Abstract
Water scarcity is a global concern that needs addressing through alternative sources. One of the approaches is the use of reclaimed water for irrigation. However, the presence of halogenated compounds and heavy metals in reclaimed water poses significant food safety threats. Therefore, a comprehensive characterization of these contaminants using a reliable method is essential. This study presents an innovative analytical technique that combines electrospray ionization (ESI) with microwave plasma ionization mass spectrometry (MPIMS), enabling the simultaneous detection of organic compounds and heavy metals. The plasma ionization process in metals exhibits novel features, unlike traditional methods, making it suitable for organic and metallic detection in complex matrices. This technique achieved a recovery rate of 78.5–123% and 79.93–119.50% for halogenated compounds and heavy metals, respectively. The limits of detection and quantification ranged from 1.5 ng mL−1 to 3.5 ng mL−1 and 4.5 ng mL−1 to 12.75 ng mL−1, respectively. Analysis of reclaimed water from three irrigation systems revealed concentrations of halogenated compounds and heavy metals below allowable levels set by national agencies, indicating manageable pollution risks. H-compounds, such as diuron and linuron, were prevalent in all samples, while zinc and lead showed higher levels in flood and sub-irrigation systems. Compared to traditional methods, ESI-MPIMS performs well and demonstrates high efficiency, good quantification, and high sensitivity in the analysis of real samples. This study shows that ESI-MPIMS is promising for on-site analysis of organic compounds and heavy metals in complex matrices and is suitable for water quality control and environmental quality assessment for pollutant screening.
Water scarcity is a global concern that needs addressing through alternative sources.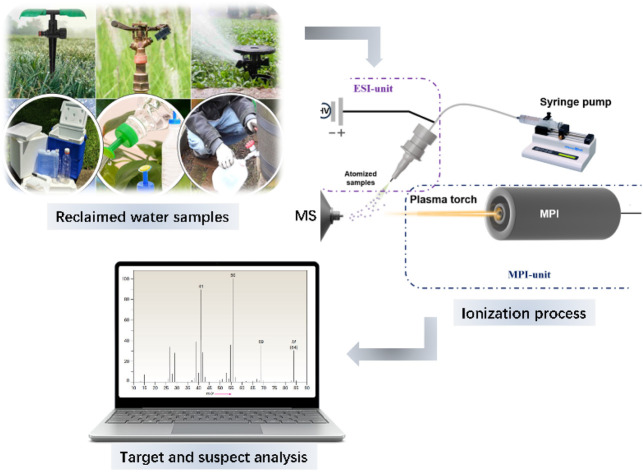
Introduction
The escalation of halogenated compounds (H-compounds) in environmental water has led to global water contamination in recent years.1 The presence of these compounds in water stems from industrial and hospital discharge,2–4 being used in drug manufacturing industries, agriculture, and veterinary applications,5 and in personal care products.6 Despite industrial sewage treatment before discharge, the full elimination of heavy metals remains challenging.7 The utilization of reclaimed water for agricultural activities alleviates water scarcity concerns and reduces the strain on freshwater resources,8 aligning with the United Nations' Sustainable Development Goals (SDGs) agenda, which advocates the global adoption of reclaimed water.9 However, the gradual accumulation of these toxicant pollutants in the reclaimed water within crops, animals, and aquatic organisms poses significant health risks.10
To address this, there is an urgent need to develop a highly sensitive analytical instrument to detect and quantify these pollutants in water. Various approaches have been proposed, such as gas chromatography-tandem mass spectrometry (GC-MS/MS), liquid chromatography-tandem mass spectrometry (LC-MS/MS),4,11–15 atomic absorption/fluorescence spectroscopy (AAS/AFS),16 inductively coupled plasma optical emission mass spectrometry (ICP-OESMS),17 and inductively coupled plasma-mass spectrometry (ICP-MS).18 However, owing to their complexity in terms of analytical sample pretreatment, they are time-consuming, labor-intensive, and expensive. A single instrument can also only achieve ionization detection of organics or elements, which is not conducive to comprehensive analysis and characterization of contamination information in recycled water.
Ambient ionization of real samples, proposed by Cook in 2004, paved the way for ambient mass spectrometry,19 Various ambient ionization techniques have been proposed, such as direct analysis in real time (DART),20 desorption atmospheric pressure chemical ionization (DAPCI),21 flowing afterglow atmospheric pressure glow discharge (FA-APGD),22 dielectric barrier discharge ionization (DBDI),23 low-temperature plasma (LTP) probes,24 microwave plasma torch (MPT),4,25 ambient flame ionization (AFI),26 and ambient electric arc ionization (AEAI),27 which are widely used for detecting pollutants in real environments and biological samples.
Among these techniques, ambient microwave plasma ionization (MPI) has shown excellent sensitivity, precision, and high level of matrix tolerance similar to ICP-MS, enabling the dissociation of charged metallic elements.4,25,28 In MPI, plasma is generated through microwave discharge and sustained by a high-frequency electromagnetic field (2.45 GHz).25,29 Whereas LTP employs dielectric barrier discharge (DBD) powered by radio frequency (RF) to produce low-power plasma.30 The modern MPI ion source is a modernized version of what Jin and colleagues designed and was further developed by anohter group at Indiana University.30,31 Since its introduction, MPI has been widely adopted with various types of linear mass spectrometers in diverse fields for analyzing chemical substances, demonstrating superior performance over traditional techniques. For example, Zhao et al.,32 utilized MPI to detect sterols in urban water, while Shuaibu,4 Chu25 and Miao33 used it to analyze drug samples in liquid solutions, showcasing MPI's potential application in analytical fields for environmental control, food analysis, and water quality assessment.34
Considering the diverse chemical compositions of the analytes, it is challenging to cover all the analytes with a single analytical mass spectrometer.
Researchers have combined different ionization techniques based on ambient plasma to broaden the detectable analyte molecules.4,28 These composite ion sources utilize distinct and collective ionization modes through parameter adjustments, allowing the identification of substances with diverse characteristics.
We hypothesize that combining MPI with electrospray ionization (ESI) techniques can effectively characterize heavy metals and H-compounds in reclaimed water with high precision. In this study, we integrated ESI with PMI-MS (ESI-MPIMS) to rapidly analyze heavy metals and H-compounds in real reclaimed water, enabling rapid detection of trace levels of these substances in complex matrices, such as environmental samples, with high sensitivity and selectivity. This profitable approach will be an alternative to traditional ion sources.
Materials and methods
Chemicals and standards
Diuron, flumequine, linuron, norfloxacin, 2,2-dichloroacetamide, 2-bromo-5-chlorophenol, and 3,5-diiodosalicylic acid were purchased from Sigma-Aldrich (Darmstadt, Germany). Mn, Ni, Pb, and Zn standard solutions were purchased from Beijing North Weiye Metrology Technology Research Institute Co., Ltd (Shanghai, China). High-purity argon (99.999% purity) was purchased from Shanghai Ji Liang Standard Reference Gases Co., Ltd (Shanghai, China). Methanol and acetone were purchased from Aladdin Co., Ltd (Shanghai, China). High-purity water from a Millipore-Q-plus water purification system (Millipore, Billerica, MA, USA) was used for this work. Linuron, norfloxacin, 2,2 dichloroacetamide, and 2-bromo-5-chlorophenol were dissolved and diluted in water and methanol at 1 : 1; diuron and 3,5-diiodosalicylic acid were diluted in acetone solution; and flumequine was dissolved and diluted in ethanol to obtain a working solution at concentrations of 50, 40, 30, 20, 10, 5, and 1 μg L−1. Water and methanol at a ratio of 1 : 1 were used to prepare standard solutions of heavy metals at concentrations of 50, 40, 30, 20, 10, 5, and 1 μg L−1. These standard solutions were used to analyze the instrument's performance and plot the quantification curves. All standards were stored at 4 °C.
Study sites and sample collection
A total of 18 samples were collected directly from irrigation farmlands located in three cities within Zhejiang and Jiangsu Provinces: Hangzhou, Wenzhou, and Xuzhou. The geographic locations of the sampling sites are shown in Fig. S1.† These samples were categorized into three groups: two samples each from the flood irrigation system, lateral move irrigation system, and sub-irrigation system. The first group includes six samples from the flood irrigation system. The second group comprises six samples from the lateral move irrigation system. The third group contains six samples from the sub-irrigation system (Tables 2–4). Water samples were collected within three days using a 3 Liter water sampling bottle kit (Beijing Grasp Technology, China) to obtain 2 Liters of water from the taps in the respective fields. The collected samples were immediately stored at 4 °C and transferred to the laboratory for processing within 24 hours.
Molecular ions of 2,2-dichloroacetamide, diuron, flumequine, and 2-bromo-5-chlorophenol detected by MPT-MS in water samples collected from irrigation farmlanda.
| S/N | Samples | Spiked (ng L−1) | 2,2,-Dichroloacetamide ion (m/z 127) | Diuron ion (m/z 232) | Flumequine ion (m/z 262) | 2-Bromo-5-chlorophenol ion (m/z 207) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | |||
| 1 | Flood irrigation system | 5 | 1.84 | 7.08 | 104.80 | 3.15 | 7.51 | 87.20 | 2.68 | 7.52 | 96.80 | 2.5 | 7.84 | 106.76 |
| 2 | 5 | 2.97 | 7.86 | 97.80 | 1.89 | 8.04 | 123.00 | 2.55 | 7.87 | 106.40 | 2.93 | 7.17 | 84.88 | |
| 3 | 5 | 3.05 | 7.42 | 87.40 | 2.51 | 7.95 | 108.80 | 3.25 | 8.84 | 111.80 | 1.04 | 5.61 | 91.45 | |
| 4 | 5 | 2.81 | 6.99 | 83.60 | 2.56 | 6.71 | 83.00 | 4.15 | 9.10 | 99.00 | 1.11 | 6.11 | 99.83 | |
| 5 | 5 | 2.04 | 7.09 | 101.00 | 3.31 | 7.89 | 91.60 | 3.85 | 8.89 | 100.80 | 1.03 | 5.22 | 83.92 | |
| 6 | 5 | 1.43 | 6.01 | 91.60 | 3.33 | 8.02 | 93.80 | 2.25 | 7.63 | 107.60 | 2.4 | 7.62 | 104.46 | |
| 1 | Lateral move irrigation system | 5 | 1.59 | 5.95 | 87.2 | 2.52 | 8.11 | 111.80 | — | 3.99 | 79.8 | 0.78 | 5.54 | 95.2 |
| 2 | 5 | — | 4.95 | 99 | 1.84 | 7.3 | 109.20 | 3.25 | 7.87 | 92.4 | — | 5.02 | 100.4 | |
| 3 | 5 | 3.03 | 7.61 | 91.6 | 2.02 | 8.01 | 119.80 | 0.98 | 6.67 | 113.8 | — | 5.33 | 106.6 | |
| 4 | 5 | — | 4.84 | 96.8 | 2.00 | 6.89 | 97.80 | — | 5.64 | 112.8 | — | 5.93 | 118.6 | |
| 5 | 5 | 2.04 | 6.45 | 88.2 | 1.78 | 7.11 | 106.60 | 2.75 | 7.68 | 98.6 | 0.53 | 5.54 | 100.2 | |
| 6 | 5 | 2.06 | 6.01 | 79 | 0.77 | 6.01 | 104.80 | 3.97 | 8.82 | 97 | 1.64 | 6.00 | 87.2 | |
| 1 | Sub-irrigation systems | 5 | 1.59 | 6.85 | 105.20 | 2.61 | 7.22 | 92.20 | 0.85 | 6.53 | 113.60 | 1.57 | 6.15 | 91.57 |
| 2 | 5 | 2.01 | 6.11 | 82.00 | 3.04 | 7.69 | 93.00 | 1.35 | 6.39 | 100.80 | 4.02 | 8.19 | 83.30 | |
| 3 | 5 | 3.02 | 7.89 | 97.40 | 1.36 | 7.48 | 122.40 | 1.66 | 7.20 | 110.80 | 2.5 | 6.54 | 80.80 | |
| 4 | 5 | 2.91 | 7.11 | 84.00 | 2.23 | 8.11 | 117.60 | 3.77 | 8.96 | 103.80 | 2.97 | 8.62 | 113.14 | |
| 5 | 5 | 2.18 | 7.02 | 96.80 | 2.00 | 7.18 | 103.60 | 2.75 | 7.28 | 90.60 | 1.16 | 6.71 | 111.03 | |
| 6 | 5 | 4.17 | 8.86 | 93.80 | 0.88 | 6.21 | 106.60 | 1.15 | 6.75 | 112.00 | 2.31 | 7.96 | 113.15 | |
—: not detected.
Molecular ions of norfloxacin, linuron, and 3,5-diiodosalicylic acid detected by MPT-MS in water samples collected from irrigation farmlanda.
| S/N | Samples | Norfloxacin ion (m/z 320) | Linuron ion (m/z 248) | 3,5-Diiodosalicylic acid ion (m/z 390) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked (ng L−1) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Spiked (ng L−1) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Spiked (ng L−1) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | ||
| 1 | Flood irrigation system | 2 | — | 1.98 | 99 | 10 | 2.15 | 11.24 | 90.90 | 5 | — | 5.81 | 116.2 |
| 2 | 2 | 0.98 | 3.11 | 106.5 | 10 | 2.04 | 12.11 | 100.70 | 5 | 2.04 | 8.03 | 119.8 | |
| 3 | 2 | 1.11 | 2.89 | 89 | 10 | 1.65 | 9.81 | 81.60 | 5 | 2.65 | 8 | 107 | |
| 4 | 2 | 1.13 | 2.78 | 82.5 | 10 | 3.32 | 11.69 | 83.70 | 5 | — | 4.52 | 90.4 | |
| 5 | 2 | — | 4.05 | 202.5 | 10 | 2.95 | 13.05 | 101.00 | 5 | — | 5.05 | 101 | |
| 6 | 2 | 1.43 | 3.11 | 84.00 | 10 | 1.81 | 10.85 | 90.40 | 5 | — | 4.91 | 98.2 | |
| 1 | Lateral move irrigation system | 2 | 1.59 | 4.01 | 121 | 10 | 1.59 | 12.56 | 109.70 | 5 | 1.59 | 7.06 | 109.4 |
| 2 | 2 | 1.24 | 3.31 | 103.5 | 10 | 2.81 | 11.49 | 86.80 | 5 | — | 4.49 | 89.8 | |
| 3 | 2 | — | 2.04 | 102 | 10 | 0.79 | 9.84 | 90.50 | 5 | 0.79 | 6.04 | 105 | |
| 4 | 2 | — | 2.04 | 102 | 10 | 0 | 9.81 | 98.10 | 5 | — | 5.11 | 102.2 | |
| 5 | 2 | 1.05 | 2.86 | 90.5 | 10 | 2.21 | 11.08 | 88.70 | 5 | — | 6.95 | 139 | |
| 6 | 2 | 0.65 | 3.01 | 118 | 10 | 3.1 | 12.76 | 96.60 | 5 | 3.1 | 8.04 | 98.8 | |
| 1 | Sub-irrigation systems | 2 | 1.25 | 3.37 | 106.00 | 10 | 0.51 | 11.31 | 108.00 | 5 | 0.51 | 5.91 | 108.00 |
| 2 | 2 | — | 2.01 | 100.50 | 10 | 1.09 | 11.48 | 103.90 | 5 | 1.09 | 7 | 118.20 | |
| 3 | 2 | 1.13 | 3.22 | 104.50 | 10 | 1.33 | 12.07 | 107.40 | 5 | 1.33 | 6.52 | 103.80 | |
| 4 | 2 | 0.71 | 3.01 | 115.00 | 10 | 3.02 | 12.1 | 90.80 | 5 | 3.02 | 7.58 | 91.20 | |
| 5 | 2 | 1.0 | 2.78 | 89.00 | 10 | 1.75 | 10.71 | 89.60 | 5 | 1.75 | 6.56 | 96.20 | |
| 6 | 2 | 1.23 | 2.96 | 86.50 | 10 | 0.91 | 9.89 | 89.80 | 5 | 0.91 | 6.11 | 104.00 | |
—: not detected.
Molecular ions of zinc, manganese, nickel, and lead detected by MPT-MS in water samples collected from irrigation farmlanda.
| S/N | Samples | Zinc ion | Manganese ion | Nickel ion | Lead ion | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked (ng L−1) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Spiked (ng L−1) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Spiked (ng L−1) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | Spiked (ng L−1) | Original (ng L−1) | Detected (ng L−1) | Recovery (%) | ||
| 1 | Flood irrigation system | 20 | 2.5 | 19.95 | 87.25 | 20 | 0.14 | 20.19 | 100.25 | 15 | 0.51 | 15.01 | 96.67 | 10 | 2.89 | 13.50 | 106.10 |
| 2 | 20 | 3.5 | 21.09 | 87.95 | 20 | 0.32 | 19.51 | 95.95 | 15 | 1.2 | 14.86 | 91.07 | 10 | 3.08 | 12.95 | 98.70 | |
| 3 | 20 | 1.27 | 20.81 | 97.70 | 20 | 1.27 | 20.93 | 98.30 | 15 | 0.22 | 14.09 | 92.47 | 10 | 2.42 | 12.84 | 104.20 | |
| 4 | 20 | 3.5 | 21.72 | 91.10 | 20 | 0.15 | 18.44 | 91.45 | 15 | 3.36 | 16.99 | 90.87 | 10 | 2.25 | 12.59 | 103.40 | |
| 5 | 20 | 3.07 | 19.45 | 81.90 | 20 | 0.85 | 20.53 | 98.40 | 15 | 2.44 | 18.09 | 104.33 | 10 | 1.98 | 12.78 | 108.00 | |
| 6 | 20 | 5.5 | 22.01 | 82.55 | 20 | 0.24 | 22.97 | 113.65 | 15 | 2.5 | 15.15 | 84.33 | 10 | 3.25 | 14.50 | 112.5 | |
| 1 | Lateral move irrigation system | 20 | 2.01 | 22.15 | 100.70 | 20 | 0.77 | 20.86 | 100.45 | 15 | 2.08 | 15.95 | 92.47 | 10 | 3.50 | 14.25 | 107.50 |
| 2 | 20 | 3.25 | 22.95 | 98.50 | 20 | — | 18.5 | 92.5 | 15 | 0.91 | 14.19 | 88.53 | 10 | 3.01 | 12.38 | 93.70 | |
| 3 | 20 | 2.08 | 23.02 | 104.70 | 20 | 9.69 | 29.28 | 97.95 | 15 | 1.05 | 13.9 | 85.67 | 10 | 1.14 | 13.09 | 119.50 | |
| 4 | 20 | 1.35 | 22.00 | 103.25 | 20 | — | 17.58 | 87.9 | 15 | 1.78 | 14.84 | 87.07 | 10 | 2.85 | 11.97 | 91.20 | |
| 5 | 20 | 1.35 | 19.09 | 88.70 | 20 | 0.52 | 20.86 | 101.7 | 15 | — | 14.56 | 97.10 | 10 | 4.69 | 14.50 | 98.10 | |
| 6 | 20 | 1.41 | 19.90 | 92.45 | 20 | 1.63 | 21.33 | 98.5 | 15 | 2.04 | 17.98 | 106.27 | 10 | 1.27 | 11.01 | 97.40 | |
| 1 | Sub-irrigation systems | 20 | 3.20 | 24.56 | 106.80 | 20 | 0.36 | 21.48 | 105.60 | 15 | 1.01 | 14.05 | 86.933 | 10 | 3.50 | 12.22 | 87.20 |
| 2 | 20 | 2.69 | 23.96 | 106.35 | 20 | 1.06 | 23.55 | 112.45 | 15 | 0.36 | 15.9 | 103.60 | 10 | 2.41 | 12.69 | 102.80 | |
| 3 | 20 | 2.36 | 22.55 | 100.95 | 20 | 1.25 | 20.86 | 98.05 | 15 | 0.29 | 14.69 | 96.00 | 10 | 3.62 | 12.48 | 88.60 | |
| 4 | 20 | 3.05 | 22.02 | 94.85 | 20 | 1.36 | 23.99 | 113.15 | 15 | — | 17.01 | 113.40 | 10 | 3.49 | 13.11 | 96.20 | |
| 5 | 20 | 1.39 | 21.94 | 102.75 | 20 | 1.15 | 22.05 | 104.5 | 15 | 1.96 | 19.02 | 113.73 | 10 | 2.49 | 12.18 | 96.90 | |
| 6 | 20 | 2.35 | 21.36 | 95.05 | 20 | — | 23.32 | 116.6 | 15 | — | 13.86 | 92.40 | 10 | 4.75 | 15.50 | 107.50 | |
—: not detected.
Instrument
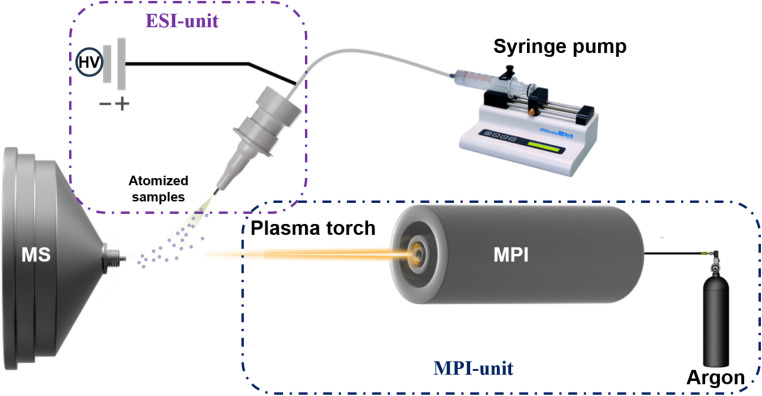
The novel flexible microwave plasma ionization mass spectrometry system integrated with electrospray ionization (ESI-MPI-MS) consists of a two-unit microwave plasma generator and an electrospray ionization source, as shown in Fig. 1. The microwave plasma torch comprised three concentric tubes: an outer tube for microwave input, an intermediate tube for supporting gas, and a central tube for working gas.4 Argon gas flows through the intermediate and central tubes as supporting and working gases, respectively. The argon flow rate was controlled using a rotameter (Horiba Metron, Beijing, China). The system was operated at a microwave frequency of 2.5 GHz with adjustable power ranging from 0 to 200 W to generate discharge. Subsequently, an approximately cone-shaped white–purple plasma plume was drawn out of the chamber to the sample surface through the quartz tube. The MPI and ESI were set at an optimized angle of 64°, with their focal pointing positions directly aligned on the three-dimensional adjustable stage, enabling precise alignment with the mass spectrometer inlet. The samples were injected using a syringe pump at a flow rate of 5–10 μL min−1. We generated nebulized atomized samples from the ESI spray and fed them into different regions of white–purple cone-shaped plasma tips for direct ionization. In this study, a linear ion trap mass spectrometer (Thermo Electron San Jose Instruments, U.S.A.) was used for ion detection and data acquisition was performed using Xcalibur® software (version 2.2 SP1.48; TFS, San Jose, CA, USA) embedded in the instrument for all experiments. The instrument parameters for the experiment were set as follows: capillary temperature: −8 V; capillary temperature: 300 °C; tube lens voltage: −120 V; microscan: 3. The ESI voltage (DC) used in the control experiment was 3.0 kV.
Fig. 1. Schematic diagram of the experimental setup. The system comprises two units: ESI unit for atomizing samples connected to a syringe pump to control the sample flow. MPI-unit for ionizing the atomized sample before entering the mass analyzer.
Result and discussion
Previous studies have shown that MPI-MS can be used to directly analyze environmental H-compounds pollutants with little or no pretreatment.4,25 Here, ESI-MPIMS was used directly for the detection of H-compounds (2,2,dichroloacetamide, norfloxacin, diuron, linuron, 3,5-diiodosalicylic acid, flumequine, and 2-bromo-5-chlorophenol) and heavy metal (zinc, manganese, nickel, and lead) pollutants in reclaimed water.
The system consists of two units (ESI-unit and MPI-unit), as shown in Fig. 1. ESI assists in delivering highly nebulized atomized samples to the ionization region with high injection efficiency. The high-temperature plasma tips come into contact with atomized samples in the ionization region, undergo desorption and are fully ionized by the plasma torch through a series of reactions with atmospheric components in the ionization region, which become activated and generate cations, active free radicals, high-energy electrons, and metastable particles.
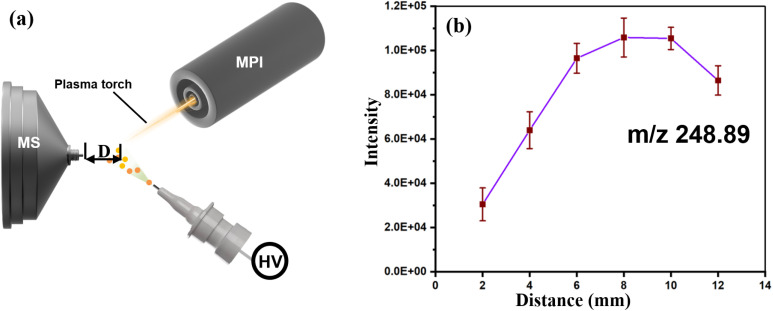
To obtain good ionization efficiency for H-compounds and heavy metals in an ambient mass spectrometer, proper optimization of the experimental data and the physical setup of the system are needed. According to Fig. 2, the linuron (248.89 m/z) signal intensity increases with increasing distance from the ionization region to the MS inlet (Fig. 2b). The intensity reaches a peak at 7.8 to 9.6 mm and then starts to decrease significantly. A simplified scheme of the system showing the adjustable parameters is shown in Fig. 3a, and a graph of the 248.89 m/z signal intensity and plasma energy is shown in Fig. 3b. The signal intensity steadily increases as the plasma energy increases from 75 to 115 W. This suggests that plasma beams with sufficient energy are necessary for the best ionization of both H-compounds and heavy metals.
Fig. 2. System optimization. (a) A schematic representation of the system illustrating the adjustable distance (D) between the plasma torch and the mass spectrometer (MS) inlet. (b) Influence of the distance (D) on the intensity of linuron product ions (m/z 248.89). Optimal ionization is achieved at an intermediate distance. Error bars indicate the standard deviation of intensity measurements at each distance.
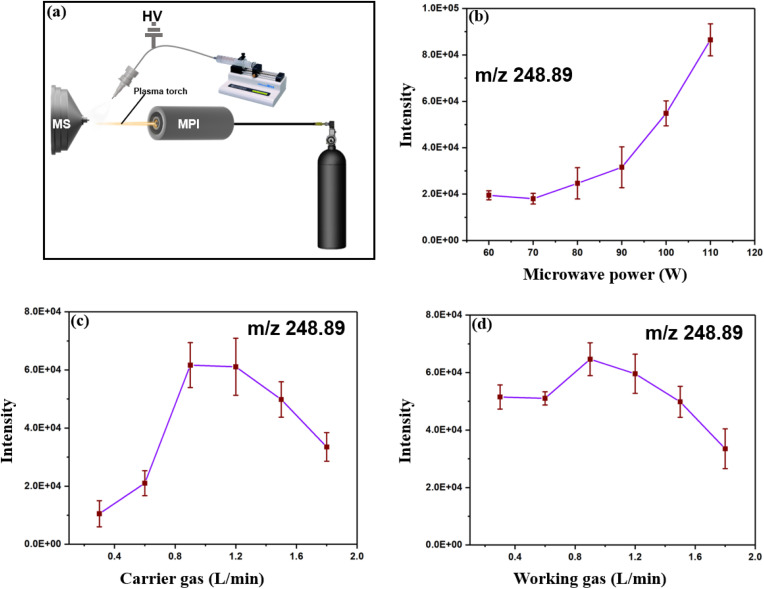
Fig. 3. Optimization of the experimental system parameters. (a) A schematic of the system highlighting the ESI, MPI, syringe pump, and gas cylinder for the carrier and working (b) Influence of microwave plasma power on the intensity of linuron product ions (m/z 248.89), showing a positive correlation between power and ion intensity up to 110 W. (c) Effect of carrier gas flow rate (L min−1) on ion intensity, with optimal ionization observed near 0.8 L min−1. (d) Influence of working gas flow rate (L min−1) on ion intensity, with peak intensity around 0.9 L min−1. Error bars in (b)–(d) represent the standard deviation of intensity measurements at each setting.
For the working gas, the signal intensity at 248.89 m/z was stable from 0.4 to 0.6 L min−1 and then increased from 0.65 to 0.9 L min−1 but decreased. This may be a result of the high flow of Ar, which lowers the plasma temperature and influences the ionization ability of the plasma (Fig. 3c). The flow rate of the carrier gas influences the particle dimension of the spray plume and subsequently affects the ionization efficiency of the plasma torch (Fig. 3d). The optimized parameters were as follows: MS inlet distance, 8.5 mm; microwave energy, 115 W; working gas flow rate, 0.8 L min; and carrier gas flow rate, 0.7 L min−1.
Analytical performance
Preliminary experiments were conducted to validate the quantification performance, reliability, and analytical power of the ESI-MPIMS system for detecting 7 H-compounds (2,2,dichroloacetamide, norfloxacin, diuron, linuron, 3,5-diiodosalicylic acid, flumequine, and 2-bromo-5-chlorophenol) and 4 heavy metals (zinc, manganese, nickel, and lead). ESI-MPIMS was used to directly quantify 7 standards of H-compounds and 4 heavy metals, which demonstrated good linearity with R2 values ranging from 0.977 to 0.998 over a linear range of 1–50 μg L−1 (Fig. S2 and S3†). The limits of detection (LODs) and limits of quantification (LOQs) ranged from 58.88 ng mL−1 to 132.59 ng mL−1 and from 196.22 ng mL−1 to 441.70 ng mL−1 (Tables 5 and 6), respectively.
Analytical quantitative results of H-compounds (2,2,-dichroloacetamide, norfloxacin, linuron, 3,5-diiodosalicylic acid, 2-bromo-5-chlorophenol, diuron, and flumequine) for correlation coefficients (R2), relative standard deviations (RSDs), and limit of detection and limit of quantification (LOD and LOQ) of the method.
| H-compounds | Equations | R 2 | Linear range (μg L−1) | RSD (n = 10, %) | LOD (ng mL−1) | LOQ (ng mL−1) |
|---|---|---|---|---|---|---|
| 2,2,-Dichroloacetamide | y = 12 840.1x + 15 878.5 | 0.994 | 1–50 | 12.09 | 1.52 | 4.94 |
| Norfloxacin | y = 3568.4x + 5679.2 | 0.998 | 1–50 | 6.89 | 1.98 | 6.44 |
| Diuron | y = 10 418.2x + 3837.6 | 0.996 | 1–50 | 8.91 | 2.55 | 8.29 |
| Linuron | y = 13 643.1x + 19 225.3 | 0.993 | 1–50 | 9.91 | 2.91 | 9.47 |
| 3,5-Diiodosalicylic acid | y = 36 820.4x − 31 005.7 | 0.995 | 1–50 | 8.79 | 1.64 | 5.33 |
| Flumequine | y = 13 563.6x + 11 020.7 | 0.995 | 1–50 | 11.54 | 1.71 | 5.56 |
| 2-Bromo-5-chlorophenol | y = 10 146.8x − 5788.6 | 0.985 | 1–50 | 12.31 | 1.85 | 6.02 |
Analytical quantitative results of calibration heavy metals (zinc, manganese, nickel, and lead) for correlation coefficients (R2), relative standard deviations (RSDs), and limit of detection and limit of quantification (LOD and LOQ) of the method.
| H-compounds | Equations | R 2 | Linear range (μg L−1) | RSD (n = 10, %) | LOD (ng mL−1) | LOQ (ng mL−1) |
|---|---|---|---|---|---|---|
| Zinc | y = 1569.2x − 291.8 | 0.992 | 1–50 | 9.06 | 3.07 | 9.99 |
| Manganese | y = 2193.2x − 1301.2 | 0.983 | 1–50 | 8.19 | 1.86 | 6.05 |
| Nickel | y = 1286.3x + 4448.9 | 0.995 | 1–50 | 9.88 | 1.55 | 5.45 |
| Lead | y = 11 981.3x − 2511.7 | 0.977 | 1–50 | 10.11 | 3.49 | 11.35 |
ESI-MPI-MS desorption and ionization principle of halogenated compounds
Fig. S4† displays the results of the H-compounds mass spectrum. Table S1† presents the basic physicochemical properties. Four compounds (2,2-dichloroacetamide, norfloxacin, and flumequine) show a protonated molecular ion peak at [M + H]+, with m/z values of 127, 320, and 261, respectively, which shows that the ionization process involved proton transfer reactions; three compounds (diuron, linuron, and 5-bromo-2-chlorophenol) show protonated and deprotonated molecular ion peaks at [M + H]+ and at [M − H]−, with corresponding m/z values of 232/234, 248/250, and 205/207, respectively. The mass spectra were collected, and MS/MS spectra were obtained to confirm the fragmentation of the ions, as presented in Fig. S5.†
According to the ionization principles used in this study, ESI provides atomized-nebulized samples with high injection efficiency. This also increases the contact area between the sample and the MPI plasma tip. This creates ionization conditions with high concentrations of protons ionized from substances in the ambient environment (such as water vapor). The plasma tips provide the ionization environment with a high temperature sufficient for desorption of the atomized sample according to the ESI. Active free radicals, high-energy electrons, and metastable particles bombard each other.
Analysis of heavy metal ions via ESI-MPI-MS
In general, the metal ions in MPI plasma become a regular form of M(NO3)n with the addition of several water molecules or OH groups. In this scenario, M represents a metal element, the NO3 group is electrophilic, and the metal ions serve as electron donors. Nitrate radicals generally come from ambient air rather than from sample solutions.35 Furthermore, depending on the value of n, the M(NO3)n group can act as either a cation or an anion; this characteristic allows for the detection of metal elements in both positive and negative modes. The ionization of heavy metals primarily occurs in the inner plasma of a microwave plasma torch at a high temperature, which is similar to ICP ionization.
Comparison with other analytical methods direct analysis of real samples
In this study, a novel ESI-MP linear ion trap mass spectrometer is used to directly analyze real water samples collected from three different irrigation systems (flood irrigation, lateral move irrigation, and sub-irrigation systems). Tables 2–4 provide a summary of the results for the detected compounds and metallic elements. Semiquantitative performance for this method was performed in both positive and negative mode for the real samples, and clear spectra were obtained in negative mode due to less noise interference from the matrices of the adjacent compounds and a much simpler MPI spectral structure, in line with Xiong.35 To prove the capacity of the developed method for the detection of H-compounds and metallics elements, the concentrations of the mentioned pollutants in real samples were measured. Furthermore, the major ions generated by the detected compounds, such as 2,2-dichroloacetamide (m/z 127), norfloxacin (m/z 320), diuron (m/z 232), linuron (m/z 248), 3,5-diiodosalicylic acid (m/z 389), flumequine (m/z 262), and 2-bromo-5-chlorophenol (m/z 207), were verified by CID via ESI-MPIMS/MS in real samples. Moreover, for the recovery experiment, all the target analytes in the samples spiked with the corresponding standard solutions at different concentrations (20, 10, and 5 μg L−1) were prepared, and the percentage recovery for the seven H-compounds ranged from 78.5% to 123%, while the percentage recovery for the four heavy metals ranged from 79.93% to 119.50%. As obviously suggested from the real sample, the average levels of the trace compounds and metallic elements were lower than the allowable values set by national agencies, suggesting that the current pollution risk is below the controllable level. Plasma-based ion sources exhibit a certain level of tolerance toward different matrices,36 which enables the direct analysis of environmental samples with little or no sample extraction.
Through a recovery experiment involving 18 samples from three different irrigation systems, the results showed significant differences in the detection of various ions. Compared to samples from the flood irrigation system, linuron ion and diuron ion were detected in substantial amounts in all real samples, whereas 2-bromo-5-chlorophenol ion and 3,5-diiodosalicylic acid ion were significantly lower in all samples (Tables 2 and 3). Linuron ion and diuron were found in higher concentrations in samples from flooding and sub-irrigation systems and in lower concentrations in lateral move systems. Additionally, zinc and lead were present in significant amounts, with higher concentrations in both flooding and sub-irrigation systems. Comprehensive analysis revealed that H-compounds had significantly higher concentrations across all three irrigation systems, which can be attributed to their extensive use in personal care products and pharmaceuticals.4 Similarly, the presence of heavy metals in flooding and sub-irrigation systems is likely due to the widespread application of pesticides and insecticides, which dissolve into soil and water.28 This demonstrated that the method proposed in this study is practical and applicable for water control, environmental pollution control, and analysis because of its sufficiently high sensitivity and other advantages. The statistical analysis presents good consistency of with the acceptable values of the coefficient of variation and standard deviation (Tables S2 and S3†). Thus, this novel ESI-MIMS method is promising and can be adopted as an alternative approach for monitoring water quality.
Profiles of halogenated compounds and metallic elements in reclaimed water compared to previous studies
The total concentrations of seven halogenated compounds and four metallic elements measured in reclaimed water from flood irrigation, lateral move irrigation, and sub-irrigation systems ranged from 0.818–7.024 ng mL−1 and 0.091–1.015 ng mL−1; 0.104–2.12 ng mL−1 and 0.118–1.177 ng mL−1; and 0.751–8.802 ng mL−1 and 0.216–3.309 ng mL−1 respectively. H-compounds were the primary contributors to the pollutants detected in the reclaimed water. Among them, the average concentrations of diuron and linuron were the highest, 4.141 ng mL−1 in flood irrigation and 3.91 ng mL−1 in sub-irrigation, which were significant pollutants according to the hygienic standard of Drinking Water in China (GB5749-2006).
Previous studies have also highlighted halogenated compounds as predominant pollutants in wastewater from the Yangtze River regions.37,38 The water samples of the three irrigation systems all contained high concentrations of zinc and lead, with ranges of 0.011–0.280 ng mL−1 and 0.045–0.314 ng mL−1; 0.061–0.136 ng mL−1 and 0.0910–0.217 ng mL−1; and 0.151–1.031 ng mL−1 and 0.014–0.971 ng mL−1, respectively. But neither zinc nor lead levels exceeded China's drinking water standards and were within safe limits. In contrast, nickel and manganese were detected at lower concentrations, similar to the previous study.28
Pollutant levels in reclaimed and wastewater were significantly higher than those reported in drinking water (12.0–128 ng mL−1) along the same region (Yangtze River, China),38 but lower than concentrations in surface waters from major eastern China water sources.4,25,28,37 Metallic element concentrations in reclaimed water were lower than those in surface water around the chemical industrial zone in Suzhou, Jiangsu Province.4,28,39 These pollutants are more prevalent in residential areas and schools, reflecting a positive correlation with densely populated areas dominated by middle-aged and elderly individuals who frequently use these compounds.4
Tables 2–4 provide a detailed characterization of contaminants detected by ESI-MPIMS in water samples from three irrigation systems: flood, lateral move, and sub-irrigation. These tables also report the original ion concentrations and recovery rates for samples spiked with a standard concentration. Differences in concentration and recovery rates across irrigation systems highlight the impact of irrigation methods on ion persistence and detectability. Tables S2 and S3† contain statistical data on recovery rate variation and precision, with the coefficient of variation (CV) and standard deviation (Std Dev) representing relative variability, which is essential for assessing recovery consistency. Lower CV values for certain compounds in flood irrigation samples indicate more consistent recovery. Furthermore, p-values indicate no significant differences in mean recovery percentages between groups at an alpha level of 0.05, supporting the null hypothesis of equal means.
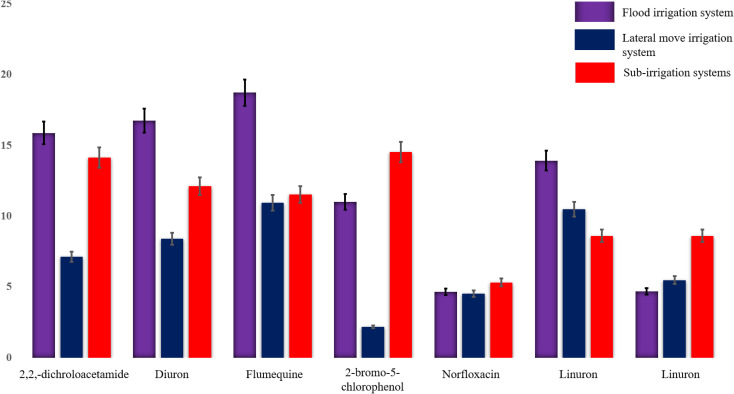
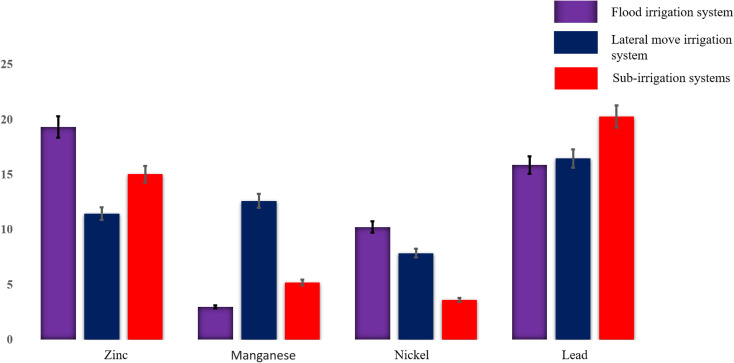
Fig. 4 and 5 illustrate concentration patterns, revealing that flood irrigation has the highest concentrations of flumequine, diuron, and 2,2-dichloroacetamide as the dominant compounds. Sub-irrigation generally follows with slightly lower concentrations of 2-bromo-5-chlorophenol, while the lateral move system consistently shows the lowest concentrations for all compounds. This pattern suggests that flood irrigation may retain the highest levels of halogenated compounds.
Fig. 4. Measured concentrations of 2,2,-dichroloacetamide, diuron, flumequine, 2-bromo-5-chlorophenol, norfloxacin, linuron, and linuron (shown in bars) in flood, lateral move, and sub-irrigation water systems using ESI-MPIMS.
Fig. 5. Measured concentrations of zinc, manganese, nickel, and lead (shown in bars) in flood, lateral move, and sub-irrigation water systems using ESI-MPIMS.
To better understand the technique, ESI operates by producing nebulized atomized samples, increasing the contact area for ionization. MPI generates a high-temperature plasma that breaks samples into smaller pieces, reducing matrix interference and enhancing sensitivity and overall quantification. This study compared current findings with previous studies, highlighting the important role of various analytical techniques in analyzing water pollutants in reclaimed and wastewater, such as liquid chromatography-mass spectrometry (LC-MS), liquid chromatography-tandem mass spectrometry (LC-MS/MS),4,11–13,15 atomic absorption/fluorescence spectroscopy (AAS/AFS),16 inductively coupled plasma optical emission mass spectrometry (ICP-OESMS),17 and inductively coupled plasma-mass spectrometry (ICP-MS)18 are conventionally applied, as summarized in Table 1. ICP-MS shows reliable quantification results, characterized by a broad dynamic range and rapid analytical speed. However, its utility is constrained by the large and costly equipment, matrix interference, sampling injection techniques, and challenges associated with the direct analysis of real samples.36 ESI-MPIMS offers less analysis time and lower sensitivity while providing stable quantification for analyzing real samples in complex matrices, such as milk tea, seawater, and Chinese spirits. Additionally, ESI-MPIMS is user-friendly and has comparable or lower limits of detection (LOD) and limits of quantification (LOQ). This demonstrates that ESI-MPIMS is a sensitive and rapid tool for analyzing real samples in environmental matrices without pretreatment.
Comparison of the proposed technique with conventional methods.
| Technique | Sample extraction | Extraction time (min) | Sensitivity (ng mL−1) | Volume consumption (μL) | References |
|---|---|---|---|---|---|
| GC-MS | Needed | >60 | 150 | 20–30 | 12 |
| PSI-MS | Needed | 5–15 | 350 | 500 | 40 |
| PESI-MS | — | 5–15 | 500 | >80 | 41 |
| LC-MS/MS | Needed | >60 | 98 | 60–90 | 14 |
| AAS/AFS | Needed | ≥30 | 56 | 40 | 42 |
| ICP-OESMS | Needed | ≥15 | 20 | 60 | 17 |
| ICP-MS | Needed | ≥45 | 20 | 60 | 18 |
| ESI-MPIMS | — | <0.5 | 3.5 | <0.2 | This study |
Conclusion
In this study, a novel ESI-MPIMS technique coupled with LTQ mass spectrometry was established for the direct detection of halogenated compounds along with heavy metal elements in treated recycled water. In particular, we developed a rapid, less time-consuming, and cost-effective technique based on an ambient mass spectrometer. The mechanism of ESI-MPIMS involves ESI to provide atomized-nebulized samples with high injection efficiency to the plasma tip. The plasma tips provide the ionization environment by providing a high temperature sufficient for desorption and engulfing the matrices of the atomized samples, as well as active free radicals, high-energy electrons, and metastable particles, which improve the ionization process. The effectiveness of the methods is demonstrated by good semi-quantitative performance (R2 = 0.977–0.998), achieving recovery rates of 78.5–123% for H-compounds and 79.93–119.50% for heavy metals. The method exhibited excellent repeatability and high sensitivity, with limits of detection (LODs) ranging from 1.5 ng mL−1 to 3.5 ng mL−1 and limits of quantification (LOQs) ranging from 4.5 ng mL−1 to 12.75 ng mL−1.
This work paves the way toward the use of ambient mass spectrometers in the simultaneous analysis of H-compounds and heavy metal pollutants for water control. The developed method facilitates the quantitative analysis of water samples, which promotes the use of water standards. ESI-MPIMS was successfully applied to quantitatively analyze real water samples collected from three irrigation system farmlands at three sites in Zhejiang and Jiangsu Provinces, China. The analysis revealed that halogenated compounds, such as diuron and linuron, were the most prevalent pollutants, with significant concentrations observed across all irrigation systems. Zinc and lead were also detected at higher levels, particularly in flood and sub-irrigation systems. The quantification and recovery validation of the seven H-compounds and four heavy metal elements indicate that ESI-MPIMS is non-susceptible to complex matrices, is less vulnerable, and is more suitable for direct and rapid identification of various compounds with high sensitivity and reproducibility via ambient mass spectrometry, even in a very complex matrix, which will fill the gap in the analysis of pollutants in recycled wastewater.
Data availability
We confirm that the data supporting the findings of this study are available within the main article and ESI.†
Author contributions
NSS: conceptualization, methodology, investigation, data curation, software, validation, writing – original draft and editing. FC, GZ, AAM, and AMI: conceptualization, investigation, and review and editing. XW, BBI, and GZ: supervision and editing. SAAA and MGI: data analysis, and figure drawing. GZ, XW, NSS, and AAM: investigation, review and editing. XW and GZ: supervision, resources, writing – reviewing and editing, project administration, and funding acquisition. All authors read and approved the manuscript.
Conflicts of interest
The authors declare no competing interests.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China with grant no. 62174147, 21927810, Key Research and Development Project of Zhejiang with grant no. 2024C03114, and Dr Li Dak Sum & Yip Yio Chin Development Fund for Regenerative Medicine, Zhejiang University.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra04995k
References
- Xu R. Xie Y. Tian J. Chen L. J. Cleaner Prod. 2021;283:124645. [Google Scholar]
- Dey S., Bano F. and Malik A., Pharmaceuticals and Personal Care Product (PPCP) Contamination-A Global Discharge Inventory, Elsevier Inc., 2019 [Google Scholar]
- Dai G. Wang B. Fu C. Dong R. Huang J. Deng S. Wang Y. Yu G. Environ. Sci.: Processes Impacts. 2016;18:445–455. doi: 10.1039/c6em00018e. [DOI] [PubMed] [Google Scholar]
- Shuaibu N. S. Zhao G. Chu F. Wang X. Environ. Sci. Pollut. Res. 2023;30:108263–108273. doi: 10.1007/s11356-023-30018-5. [DOI] [PubMed] [Google Scholar]
- Jeschke P. Eur. J. Org. Chem. 2022;2022:1–11. [Google Scholar]
- Khalil M., Iqbal M., Turan V., Tauqeer H. M., Farhad M., Ahmed A. and Yasin S., Household Chemicals and Their Impact, Elsevier Inc., 2022 [Google Scholar]
- Vidu R. Matei E. Predescu A. M. Alhalaili B. Pantilimon C. Tarcea C. Predescu C. Toxics. 2020;8:1–37. doi: 10.3390/toxics8040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L. Jiao W. Chen X. Chen W. J. Environ. Sci. 2011;23:1585–1593. doi: 10.1016/s1001-0742(10)60627-4. [DOI] [PubMed] [Google Scholar]
- U. Nations, WATER, 2016
- Brázová T. Šalamún P. Miklisová D. Šestinová O. Findoráková L. Hanzelová V. Oros M. Bull. Environ. Contam. Toxicol. 2021;106:485–492. doi: 10.1007/s00128-021-03114-w. [DOI] [PubMed] [Google Scholar]
- Chen L. Yan X. Zhou X. Peng P. Sun Q. Zhao F. TrAC, Trends Anal. Chem. 2023;160:116976. [Google Scholar]
- Liu Q. Zhao Z. Li H. Su M. xuan Liang S. Ecotoxicol. Environ. Saf. 2020;207:111237. doi: 10.1016/j.ecoenv.2020.111237. [DOI] [PubMed] [Google Scholar]
- Ofrydopoulou A. Nannou C. Evgenidou E. Lambropoulou D. J. Chromatogr. A. 2021;1652:462369. doi: 10.1016/j.chroma.2021.462369. [DOI] [PubMed] [Google Scholar]
- Qu L. Huang H. Xia F. Liu Y. Dahlgren R. A. Zhang M. Mei K. Environ. Pollut. 2018;237:639–649. doi: 10.1016/j.envpol.2018.02.020. [DOI] [PubMed] [Google Scholar]
- Sani S. N. Zhou W. Ismail B. B. Zhang Y. Chen Z. Zhang B. Bao C. Zhang H. Wang X. Cancers. 2023;15:1186. doi: 10.3390/cancers15041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escandar G. M. Olivieri A. C. ACS Omega. 2022;7:39574–39585. doi: 10.1021/acsomega.2c05215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraji M. Yamini Y. Saleh A. Rezaee M. Ghambarian M. Hassani R. Anal. Chim. Acta. 2010;659:172–177. doi: 10.1016/j.aca.2009.11.053. [DOI] [PubMed] [Google Scholar]
- Dulama I. D. Radulescu C. Chelarescu E. D. Stihi C. Bucurica I. A. Teodorescu S. Stirbescu R. M. Gurgu I. V. Let D. D. Stirbescu N. M. Rom. J. Phys. 2017;62:1–9. [Google Scholar]
- Takáts Z. Wiseman J. M. Gologan B. Cooks R. G. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- Eberherr W. Buchberger W. Hertsens R. Klampfl C. W. Anal. Chem. 2010;82:5792–5796. doi: 10.1021/ac1008496. [DOI] [PubMed] [Google Scholar]
- Wu Z. Chen H. Wang W. Jia B. Yang T. Zhao Z. Ding J. Xiao X. J. Agric. Food Chem. 2009;57:9356–9364. doi: 10.1021/jf9018504. [DOI] [PubMed] [Google Scholar]
- Jecklin M. C. Gamez G. Zenobi R. Analyst. 2009;134:1629–1636. doi: 10.1039/b819560a. [DOI] [PubMed] [Google Scholar]
- Na N. Zhao M. Zhang S. Yang C. Spectrometry. 2002;18(10):1859–1862. doi: 10.1016/j.jasms.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Garcia-Reyes J. F. Harper J. D. Salazar G. A. Charipar N. A. Ouyang Z. Cooks R. G. Anal. Chem. 2011;83:1084–1092. doi: 10.1021/ac1029117. [DOI] [PubMed] [Google Scholar]
- Chu F. Zhao G. Li W. Wei W. Chen W. Ma Z. Gao Z. Shuaibu N. S. Luo J. Yu B. Feng H. Pan Y. Wang X. Anal. Chem. 2023;95(3):2004–2010. doi: 10.1021/acs.analchem.2c04469. [DOI] [PubMed] [Google Scholar]
- Li Z. Zhang F. Zhao J. Liu X. Chen X. Su Y. Guo Y. Talanta. 2018;182:241–246. doi: 10.1016/j.talanta.2018.01.071. [DOI] [PubMed] [Google Scholar]
- Gao Y. Li Y. Zhan B. He Q. Zhu H. Chen W. Yin Q. Feng H. Pan Y. Analyst. 2021;146:5682–5690. doi: 10.1039/d1an00872b. [DOI] [PubMed] [Google Scholar]
- Zhao G. Yang M. Zhang T. Jia B. Xu L. Cheng P. Anal. Chim. Acta. 2024;1304:1–9. doi: 10.1016/j.aca.2024.342531. [DOI] [PubMed] [Google Scholar]
- Miao M. Zhao G. Li X. Jung D. Ping C. J. Mass Spectrom. 2018:189–194. doi: 10.1002/jms.4055. [DOI] [PubMed] [Google Scholar]
- Adamovich I. Baalrud S. D. Bogaerts A. Bruggeman P. J. Cappelli M. Colombo V. Czarnetzki U. Ebert U. Eden J. G. Favia P. Graves D. B. Hamaguchi S. Hieftje G. Hori M. Kaganovich I. D. Kortshagen U. Kushner M. J. Mason N. J. Mazouffre S. Thagard S. M. Metelmann H. R. Mizuno A. Moreau E. Murphy A. B. Niemira B. A. Oehrlein G. S. Petrovic Z. L. Pitchford L. C. Pu Y. K. Rauf S. Sakai O. Samukawa S. Starikovskaia S. Tennyson J. Terashima K. Turner M. M. Van De Sanden M. C. M. Vardelle A. J. Phys. D Appl. Phys. 2017;50(32):323001. [Google Scholar]
- Jin Q. Zhu C. Border M. W. Hieftje G. M. Spectrochim. Acta, Part B. 1991;46:417–430. [Google Scholar]
- Zhao G. Chu F. Zhou J. J. Mass Spectrom. 2022;57(2):1–7. doi: 10.1002/jms.4809. [DOI] [PubMed] [Google Scholar]
- Miao M. Zhao G. Wang Y. Xu L. Dong J. Cheng P. Rapid Commun. Mass Spectrom. 2017;31:2092–2100. doi: 10.1002/rcm.7991. [DOI] [PubMed] [Google Scholar]
- Jiang T. Jiang F. Liu H. Yuan L. Mo T. Arabian J. Chem. 2020;13:7939–7952. [Google Scholar]
- Xiong X. Jiang T. Qi W. Zuo J. Yang M. Fei Q. Xiao S. Yu A. Zhu Z. Chen H. Int. J. Anal. Chem. 2015;2015:1–10. doi: 10.1155/2015/156509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild M. Gundlach-Graham A. Menon A. Jevtic J. Pikelja V. Tanner M. Hattendorf B. Günther D. Anal. Chem. 2018;90:13443–13450. doi: 10.1021/acs.analchem.8b03251. [DOI] [PubMed] [Google Scholar]
- Pan Y. Zhang H. Cui Q. Sheng N. Yeung L. W. Y. Sun Y. Guo Y. Dai J. Environ. Sci. Technol. 2018;52:7621–7629. doi: 10.1021/acs.est.8b00829. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Zhou Y. Zhang A. Li J. Yu J. Dou Y. He J. Kong D. Ecotoxicol. Environ. Saf. 2021;218:112289. doi: 10.1016/j.ecoenv.2021.112289. [DOI] [PubMed] [Google Scholar]
- Yao J. Sheng N. Guo Y. Yeung L. W. Y. Dai J. Pan Y. Environ. Sci. Technol. 2022;56:7986–7996. doi: 10.1021/acs.est.2c00891. [DOI] [PubMed] [Google Scholar]
- Kim D. Yong S. Geon J. Cha S. Hyuk U. Kim S. J. Hazard. Mater. 2018;359:421–428. doi: 10.1016/j.jhazmat.2018.07.060. [DOI] [PubMed] [Google Scholar]
- Hiraoka K. Ariyada O. Usmanov D. T. Chen L. C. Ninomiya S. Yoshimura K. Takeda S. Yu Z. Mandal M. K. Wada H. Rankin-Turner S. Nonami H. Mass Spectrom. 2020;9:1–21. doi: 10.5702/massspectrometry.A0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W. Ma Y. Zhao W. Feng Y. Wang N. Si Z. Anal. Bioanal. Chem. 2003;377:681–684. doi: 10.1007/s00216-003-2132-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We confirm that the data supporting the findings of this study are available within the main article and ESI.†