Abstract
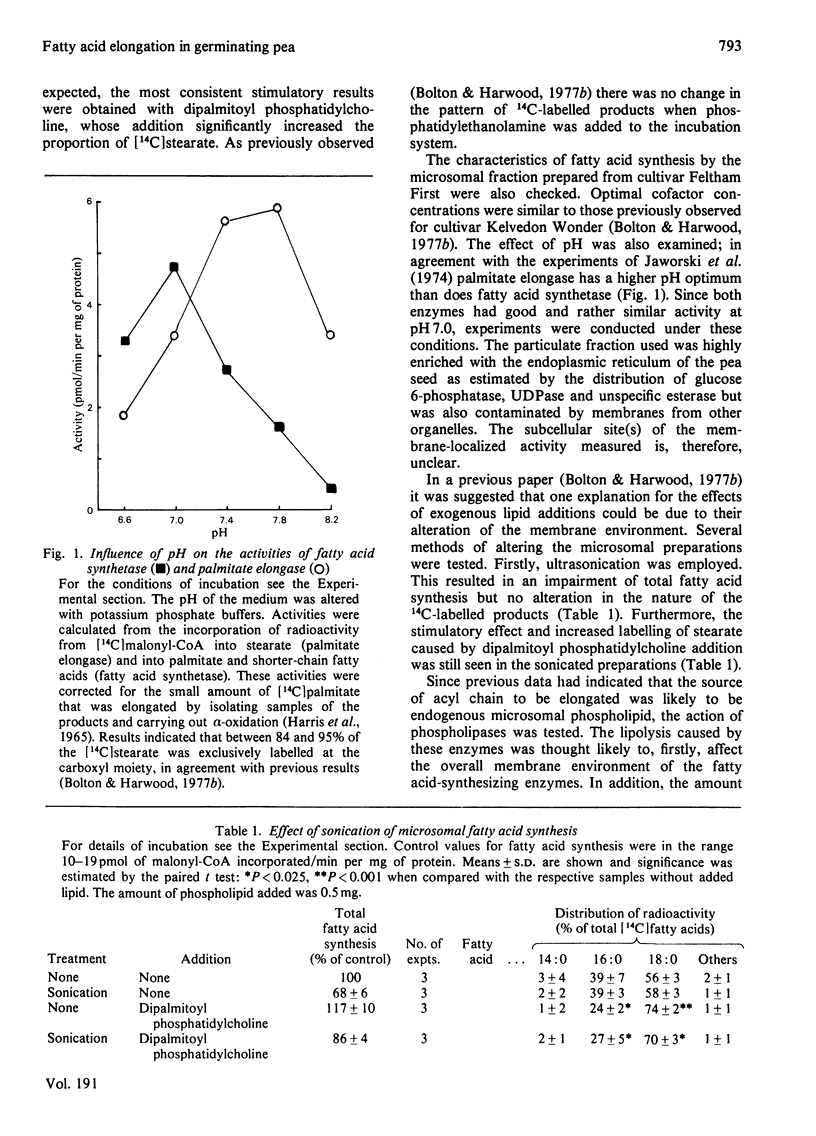
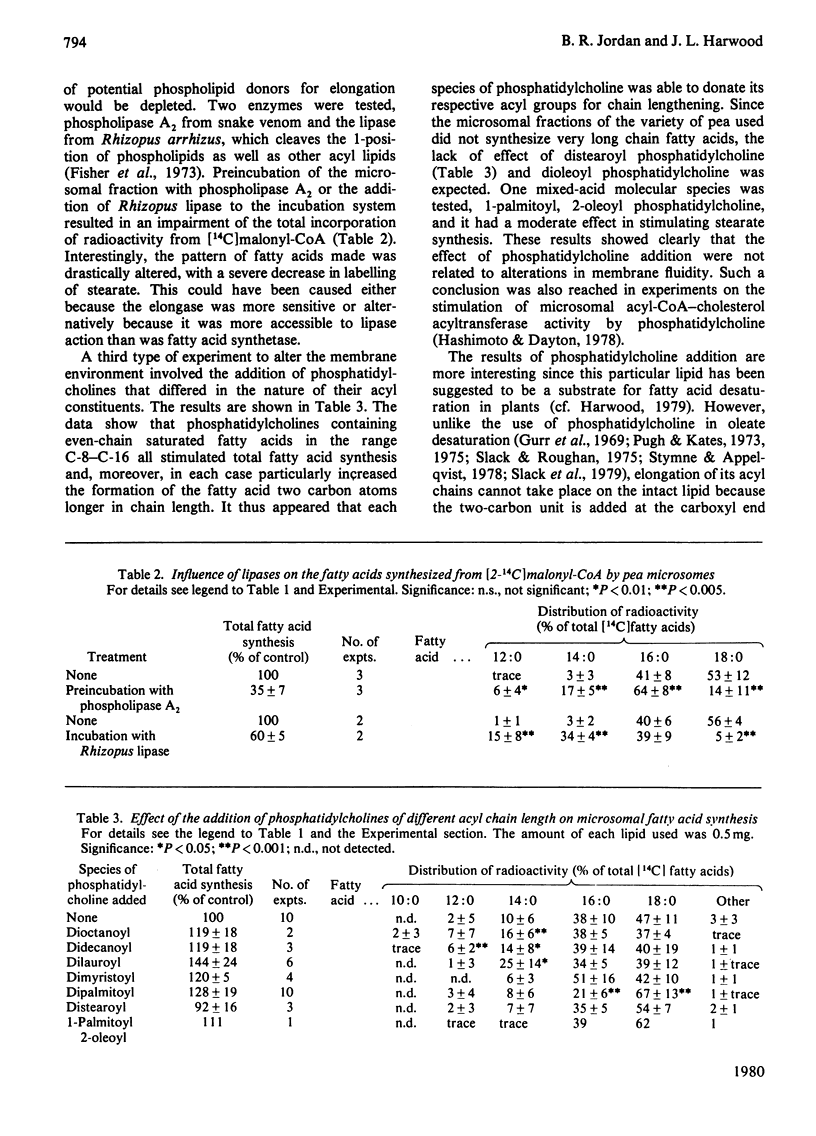
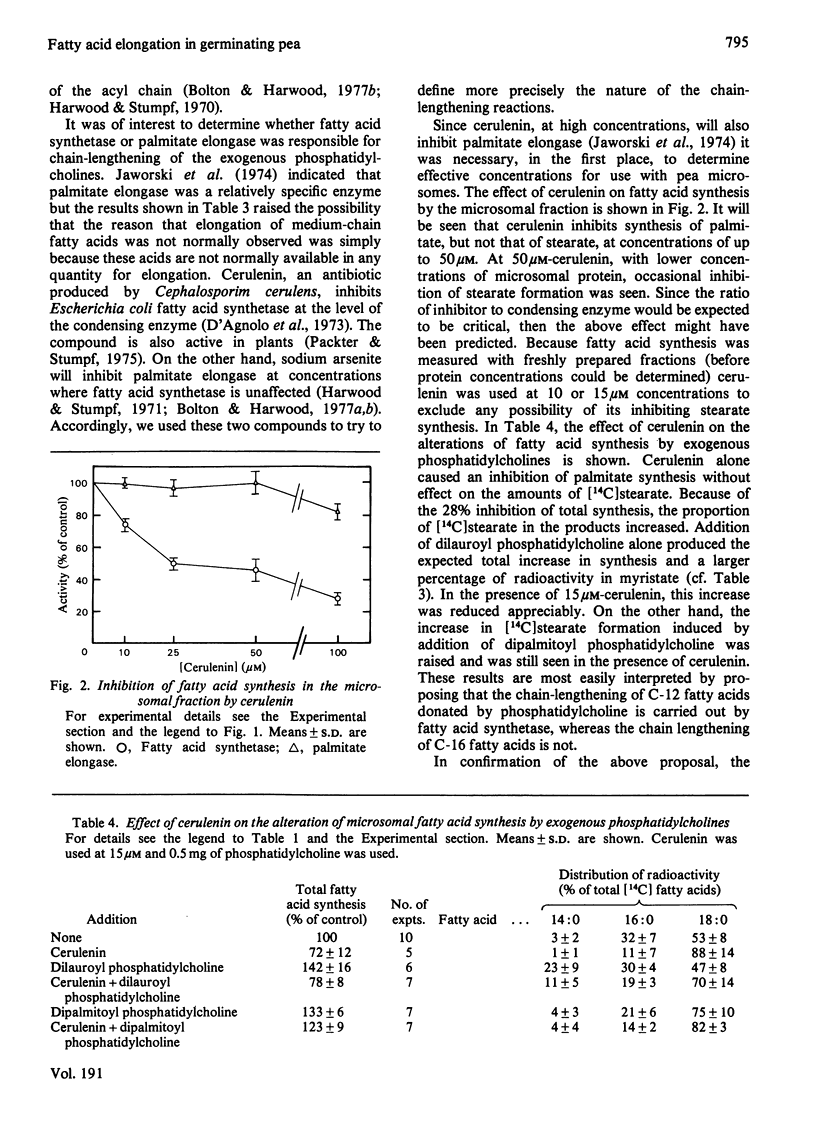
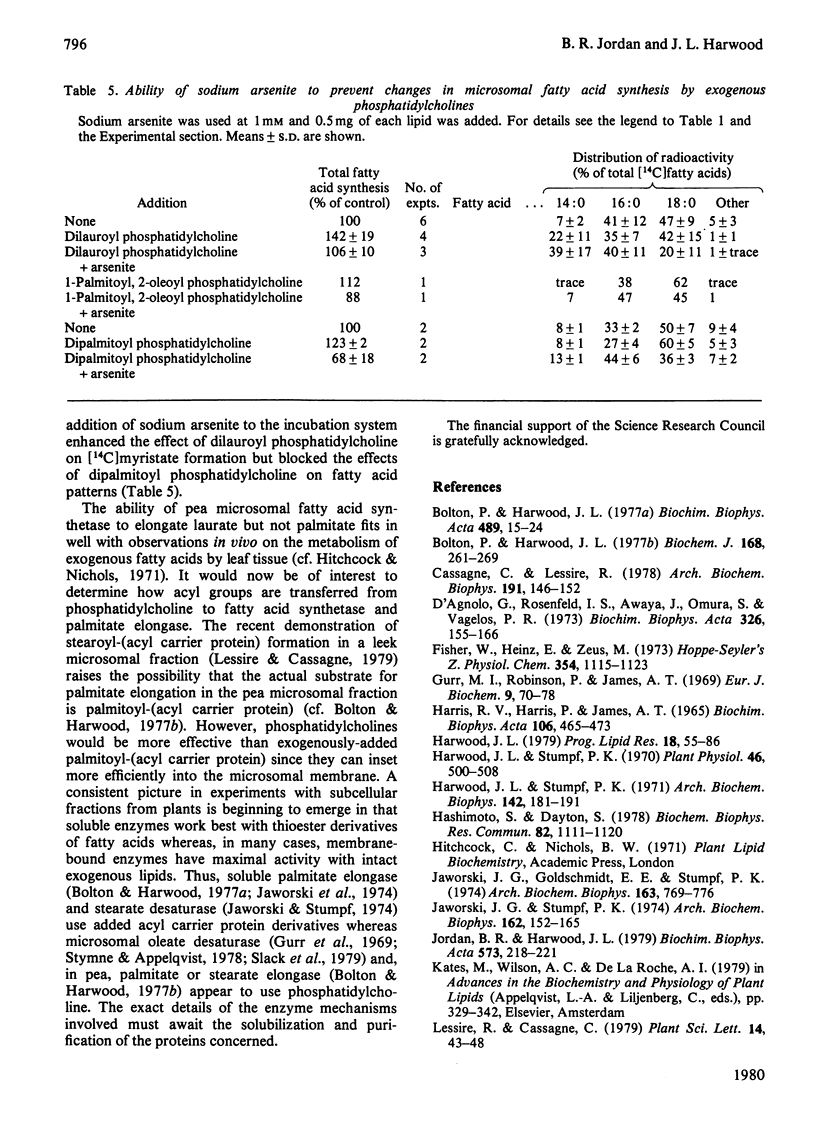
The synthesis of fatty acids from [14C]malonyl-CoA was studied with a high-speed particulate fraction from germinating pea (Pisum sativum). The variety used (Feltham First) produced mainly saturated fatty acids with palmitate (30--40%) and stearate (40--60%) predominating. Several palmitate-containing lipids stimulated overall synthesis and, in addition, increased the percentage of label in stearate. The production of stearate was severely inhibited by preincubation of the microsomal fraction with snake venom phospholipase A2 or by incubation with Rhizopus arrhizus lipase. Addition of a series of di-saturated phosphatidylcholines, with different acyl constituents, resulted in stimulation of overall fatty acid synthesis as well as an increase in the radiolabelling of the fatty acid two carbon atoms longer than the acyl chain added. This chain lengthening of fatty acids donated from phosphatidylcholine was due to the action of both fatty acid synthetase and palmitate elongase. The latter would utilize dipalmitoyl phosphatidylcholine and was sensitive to arsenite whereas fatty acid synthetase would use dilauroyl phosphatidylcholine and was sensitive to cerulenin. The results are discussed in relation to previous data obtained in vivo on plant fatty acid synthesis and current suggestions for the role of phosphatidylcholine in this process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton P., Harwood J. L. Fatty acid biosynthesis by a particulate preparation from germinating pea. Biochem J. 1977 Nov 15;168(2):261–269. doi: 10.1042/bj1680261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton P., Harwood J. L. Some characteristics of soluble fatty acid synthesis in germinating pea seeds. Biochim Biophys Acta. 1977 Oct 24;489(1):15–24. doi: 10.1016/0005-2760(77)90227-2. [DOI] [PubMed] [Google Scholar]
- Cassagne C., Lessire R. Biosynthesis of saturated very long chain fatty acids by purified membrane fractions from leek epidermal cells. Arch Biochem Biophys. 1978 Nov;191(1):146–152. doi: 10.1016/0003-9861(78)90076-0. [DOI] [PubMed] [Google Scholar]
- D'Agnolo G., Rosenfeld I. S., Awaya J., Omura S., Vagelos P. R. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim Biophys Acta. 1973 Nov 29;326(2):155–156. doi: 10.1016/0005-2760(73)90241-5. [DOI] [PubMed] [Google Scholar]
- Fischer W., Heinz E., Zeus M. The suitability of lipase from Rhizopus arrhizus delemar for analysis of fatty acid distribution in dihexosyl diglycerides, phospholipids and plant sulfolipids. Hoppe Seylers Z Physiol Chem. 1973 Sep;354(9):1115–1123. doi: 10.1515/bchm2.1973.354.2.1115. [DOI] [PubMed] [Google Scholar]
- Gurr M. I., Robinson M. P., James A. T. The mechanism of formation of polyunsaturated fatty acids by photosynthetic tissue. The tight coupling of oleate desaturation with phospholipid synthesis in Chlorella vulgaris. Eur J Biochem. 1969 May 1;9(1):70–78. doi: 10.1111/j.1432-1033.1969.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Harris R. V., James A. T. The fatty acid metabolism of Chlorella vulgaris. Biochim Biophys Acta. 1965 Dec 2;106(3):465–473. doi: 10.1016/0005-2760(65)90063-9. [DOI] [PubMed] [Google Scholar]
- Harwood J. L., Stumpf P. K. Fat Metabolism in Higher Plants: XL. Synthesis of Fatty Acids in the Initial Stage of Seed Germination. Plant Physiol. 1970 Oct;46(4):500–508. doi: 10.1104/pp.46.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. L. The synthesis of acyl lipids in plant tissues. Prog Lipid Res. 1979;18(2):55–86. doi: 10.1016/0163-7827(79)90006-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Dayton S. Stimulation of acyl-CoA:cholesterol acyltransferase activity in rat liver microsomes by phosphatidylcholine. Biochem Biophys Res Commun. 1978 Jun 29;82(4):1111–1120. doi: 10.1016/0006-291x(78)90302-9. [DOI] [PubMed] [Google Scholar]
- Jaworski J. G., Goldschmidt E. E., Stumpf P. K. Fat metabolism in higher plants. Properties of the palmityl acyl carrier protein: stearyl acyl carrier protein elongation system in maturing safflower seed extracts. Arch Biochem Biophys. 1974 Aug;163(2):769–776. doi: 10.1016/0003-9861(74)90539-6. [DOI] [PubMed] [Google Scholar]
- Jaworski J. G., Stumpf P. K. Fat metabolism in higher plants. Properties of a soluble stearyl-acyl carrier protein desaturase from maturing Carthamus tinctorius. Arch Biochem Biophys. 1974 May;162(1):158–165. doi: 10.1016/0003-9861(74)90114-3. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Harwood J. L. alpha-Hydroxylation of newly synthesised fatty acids by a soluble fraction from germinating pea. Biochim Biophys Acta. 1979 Apr 27;573(1):218–221. doi: 10.1016/0005-2760(79)90190-5. [DOI] [PubMed] [Google Scholar]
- Macey M. J., Stumpf P. K. Fat Metabolism in Higher Plants XXXVI: Long Chain Fatty Acid Synthesis in Germinating Peas. Plant Physiol. 1968 Oct;43(10):1637–1647. doi: 10.1104/pp.43.10.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packter N. M., Stumpf P. K. Fat metabolism in higher plants. Production of short- and medium-chain acyl-acyl carrier protein by spinach stroma preparations treated with cerulenin. Biochim Biophys Acta. 1975 Dec 17;409(3):274–282. [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Characterization of a membrane-bound phospholipid desaturase system of candida lipolytica. Biochim Biophys Acta. 1975 Mar 24;380(3):442–453. doi: 10.1016/0005-2760(75)90112-5. [DOI] [PubMed] [Google Scholar]
- Pugh E. L., Kates M. Desaturation of phosphatidylcholine and phosphatidylethanolamine by a microsomal enzyme system from Candida lipolytica. Biochim Biophys Acta. 1973 Sep 25;316(3):305–316. doi: 10.1016/0005-2760(73)90071-4. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Browse J. Evidence for an oleoyl phosphatidylcholine desaturase in microsomal preparations from cotyledons of safflower (Carthamus tinctorius) seed. Biochem J. 1979 Jun 1;179(3):649–656. doi: 10.1042/bj1790649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G. The kinetics of incorporation in vivo of (14C)acetate and (14C)carbon dioxide into the fatty acids of glycerolipids in developing leaves. Biochem J. 1975 Nov;152(2):217–228. doi: 10.1042/bj1520217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S., Appelqvist L. A. The biosynthesis of linoleate from oleoyl-CoA via oleoyl-phosphatidylcholine in microsomes of developing safflower seeds. Eur J Biochem. 1978 Oct;90(2):223–229. doi: 10.1111/j.1432-1033.1978.tb12594.x. [DOI] [PubMed] [Google Scholar]


