Abstract
The kinetics of the reactions of the active-centre thiol groups of papain (EC 3.4.22.2) and ficin (EC 3.4.22.3) with the two-protonic-state reactivity probes 2,2'-dipyridyl disulphide, n-propyl 2-pyridyl disulphide and 4-(N-aminoethyl 2'-pyridyl disulphide)- 7-nitrobenzo-2-oxa-1,3-diazole (compound I) were studied over a wide range of pH. Differences between the reactivities of ficin and papain towards the cationic forms of the alkyl 2-pyridyl disulphide probes suggest that ficin contains a cationic site without exact analogue in papain, and the striking difference in the shapes of the pH-rate profiles for the reactions of the two enzymes with compound (1) suggests differences in the mobilities or dispositions of the active-centre histidine imidazole groups with respect to relevant hydrophobic binding areas. The evidence from reactivity-probe studies that the papain catalytic mechanism involves substantial repositioning of the active-centre imidazole group during the catalytic act does not apply also to ficin. If ficin contains an aspartic acid residue analogous to aspartic acid-158 in papain, the pKa of its carboxy group is probably significantly lower than the pKa of the analogous group in papain.
Full text
PDF

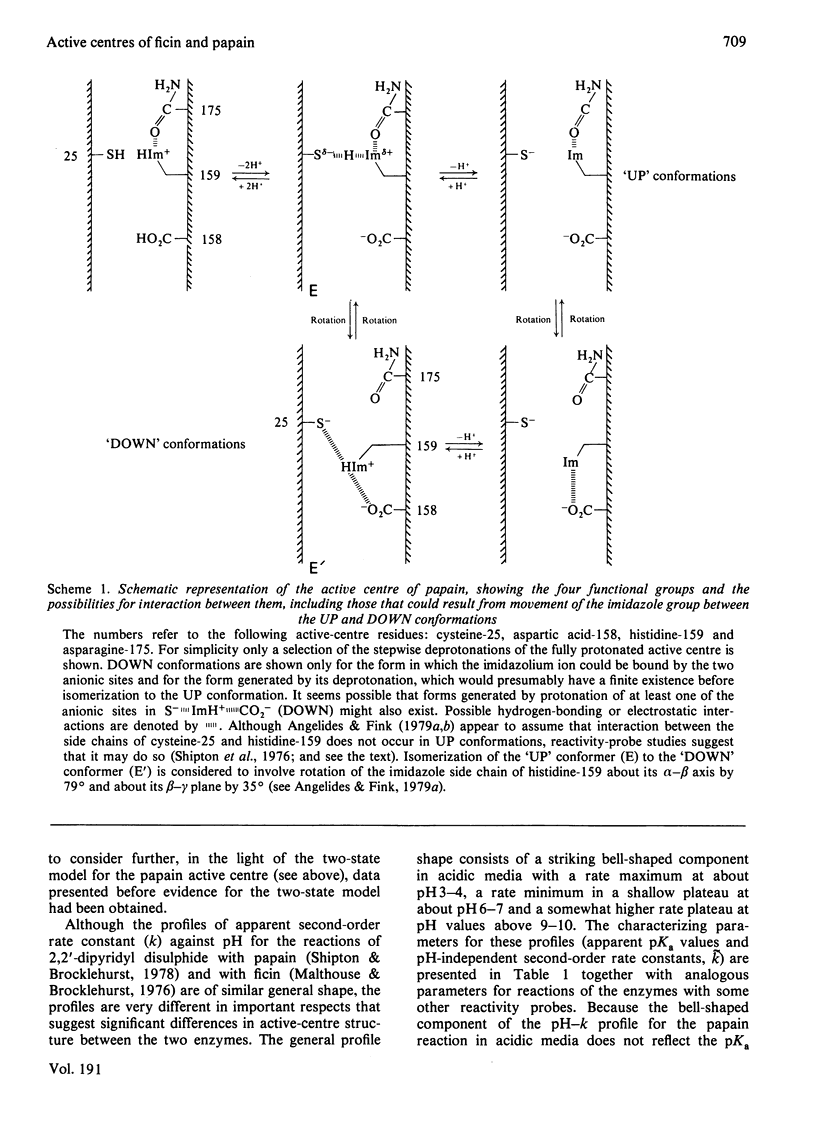

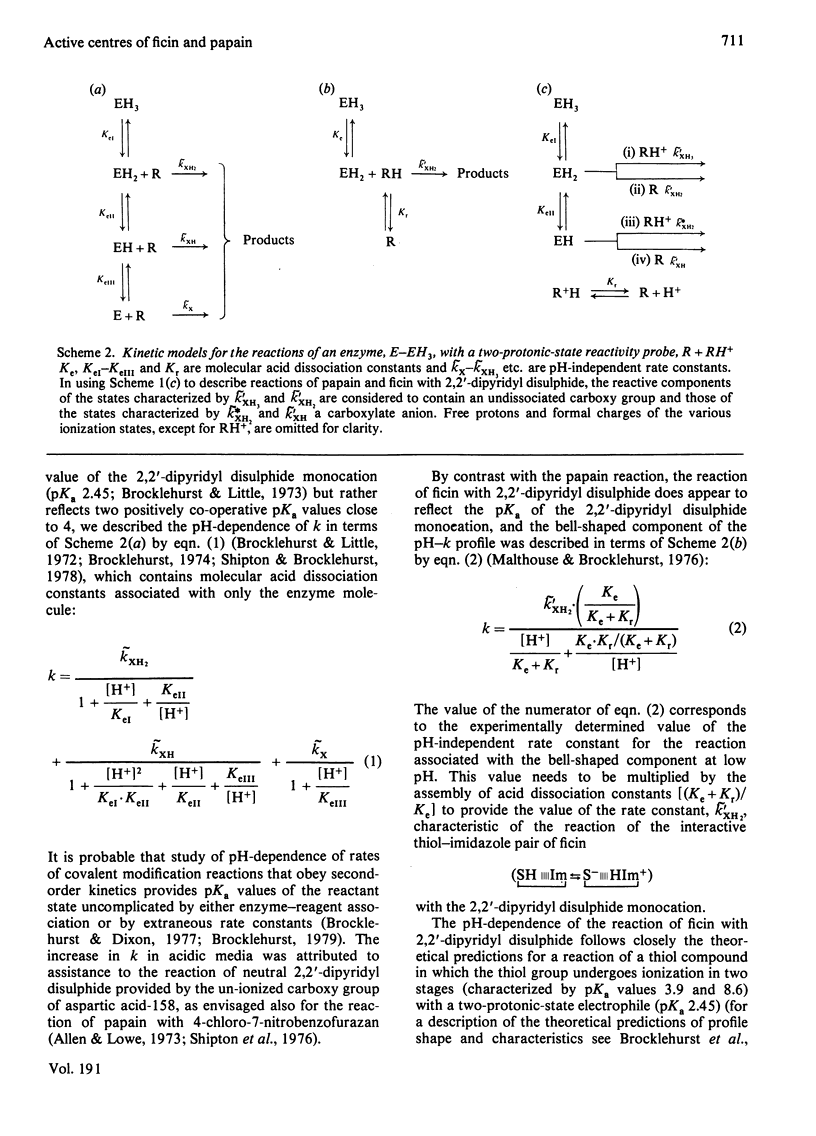
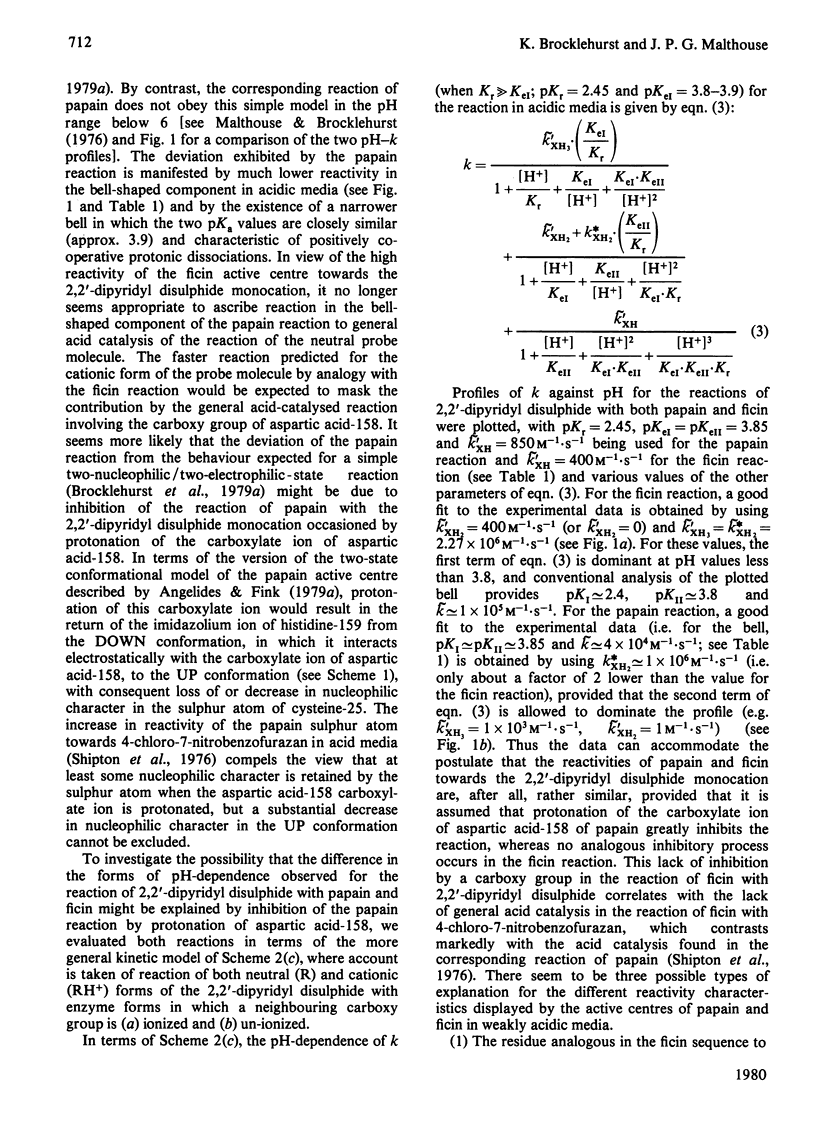
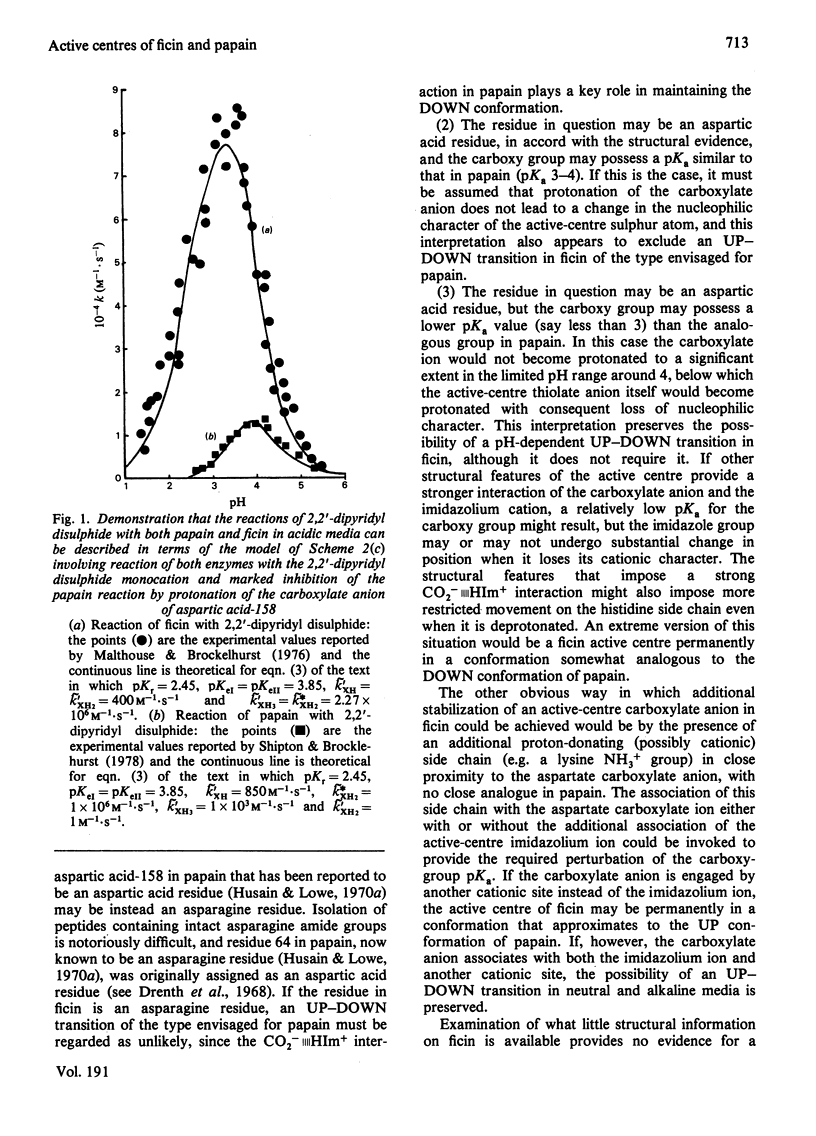




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alecio M. R., Dann M. L., Lowe G. The specificity of the S1' subsite of papain. Biochem J. 1974 Aug;141(2):495–501. doi: 10.1042/bj1410495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G., Lowe G. Investigation of the active site of papain with fluorescent probes. Biochem J. 1973 Aug;133(4):679–686. doi: 10.1042/bj1330679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelides K. J., Fink A. L. Cryoenzymology of papain: reaction mechanism with an ester substrate. Biochemistry. 1978 Jun 27;17(13):2659–2668. doi: 10.1021/bi00606a032. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Fink A. L. Mechanism of Action of papain with a specific anilide substrate. Biochemistry. 1979 May 29;18(11):2355–2363. doi: 10.1021/bi00578a034. [DOI] [PubMed] [Google Scholar]
- Angelides K. J., Fink A. L. Mechanism of thiol protease catalysis: detection and stabilization of a tetrahedral intermediate in papain catalysis. Biochemistry. 1979 May 29;18(11):2363–2369. doi: 10.1021/bi00578a035. [DOI] [PubMed] [Google Scholar]
- Baker E. N. Structure of actinidin: details of the polypeptide chain conformation and active site from an electron density map at 2-8 A resolution. J Mol Biol. 1977 Sep 25;115(3):263–277. doi: 10.1016/0022-2836(77)90154-1. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. The pH-dependence of second-order rate constants of enzyme modification may provide free-reactant pKa values. Biochem J. 1977 Dec 1;167(3):859–862. doi: 10.1042/bj1670859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Herbert J. A., Norris R., Suschitzky H. Evidence for association-activation effects in reactions of papain from studies on its reactivity towards isomeric two-protonic-state reactivity probes. Biochem J. 1979 Nov 1;183(2):369–373. doi: 10.1042/bj1830369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactions of papain and of low-molecular-weight thiols with some aromatic disulphides. 2,2'-Dipyridyl disulphide as a convenient active-site titrant for papain even in the presence of other thiols. Biochem J. 1973 May;133(1):67–80. doi: 10.1042/bj1330067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Little G. Reactivities of the various protonic states in the reactions of papain and of L-cysteine with 2,2'- and with 4,4'- dipyridyl disulphide: evidence for nucleophilic reactivity in the un-ionized thiol group of the cysteine-25 residue of papain occasioned by its interaction with the histidine-159-asparagine-175 hydrogen-bonded system. Biochem J. 1972 Jun;128(2):471–474. doi: 10.1042/bj1280471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P. Mechanism of the reaction of papain with substrate-derived diazomethyl ketones. Implications for the difference in site specificity of halomethyl ketones for serine proteinases and cysteine proteinases and for stereoelectronic requirements in the papain catalytic mechanism. Biochem J. 1978 Nov 1;175(2):761–764. doi: 10.1042/bj1750761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Malthouse J. P., Shipton M. Evidence that binding to the s2-subsite of papain may be coupled with catalytically relevant structural change involving the cysteine-25-histidine-159 diad. Kinetics of the reaction of papain with a two-protonic-state reactivity probe containing a hydrophobic side chain. Biochem J. 1979 Nov 1;183(2):223–231. doi: 10.1042/bj1830223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Stuchbury T., Malthouse J. P. Reactivities of neutral and cationic forms of 2,2'-dipyridyl disulphide towards thiolate anions. Detection of differences between the active centres of actinidin, papain and ficin by a three-protonic-state reactivity probe. Biochem J. 1979 Nov 1;183(2):233–238. doi: 10.1042/bj1830233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K. The equilibrium assumption is valid for the kinetic treatment of most time-dependent protein-modification reactions. Biochem J. 1979 Sep 1;181(3):775–778. doi: 10.1042/bj1810775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Swen H. M., Wolthers B. G. Structure of papain. Nature. 1968 Jun 8;218(5145):929–932. doi: 10.1038/218929a0. [DOI] [PubMed] [Google Scholar]
- Drenth J., Kalk K. H., Swen H. M. Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry. 1976 Aug 24;15(17):3731–3738. doi: 10.1021/bi00662a014. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. A reinvestigation of residues 64-68 and 175 in papain. Evidence that residues 64 and 175 are asparagine. Biochem J. 1970 Feb;116(4):689–692. doi: 10.1042/bj1160689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Evidence for histidine in the active site of papain. Biochem J. 1968 Aug;108(5):855–859. doi: 10.1042/bj1080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The amino acid sequence around the active-site cysteine and histidine residues, and the buried cysteine residue in ficin. Biochem J. 1970 Apr;117(2):333–340. doi: 10.1042/bj1170333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. The location of the active-site histidine residue in the primary sequence of papain. Biochem J. 1968 Aug;108(5):861–866. doi: 10.1042/bj1080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortt A. A., Liu T. Y. On the mechanism of action of streptococcal proteinase. 3. The effect of pH, organic solvents, and deuterium oxide on the proteinase-catalyzed hydrolysis of N-acylamino acid esters. Biochemistry. 1973 Jan 16;12(2):338–345. doi: 10.1021/bi00726a025. [DOI] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. A kinetic method for the study of solvent environments of thiol groups in proteins involving the use of a pair of isomeric reactivity probes and a differential solvent effect. Investigation of the active centre of ficin by using 2,2'- and 4,4'- dipyridyl disulphides as reactivity probes. Biochem J. 1980 Jan 1;185(1):217–222. doi: 10.1042/bj1850217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Intramolecular inhibition by enzyme of site-specific modification reactions can mask pKa values characteristic of the reaction pathway: do the side chains of aspartic acid-158 and lysine-156 of papain form an ion-pair? [proceedings]. Biochem Soc Trans. 1978;6(1):250–252. doi: 10.1042/bst0060250. [DOI] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Preparation of fully active ficin from Ficus glabrata by covalent chromatography and characterization of its active centre by using 2,2'-depyridyl disulphide as a reactivity probe. Biochem J. 1976 Nov;159(2):221–234. doi: 10.1042/bj1590221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. A., Alden R. A., Birktoft J. J., Freer T., Kraut J. Re-examination of the charge relay system in subtilisin comparison with other serine proteases. J Biol Chem. 1977 Dec 25;252(24):8875–8883. [PubMed] [Google Scholar]
- Petkov D., Christova E., Stoineva I. Catalysis and leaving group binding in anilide hydrolysis by chymotrypsin. Biochim Biophys Acta. 1978 Nov 10;527(1):131–141. doi: 10.1016/0005-2744(78)90262-0. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the active site of proteases. 3. Mapping the active site of papain; specific peptide inhibitors of papain. Biochem Biophys Res Commun. 1968 Sep 6;32(5):898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Shipton M., Brochlehurst K. Characterization of the papain active centre by using two-protonic-state electrophiles as reactivity probes. Evidence for nucleophilic reactivity in the un-interrupted cysteine-25-histidine-159 interactive system. Biochem J. 1978 May 1;171(2):385–401. doi: 10.1042/bj1710385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Stuchbury T., Brocklehurst K. 4-Chloro-7-nitrobenzo-2-oxa-1,3-diazole as a reactivity probe for the investigation of the thiol proteinases. evidence that ficin and bromelain may lack carboxyl groups conformationally equivalent to that of aspartic acid-158 of papain. Biochem J. 1976 Nov;159(2):235–244. doi: 10.1042/bj1590235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuchbury T., Shipton M., Norris R., Malthouse J. P., Brocklehurst K., Herbert J. A., Suschitzky H. A reporter group delivery system with both absolute and selective specificity for thiol groups and an improved fluorescent probe containing the 7-nitrobenzo-2-oxa-1,3-diazole moiety. Biochem J. 1975 Nov;151(2):417–432. doi: 10.1042/bj1510417. [DOI] [PMC free article] [PubMed] [Google Scholar]


