for protein binding sites. Immunochemistry. 1978 Dec;15(12):909–936. doi: 10.1016/0161-5890(78)90126-8. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Sakata S., Kazim A. L. Localization and verification by synthesis of five antigenic sites of bovine serum albumin. Biochem J. 1979 May 1;179(2):327–331. doi: 10.1042/bj1790327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atassi M. Z., Saplin B. J. Immunochemistry of sperm whale myoglobin. I. The specific interaction of some tryptic peptides and of peptides containing all the reactive regions of the antigen. Biochemistry. 1968 Feb;7(2):688–698. doi: 10.1021/bi00842a026. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Saplin B. J. Immunochemistry of sperm whale myoglobin. X. Regions responsible for immunochemical cross-reaction with finback whale myoglobin. Some general conclusions concerning immunochemical cross-reaction of proteins. Biochemistry. 1971 Dec 7;10(25):4740–4747. doi: 10.1021/bi00801a021. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Skalski D. J. Immunochemistry of some artificial human hemoglobins. Immunochemistry. 1969 Jan;6(1):25–34. doi: 10.1016/0019-2791(69)90175-x. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Smith J. A. A proposal for the nomenclature of antigenic sites in peptides and proteins. Immunochemistry. 1978 Aug;15(8):609–610. doi: 10.1016/0161-5890(78)90016-0. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Tarlowski D. P., Paull J. H. Immunochemistry of sperm whale myoglobin. VII. Correlation of immunochemical cross-reaction of eight myoglobins with structural similarity and its dependence on conformation. Biochim Biophys Acta. 1970 Dec 22;221(3):623–635. doi: 10.1016/0005-2795(70)90234-5. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Thomas A. V. Immunochemistry of sperm whale myoglobin. IV. The role of the arginine residues in the conformation and differentiation of their roles in the antigenic reactivity. Biochemistry. 1969 Aug;8(8):3385–3394. doi: 10.1021/bi00836a037. [DOI] [PubMed] [Google Scholar]
- Castillo O., Jones L. T., Lehmann H. The myoglobin of an echidna (Tachyglossus aculeatus aculeatus). Biochim Biophys Acta. 1978 Apr 26;533(2):289–292. doi: 10.1016/0005-2795(78)90375-6. [DOI] [PubMed] [Google Scholar]
- Dautrevaux M., Boulanger Y., Han K., Biserte G. Structure covalente de la myoglobine de cheval. Eur J Biochem. 1969 Dec;11(2):267–277. doi: 10.1111/j.1432-1033.1969.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Deconinck M., Peiffer S., Depreter J., Paul C., Schnek A. G., Leonis J. The primary sequence of chicken myoglobin (Gallus gallus). Biochim Biophys Acta. 1975 Apr 29;386(2):567–575. doi: 10.1016/0005-2795(75)90300-1. [DOI] [PubMed] [Google Scholar]
- DiMarchi R. D., Wang C. C., Hemenway J. B., Gurd F. R. Complete amino acid sequence of the major component myoglobin of finback whale (Balaenoptera physalus). Biochemistry. 1978 May 16;17(10):1968–1970. doi: 10.1021/bi00603a026. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. IV. Demonstration of conformational differences between alpha-lactalbumin and lysozyme. Biochim Biophys Acta. 1971 Apr 27;236(1):131–141. doi: 10.1016/0005-2795(71)90158-9. [DOI] [PubMed] [Google Scholar]
- Han K. K., Dautrevaux M., Chaila X., Biserte G. The covalent structure of beef heart myoglobin. Eur J Biochem. 1970 Nov;16(3):465–471. doi: 10.1111/j.1432-1033.1970.tb01103.x. [DOI] [PubMed] [Google Scholar]
- Han K. K., Tetaert D., Moschetto Y., Dautrevaux M., Kopeyan C. The covalent structure of sheep-heart myoglobin. Eur J Biochem. 1972 Jun 9;27(3):585–592. doi: 10.1111/j.1432-1033.1972.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Hurrell J. G., Smith J. A., Leach S. J. The detection of five antigenically reactive regions in the soybean leghemoglobin a molecule. Immunochemistry. 1978 May;15(5):297–302. doi: 10.1016/0161-5890(78)90089-5. [DOI] [PubMed] [Google Scholar]
- Hurrell J. G., Smith J. A., Todd P. E., Leach S. J. Cross-reactivity between mammalian myoglobins: linear vs spatial antigenic determinants. Immunochemistry. 1977 Apr;14(4):283–288. doi: 10.1016/0019-2791(77)90251-8. [DOI] [PubMed] [Google Scholar]
- Jones L. T., Castillo O., Lehmann H. The myoglobin of the Cape fox (Vulpes chama). Biochim Biophys Acta. 1977 Aug 23;493(2):460–464. doi: 10.1016/0005-2795(77)90202-1. [DOI] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Antibodies against protein antigenic sites that are identical in the homologous protein of the immunized animal. Autoreactivity in rabbits of antibodies to sperm-whale myoglobin. Biochim Biophys Acta. 1977 Sep 27;494(1):277–282. doi: 10.1016/0005-2795(77)90156-8. [DOI] [PubMed] [Google Scholar]
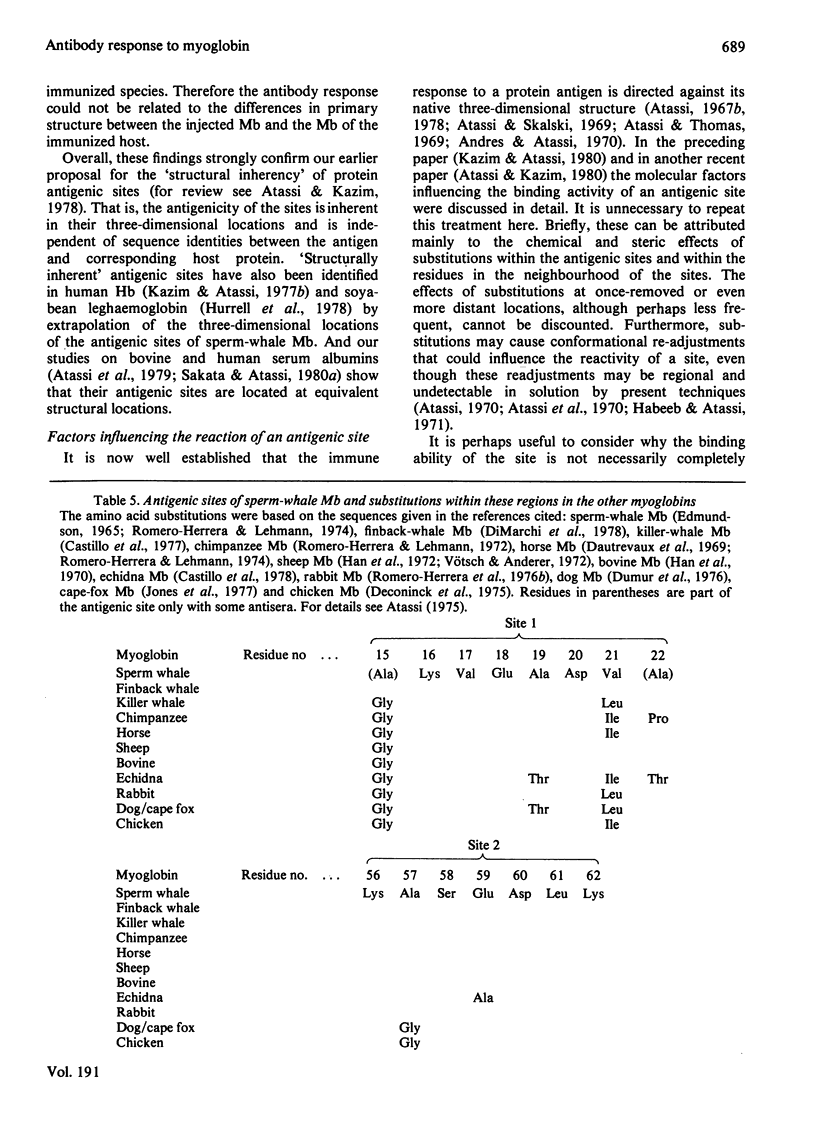
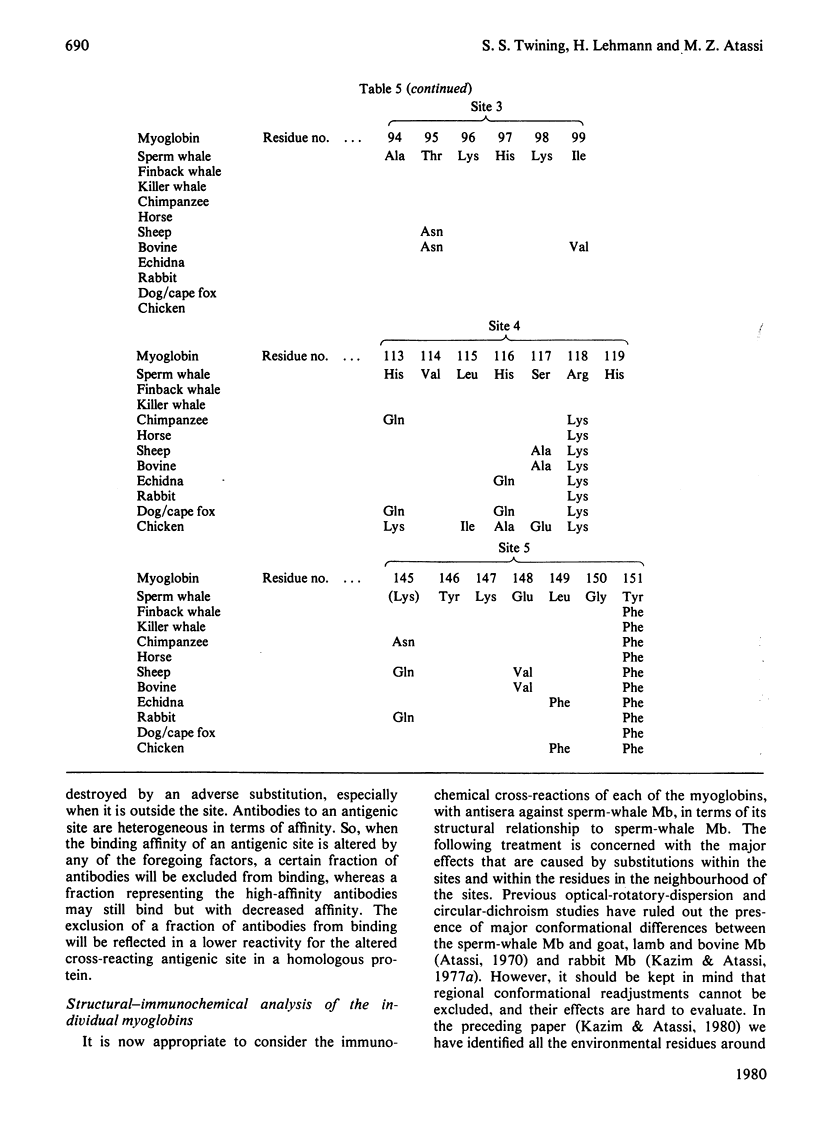
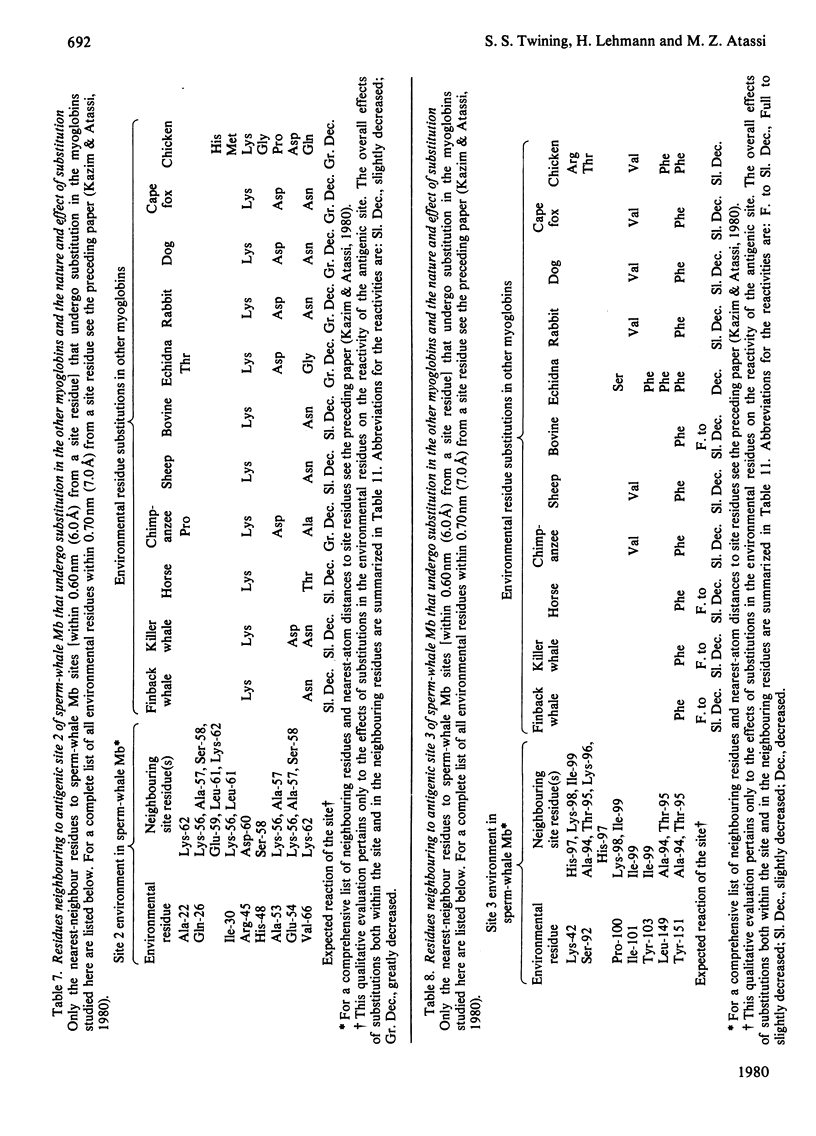
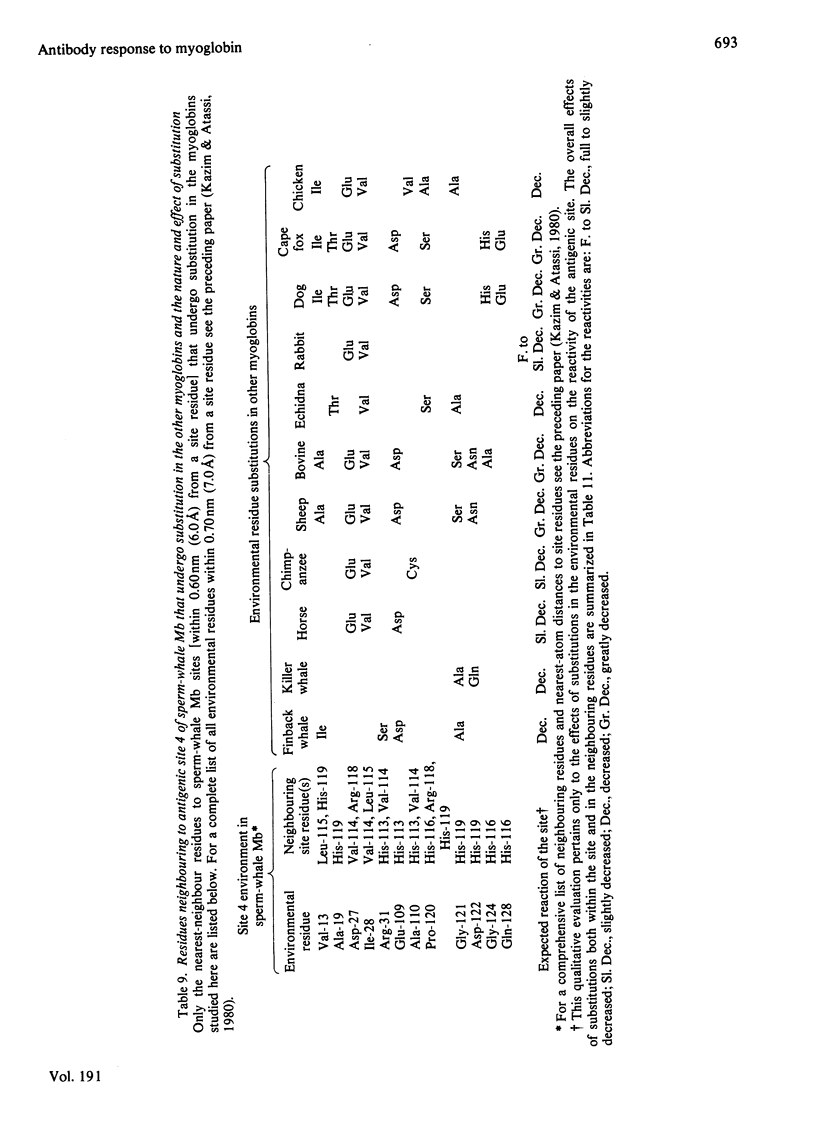
- Kazim A. L., Atassi M. Z. Nearest-neighbour analysis of myoglobin antigenic sites. Nearest-neighbour residues whose replacement can alter the environment of binding-site residue(s) and thus change their characteristics and binding capability. Biochem J. 1980 Dec 1;191(3):673–680. doi: 10.1042/bj1910673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Prediction and conformation by synthesis of two antigenic sites in human haemoglobin by extrapolation from the known antigenic structure of sperm-whale myoglobin. Biochem J. 1977 Oct 1;167(1):275–278. doi: 10.1042/bj1670275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazim A. L., Atassi M. Z. Production of autoantibodies by immunization with rabbit myglobin. Immunochemistry. 1978 Jan;15(1):67–70. doi: 10.1016/0161-5890(78)90028-7. [DOI] [PubMed] [Google Scholar]
- Koketsu J., Atassi M. Z. Immunochemistry of sperm-whale myoglobin. 18. Accurate delineation of the single reactive region in sequence 120-153 by study of synthetic peptides. Biochim Biophys Acta. 1973 Dec 6;328(2):289–302. [PubMed] [Google Scholar]
- Koketsu J., Atassi M. Z. Immunochemistry of sperm-whale myoglobin. XVI. Accurate delineation of the single region in sequence 1-55 by immunochemical studies of synthetic peptides. Some conclusions concerning antigenic structures of proteins. Immunochemistry. 1974 Jan;11(1):1–8. doi: 10.1016/0019-2791(74)90335-8. [DOI] [PubMed] [Google Scholar]
- Lee C. L., Atassi M. Z. Enzymic and immunochemical properties of lysozyme. Accurate definition of the antigenic site around the disulphide bridge 30-115 (site 3) by 'surface-simulation' synthesis. Biochem J. 1977 Dec 1;167(3):571–581. doi: 10.1042/bj1670571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Romero Herrera A. E., Lehmann H. The myoglobin of primates. II. Pan Troglodytes (chimpanzee). Biochim Biophys Acta. 1972 Aug 31;278(1):62–67. doi: 10.1016/0005-2795(72)90107-9. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Castillo O. The myoglobin of primates. VIII. Nycticebus coucang (slow loris). Biochim Biophys Acta. 1976 Feb 20;420(2):387–396. doi: 10.1016/0005-2795(76)90330-5. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Castillo O. The primary structure of the myoglobin of rabbit (Oryctolagus cuniculus) Biochim Biophys Acta. 1976 Jul 19;439(1):51–54. doi: 10.1016/0005-2795(76)90159-8. [DOI] [PubMed] [Google Scholar]
- Romero-Herrera A. E., Lehmann H., Joysey K. A., Friday A. E. On the evolution of myoglobin. Philos Trans R Soc Lond B Biol Sci. 1978 May 9;283(995):61–163. doi: 10.1098/rstb.1978.0018. [DOI] [PubMed] [Google Scholar]
- Sakata S., Atassi M. Z. Immunochemistry of serum albumin--V. A time-dependent examination of the antibody response to bovine serum albumin by the activity of its third domain. Mol Immunol. 1979 Jul;16(7):451–456. doi: 10.1016/0161-5890(79)90070-1. [DOI] [PubMed] [Google Scholar]
- Sakata S., Atassi M. Z. Immunochemistry of serum albumin. VI. A dynamic approach to the immunochemical cross reactions of proteins using serum albumins from various species as models. Biochim Biophys Acta. 1979 Feb 26;576(2):322–332. doi: 10.1016/0005-2795(79)90407-0. [DOI] [PubMed] [Google Scholar]
- Sakata S., Atassi M. Z. Immunochemistry of serum albumin. X. Five major antigenic sites of human serum albumin are extrapolated from bovine albumin and confirmed by synthetic peptides. Mol Immunol. 1980 Jan;17(1):139–142. doi: 10.1016/0161-5890(80)90134-0. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. I. Crystallographic refinement of metmyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):537–568. doi: 10.1016/s0022-2836(77)80111-3. [DOI] [PubMed] [Google Scholar]
- Twining S. S., Atassi M. Z. Antibody-combining sites can be mimicked synthetically. Surface-simulation synthesis of the immunoglobulin new combining site to the gamma-hydroxyl derivative of vitamin K1. J Biol Chem. 1978 Aug 10;253(15):5259–5262. [PubMed] [Google Scholar]
- Twining S. S., Atassi M. Z. Use of immunoadsorbents for the study of antibody binding to sperm whale myoglobin and its synthetic antigenic sites. J Immunol Methods. 1979;30(2):139–151. doi: 10.1016/0022-1759(79)90088-7. [DOI] [PubMed] [Google Scholar]
- Vötsch W., Anderer F. A. The N-terminal amino acid sequence of sheep heart myoglobin. Z Naturforsch B. 1972 Feb;27(2):157–159. doi: 10.1515/znb-1972-0210. [DOI] [PubMed] [Google Scholar]


