Abstract
Importance
According to the Centers for Disease Control and Prevention and governing bodies within the American College of Surgeons, the administration of antibiotics as prophylaxis against infection prior to a planned elective procedure is, with rare exception, routinely recommended. The goal of “getting to zero” infections remains a high priority for policymakers, practitioners, and certainly for patients.
Observations
Despite the many advances in surgical technique, skin decontamination, sterile procedure, and enhanced recovery programs, surgical site infections continue to adversely affect procedures as diverse as dental implant surgery, joint arthroplasty, and major abdominal surgery. Although surgical site infection rates are at historically low levels, progress has stalled in recent reporting periods and such infections remain disabling, costly, and occasionally lethal. Stakeholders in the field, including surgeons, infectious diseases specialists, and industry, advocate for strategies emphasizing greater levels of intraoperative sterility or broader-spectrum antibiotic coverage as the most appropriate path forward.
Conclusions and Relevance
The current emphasis on ever-increasing levels of intraoperative sterility and extended-spectrum antibiotic use are not sustainable long-term solutions. Continuing to escalate these approaches may contribute to unintended consequences including antimicrobial resistance. Principles of antimicrobial stewardship and microbiome sciences can be applied to inform a more effective and sustainable approach to infection prevention in the field of surgery.
INTRODUCTION
Surgeons’ practices toward prophylactic antibiotic administration for elective surgery reflect a perspective that prophylactic antibiotics are both safe and effective and should therefore be maximally leveraged for the prevention of surgical site infections (SSIs).1 Overall, routine preoperative antibiotic prophylaxis significantly reduces the incidence of SSIs compared with no,2,3 or delayed,4 systemic antibiotic prophylaxis in many procedures. However, in the decades since the first trials of routine surgical antibiotic prophylaxis were conducted, a new appreciation for the limitations and harms of antibiotic use has emerged and antibiotic administration for surgical prophylaxis has grown to comprise 1 in 5 of all inpatient antibiotic exposures.5 Over the same period, the prevalence of bacteria resistant to common antibiotic agents has increased in our communities and health systems, such that now up to half of all SSIs in the US are estimated to be resistant to the recommended prophylactic agent, while national SSI rates have stopped decreasing.6,7 In turn, several recent studies focused on high-risk procedures (ie, pancreas and colon surgery) have shown that use of additional and/or broader-spectrum prophylactic antibiotics can incrementally reduce SSI rates in this new context,8–12 an effect specifically associated with the expanded coverage of bacteria resistant to more narrow-spectrum agents.11,13 However, a high degree of inappropriate perioperative antibiotic use persists, both in the US and internationally.5,14,15 Such expansions in the spectrum of, duration of, and indications for perioperative antibiotics by health systems and individual surgeons represent a natural response to the persistent problem of SSIs, but are in conflict with the growing reality of antimicrobial resistance and antibiotic stewardship programs intended to constrain harm associated with their overuse.16 In this article, we review challenges, misconceptions, and opportunities in preventing SSIs in the era of escalating antibiotic resistance, and discuss the risks, benefits, and alternatives of current prophylactic antibiotic prescribing practices in the context of antimicrobial stewardship.
Proposed Framework for Stewardship of Surgical Antibiotic Prophylaxis
Although the best approaches to optimizing antimicrobial use in surgery may vary appropriately between practice settings and procedure types, we propose a general 5-part framework (Table 1), based on a survey of current literature and priority areas in this field, by which the principles of antimicrobial stewardship and the sustained effectiveness of surgical antibiotic prophylaxis can be integrated.
Table 1.
Proposed 5-Part Framework for Stewardship of Surgical Antibiotic Prophylaxis
| Framework for Stewardship of Surgical Antibiotic Prophylaxis |
|---|
|
DISCUSSION/OBSERVATION
1. Avoid antibiotics in contexts where studies indicate they are not beneficial.
The risk-benefit calculation for antibiotic use is most straightforward in clinical scenarios for which strong evidence is available to demonstrate a lack of benefit. For example, in elective laparoscopic cholecystectomy without acute inflammation, surgical antibiotic prophylaxis has demonstrated minimal or no reduction in SSIs for low-risk patients.17–20 Similarly, no benefit from routine antibiotic prophylaxis has been shown for laparoscopic sleeve gastrectomy, laparoscopic roux-en-Y gastric bypass,21 endoscopic polypectomy,22 and open or transoral thryroidectomy or parathyroidectomy.23 For outpatient procedures such as ophthalmologic, dermatologic, fertility, or dental procedures, antibiotic use remains common, but has been shown to be unnecessary in many cases.14,24–27
The challenge of identifying additional procedures for which antibiotic prophylaxis can safely be omitted in light of advances in surgical care (eg, laparoscopy and minimally invasive techniques, enhanced recovery pathways) is more complex. There is significant ethical concern related to the peeling back of a generally effective intervention.28 Trials conducted to reassess the current need for antibiotic prophylaxis in open mesh hernia repair,29 abdominal hysterectomy,30 orthognathic surgery,31 and noninstrumented spine surgery32 each concluded that antibiotics remain necessary in these contexts. A particular need for caution exists in procedures for which the consequences of a single excess infection can be devastating and irreversible. For example, one trial of a third-generation cephalosporin vs no antibiotic in craniotomy confirmed substantially higher rates of bone flap infection, meningitis, and abscess formation in the control group, with consequences among affected patients including death and coma.33 We suggest that candidate procedures for omission of antibiotic prophylaxis meet at least 2 preliminary criteria for consideration: (1) changes in technique have resulted in fundamentally lower risk of infection (eg, transperineal vs transrectal prostate biopsy34) and (2) most infections are expected to be treatable without irreversible consequence. Prior to initiating any type of study in which preprocedural prophylactic antibiotics are withheld, a consensus panel should be assembled, consisting of surgeons, infectious diseases specialists, clinical trialists, medical ethicists, and patients, to make patient safety the first and foremost concern.
Continuation of antibiotic prophylaxis into the postoperative period represents another major contributor to the global burden of inappropriate inpatient antibiotic exposure.5,14,15,35 A sufficient body of evidence now exists to broadly question the value of postoperative antibiotic prophylaxis in uncomplicated surgical procedures.36 For breast surgery,37 pediatric surgery,38 liver resection,39 cardiac surgery,40,41 pacemaker implantation,42 oromaxillofacial surgery,43 sarcoma resection,44 orthopedic surgery,45 and spine surgery,46 studies have shown no difference in SSI frequency when omitting or reducing the duration of postoperative antibiotic prophylaxis. In contrast, postoperative antibiotic durations longer than 24 hours have repeatedly been shown to be associated with increases in Clostridioides difficileinfection, acute kidney injury, and antibiotic-resistant infection.40,44,47 The Society for Healthcare Epidemiology of America/Infectious Diseases Society of America/Association for Professionals in Infection Control and Epidemiology Practice Recommendation48 on postoperative antibiotic prophylaxis has notably been revised in its most recent update to emphasize that prophylaxis should be discontinued at the time of incision closure in the operating room, irrespective of the presence of postoperative drains.
Given the demonstrated lack of benefit and significant attributable harms, postoperative antibiotic ordering practices represent a clear opportunity for immediate change. Currently, most US health systems have antimicrobial stewardship programs, but few have adopted protocols reflecting current guidelines for appropriate postoperative antibiotic prescribing or provide feedback to practitioners on postoperative prescribing patterns.16Automated decision support tools are an appealing solution to this problem, but require a level of customization not available in standard system builds.49 Without careful adaptation, routine “clicking” to pass through electronic menus and sidestep alerts and nondefault options in the interest of time has been observed50 and may perpetuate prescribing of prophylactic antibiotics when none are needed. More nuanced decision support algorithms have shown the potential to improve appropriate postoperative antibiotic use51 and should be explored as standard elements of electronic health platforms.
Consider Antimicrobial Strategies With Lower-Risk Profiles
Antimicrobial strategies involving nonsystemic administration or using agents with more favorable risk profiles should be considered. These strategies include:
Combined Oral Antibiotic and Mechanical Bowel Preparation
Several randomized clinical trials have now shown that combined preoperative oral antibiotic and mechanical bowel preparation reduce colorectal SSIs by approximately 50%, but consistent use has lagged in the US.52 Many antimicrobials used in these regimens are minimally absorbed from their key site of action in the gastrointestinal tract (neomycin and kanamycin), are not associated with C difficile infection (metronidazole), and/or are not considered high-risk agents from an antimicrobial stewardship perspective. Although there is compelling evidence for the effectiveness of this approach in SSI prevention, considerations regarding dysbiosis and selection for transmissible resistance genes53 remain given the potency of these exposures on gut microbiota.
Preoperative Decolonization
Despite many randomized clinical trials indicating that the application of mupirocin and other topical decontaminants can rid the nares of Staphylococcus aureus, the single-most prevalent SSI pathogen, its routine use and the optimal approach to implementation remain controversial. It is surprising that there is little evidence to explain the mechanism by which decolonization of the nares shortly before surgery is seemingly efficacious in preventing SSI at remote surgical sites. For example, if S aureus is present in the nares, it is also frequently present in the gastrointestinal tract and other anatomical regions more proximal to the surgical site.54,55 While awaiting improved understandings of the mechanism and most effective approach to decolonization, the risks of resistance and adverse effects from topical antimicrobials used as part of a short-term preoperative protocol appear small56 relative to their demonstrated benefit in many studies. Decolonization for several procedure groups has recently been upgraded to an “essential practice” in US consensus guidelines.48
Targeting Bacterial Virulence Rather Than Viability
Most health care–associated infections, including SSIs, are now appreciated to arise from bacteria colonizing patients prior to admission.57–59 By targeting the factors that drive these organisms from states of colonization to infection, rather than targeting the bacteria themselves, it may be possible to reduce infectious complications of surgery without the risks of dysbiosis and antimicrobial resistance that attend traditional antibiotic and antiseptic agents.60 Antivirulence pharmaceuticals for a wide variety of applications are in development, including the prevention of SSIs.61
Use of Local Antimicrobial Application to the Skin
For dermatologic surgery, the effectiveness of systemic antibiotic prophylaxis remains controversial. Most guidelines recommend administering prophylaxis either in the setting of increased risk (eg, lower extremity and groin procedures) or in the presence of existing cardiac or orthopedic prostheses.62 Recently, the effectiveness of microdose antibiotics added to local anesthetic has demonstrated a significant reduction in local infections compared with placebo, with presumably less systemic exposure.63
Maximize the Effect of Antibiotic Prophylaxis Through More Targeted Use
Despite the goal of eliminating perioperative antibiotic use in nonbeneficial scenarios, it is clear that preoperative surgical prophylaxis remains a highly important intervention in many procedures2 and that differences in the timing4 and spectrum8,64 of activity of these medications translate to clinically meaningful differences in SSIs. It is also increasingly apparent that prior infection65 or preoperative colonization66 with antibiotic-resistant bacteria is associated with substantial excess SSI risk that can be partially offset by use of a prophylactic agent to which the resistant organism is susceptible.11,13 Current guidelines for surgical prophylaxis are fairly uniform and account for high-level procedural differences,67 but not for the wide range of individual and geographical factors known to be associated with SSI microbiology and resistance. Targeted selection of prophylactic agents tailored to the patient, procedure, and surgical context potentially offers a more efficient approach to maximizing the effectiveness of prophylaxis in the age of rising antimicrobial resistance while minimizing adverse individual and population-level harms.
This framework is conceptually similar to the selective addition of vancomycin to standard prophylactic agents such as cefazolin for patients colonized with methicillin-resistant S aureus (MRSA), which is supported by limited evidence68 and included as a practice for consideration requiring further research in current guidelines.67 Extension of that paradigm to other classes of resistant bacteria has been explored. For example, a study of personalized ertapenem prophylaxis based on the results of preoperative screening for extended-spectrum β-lactamase–producing Enterobacterales suggested a benefit in colorectal surgery with no measurable increase in C difficile infection or selection for postoperative colonization with resistant strains.11,69 Surrogate clinical, procedural, or geographical factors associated with resistant infection available in electronic health records could also be leveraged to complement laboratory-based methods. Aside from the question of effectiveness, substantial barriers to realizing such an approach currently exist. The relevant populations, sampling strategies, diagnostic technologies, clinical protocols, and oversight structure remain to be defined. This issue is acknowledged, but remains to be addressed, in the latest global guidelines for SSI prevention.70
As a prerequisite to refining evidence-based protocols for surgical prophylaxis and understanding the role of antimicrobial resistance in the US, more epidemiologic data on SSI microbiology are needed. Data collected by the US Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network capture case-level pathogen and resistance patterns, but are limited in scope to colorectal surgery and abdominal hysterectomy at a national level and are not available for research or policy development outside of the CDC, limiting the potential utility of that resource. Conversely, large, accessible registries such as the American College of Surgeons National Surgical Quality Improvement Program or Society of Thoracic Surgeons National Database and commercially available research datasets capture SSI occurrence across more diverse procedure groups, but do not include corresponding laboratory data from wound cultures. Even in landmark clinical studies addressing SSIs, the microbiological results underlying the findings are often not reported.71 The absence of such data in these key sources may reflect their inherent complexity relative to other laboratory data such as hemoglobin or white blood cell measurements; however, the unrealized public health value of these results, generated daily in the course of clinical care and clinical trials, is immense.
Leverage Nonantibiotic SSI Prevention Measures
A number of complementary, nonantibiotic strategies exist to facilitate the same objective of SSI prevention served by antibiotics. Given anticipated increases in SSIs caused by bacteria resistant to standard prophylactic agents3 and the diminishing marginal returns of efforts to further sterilize the operating room environment,72–74 these alternative evidence-based strategies represent “win-win” practices that should be more consistently emphasized.
Temperature and Glucose Management
Maintenance of established targets for normothermia and euglycemia, both strongly associated with reduction in SSI risk, remains inconsistent across a wide range of practice settings and procedure types.75 The association between perioperative hyperglycemia and SSIs appears to be similar for both patients with diabetes and those without diabetes,76 indicating that broader screening for hyperglycemia in surgical patients may be beneficial.
Closed Incision Negative Pressure Wound Therapy
Randomized clinical trials of negative pressure wound therapy after primary closure of surgical incisions for the prevention of SSIs have shown effectiveness for the prevention of SSIs across various emergency and elective procedures. The overall weight of evidence has gradually shifted in favor of this technique in updates to high-profile meta-analyses77,78 and is now supported by expert recommendation in the US.58 Although significant heterogeneity exists among studies (even those performed among similar populations), it appears likely that routine use of negative pressure wound therapy will ultimately be favored for some high-risk patient and procedure groups.
Oral Hygiene
Preoperative oral hygiene interventions prior to elective surgery to address dental caries, gum disease, or ongoing mouth infections may decrease SSI rates.79 This approach considers that mouth organisms existing in chronic asymptomatic infections (eg, gingivitis, dental caries) may find their way to the operative site through the bloodstream and cause SSIs.80 The causal pathway by which this may occur and whether improving oral hygiene prior to surgery mitigates risk remain to be determined.
Dietary Prehabilitation and Probiotics
Preoperative dietary habits have been associated with differences in overall surgical complications including SSIs, anastomotic leakage, and postoperative ileus.81 Similarly, a high-fat, low-fiber “Western diet” has also been shown to be associated with increased rates of anastomotic leak in mice, but such an effect can be reversed by short-term (ie, 2-day) consumption of a plant-based, high-fiber, low-fat diet.82 Taken together, there is emerging evidence that dietary prehabilitation for high-risk patients may have benefits in reducing SSI rates via effects on the gut microbiome. Probiotics, synbiotics, and fecal transplants are also conceptually appealing avenues for preservation of protective gut flora during exposure to antibiotics and surgical stress83; however, important fundamental questions preceding human trials remain, related to safety,84 specific composition of probiotic consortia, complementation of the baseline microbiome, and other factors.
Enhanced Recovery Programs
Enhanced recovery programs and fast-track surgical pathways include many improvements in surgical care that have independently been shown to reduce SSIs. These programs include the use of minimally invasive techniques, elimination of drains, and normothermia. Although the added association of other common bundle elements (eg, nonopioid analgesia, nutritional optimization, early hospital discharge) with infection is unclear, these programs have several benefits and have been associated with reduced rates of SSIs for some groups.85 Additional evidence-based SSI prevention practices such as preoperative screening and decolonization, glucose management, appropriate use of combined antibiotic or mechanical bowel preparation, use of cephalosporins for patients with reported penicillin allergies, and avoidance of postoperative antibiotic prophylaxis could reasonably be incorporated into these pathways to facilitate practice standardization in this domain.
Consider New Models of SSI Pathogenesis
Significant further advancements in SSI prevention will likely require an improved fundamental understanding of SSI pathogenesis. Although SSIs can certainly occur as a result of direct intraoperative contamination of the wound, and changes in antiseptic practice targeting this mechanism of SSIs have yielded gains over preceding decades, current data from both the Agency for Healthcare Research and Quality and CDC indicate that rates of SSIs are no longer decreasing.6,7 Next-generation techniques being developed for microbiome science could be used to identify, characterize, and track potential pathogens before, during, and after surgery (Figure 1) to better define (1) the specific origins of strains causing SSIs, (2) the routes by which those strains achieve entry into the surgical site, and (3) the mechanisms by which SSI-causing bacteria evade current prevention measures and express virulence and antibiotic resistance traits.
Figure 1: Application of next-generation technologies to understand the pathogenesis and antimicrobial resistance patterns of SSIs.
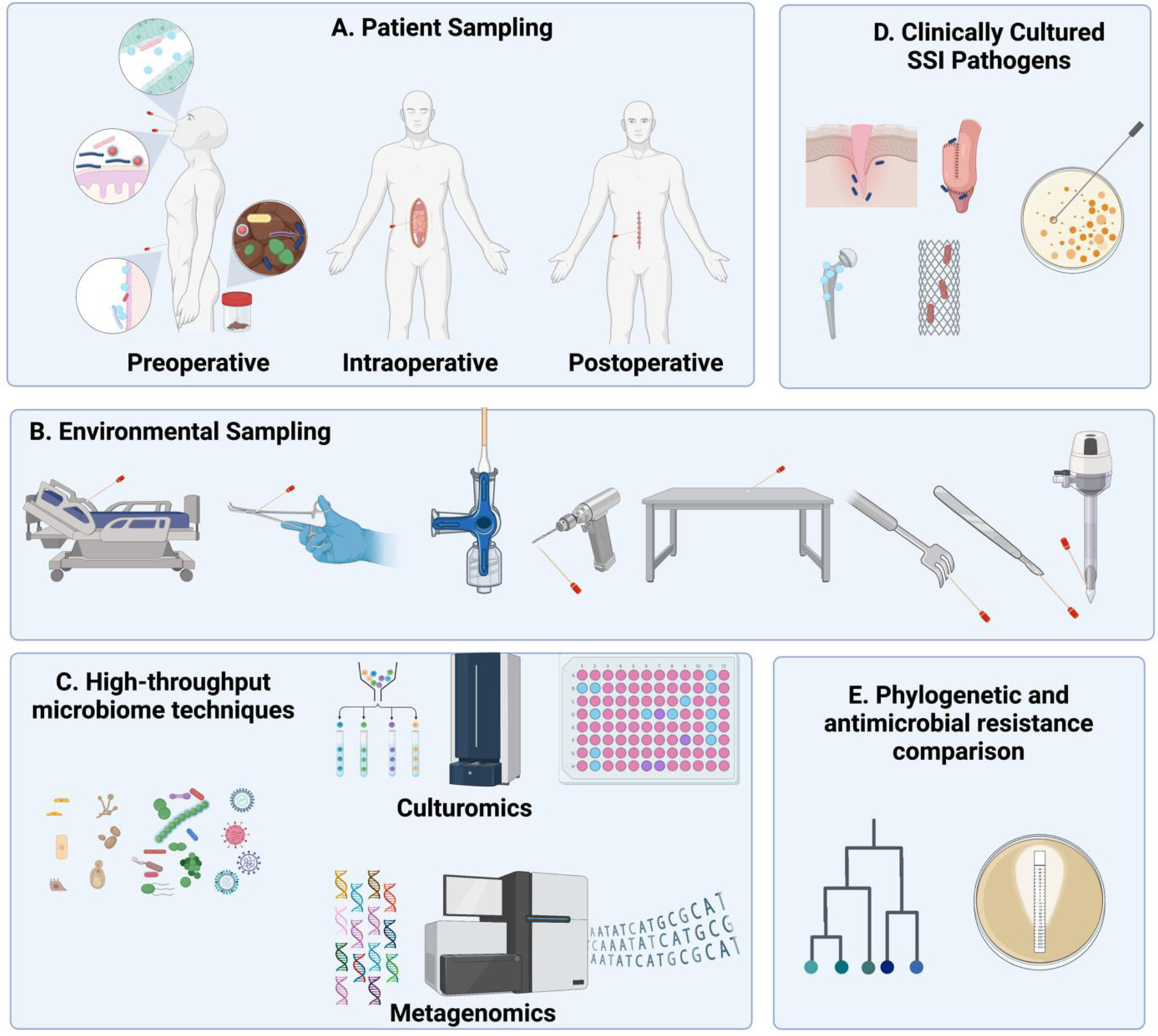
Samples from various patient- (A) and environmental (B) reservoirs can be sampled throughout the perioperative period and preserved. High-throughput microbiome analyses (C) can then be performed when an SSI develops and its associated pathogen(s) can be isolated and cultured (D). Strain-level comparisons of genome sequence and antimicrobial resistance genotypes/phenotypes of the SSI pathogen can be compared with preserved samples to examine potential sources of infection and the genetic progression of antimicrobial resistance (E).
The first step in any of these investigations will be to consider the possibility that endogenous sites of colonization may be the source of most SSIs.57 For example, among SSIs caused by S aureus, approximately 80% are genetically similar to endogenous strains colonizing the patient nares.86 However, whether this finding is true of infections caused by other SSI pathogens, how colonization or decolonization is associated with infection at a remote surgical site remains ill defined,54,55 and other outstanding questions remain. Is the presence of MRSA in the nares a surrogate for colonization more proximal to the surgical site? Or can bacteria present in high numbers in remote reservoirs be translocated to the surgical site by indirect means, as suggested by the “Trojan Horse” hypothesis of SSIs?80 For a given procedure, what are the most relevant reservoirs to target for preoperative screening and decolonization?
Standard culture methods have been used to explore these questions in the past, but are constrained in the type and number of bacteria that can be characterized, limiting practical clinical inferences into the complex pathogenesis of SSIs. In contrast, newer culturomic technologies (special media and techniques for isolating large numbers of diverse bacteria) and metagenomic technologies (sequencing-based classification of host and microbial populations) are capable of characterizing the events leading to infection with substantially higher resolution. Deploying next-generation technologies to define the distribution of relevant bacterial strains and their virulence or resistance traits over time and anatomical space may inform more effective prevention strategies beyond current uses of antibiotics and environmental sterility measures.
Conclusions
The anticipated global increase in colonization of healthy individuals with antimicrobial-resistant organisms will be reflected in a rapidly changing microbial landscape within our hospitals and communities, and demands a coordinated and proactive effort to address. Development of effective and sustainable programs for perioperative antibiotic use in SSI prevention will require incorporating antimicrobial stewardship as a key component of surgical quality. Use of new data sources and technologies to illuminate novel models of SSI pathogenesis and more targeted methods of prevention should be considered as essential elements of this approach.
ACKNOWLEDGEMENTS
One or more figures created with BioRender.com.
Funding/Support:
This study was supported in part by grant R01 2R01GM062344-22 from the National Institutes of Health/National Institute of General Medical Sciences (Dr Alverdy) and grant 5K23AR080209-02 from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (Dr Long).
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Disclosure: Dr Alverdy is the founder and chief research officer of Covira Surgical, a University of Chicago generated company
Conflict of Interest Disclosures: Dr Sawyer reported receiving personal fees from Pfizer, Merck, Molnlycke, and AbbVie outside the submitted work. Dr Alverdy reported being founder and chief scientific officer of Covira Surgical during the conduct of the study; in addition, Dr Alverdy had a patent for PCT/US19/28748 issued. No other disclosures were reported.
REFERENCES
- 1.Roberts SA, Morris AJ. Surgical antibiotic prophylaxis: more is not better. Lancet Infect Dis. 2020;20(10):1110–1111. doi: 10.1016/S1473-3099(20)30290-5 [DOI] [PubMed] [Google Scholar]
- 2.Bowater RJ, Stirling SA, Lilford RJ. Is antibiotic prophylaxis in surgery a generally effective intervention? testing a generic hypothesis over a set of meta-analyses. Ann Surg. 2009;249(4):551–556. doi: 10.1097/SLA.0b013e318199f202 [DOI] [PubMed] [Google Scholar]
- 3.Teillant A, Gandra S, Barter D, Morgan DJ, Laxminarayan R. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis. 2015;15(12):1429–1437. doi: 10.1016/S1473-3099(15)00270-4 [DOI] [PubMed] [Google Scholar]
- 4.Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013;148(7):649–657. doi: 10.1001/jamasurg.2013.134 [DOI] [PubMed] [Google Scholar]
- 5.Magill SS, O’Leary E, Ray SM, et al. ; Emerging Infections Program Hospital Prevalence Survey Team. Antimicrobial use in US hospitals: comparison of results from emerging infections program prevalence surveys, 2015 and 2011. Clin Infect Dis. 2021;72(10):1784–1792. doi: 10.1093/cid/ciaa373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research and Quality. AHRQ national scorecard on hospital-acquired conditions: final results for 2014 through 2017. 2020. Accessed May 13, 2024. https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/pfp/Updated-hacreportFInal2017data.pdf
- 7.Healthcare-associated infections (HAIs). Current HAI progress report. 2022 National and state healthcare-associated infections progress report. 2023. Centers for Disease Control and Prevention (CDC). Accessed February 3, 2023. https://www.cdc.gov/hai/data/portal/progress-report.html [Google Scholar]
- 8.D’Angelica MI, Ellis RJ, Liu JB, et al. Piperacillin-tazobactam compared with cefoxitin as antimicrobial prophylaxis for pancreatoduodenectomy: a randomized clinical trial. JAMA. 2023;329(18):1579–1588. doi: 10.1001/jama.2023.5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kone LB, Torres C, Banulescu M, Maker VK, Maker AV. Perioperative broad-spectrum antibiotics are associated with decreased surgical site infections compared to 1st-3rd generation cephalosporins after open pancreaticoduodenectomy in patients with jaundice or a biliary stent. Ann Surg. 2022;275(6):1175–1183. doi: 10.1097/SLA.0000000000004216 [DOI] [PubMed] [Google Scholar]
- 10.Deierhoi RJ, Dawes LG, Vick C, Itani KMF, Hawn MT. Choice of intravenous antibiotic prophylaxis for colorectal surgery does matter. J Am Coll Surg. 2013;217(5):763–769. doi: 10.1016/j.jamcollsurg.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 11.Nutman A, Temkin E, Harbarth S, et al. Personalized ertapenem prophylaxis for carriers of extended-spectrum β-lactamase–producing enterobacteriaceae undergoing colorectal surgery. Clin Infect Dis. 2020;70(9):1891–1897. doi: 10.1093/cid/ciz524 [DOI] [PubMed] [Google Scholar]
- 12.Hatachi T, Sofue T, Ito Y, et al. Antibiotic prophylaxis for open chest management after pediatric cardiac surgery. Pediatr Crit Care Med. 2019;20(9):801–808. doi: 10.1097/PCC.0000000000001995 [DOI] [PubMed] [Google Scholar]
- 13.Ellis RJ, Brajcich BC, Bertens KA, et al. Association between biliary pathogens, surgical site infection, and pancreatic fistula: results of a randomized trial of perioperative antibiotic prophylaxis in patients undergoing pancreatoduodenectomy. Ann Surg. 2023;278(3):310–319. doi: 10.1097/SLA.0000000000005955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ierano C, Thursky K, Marshall C, et al. Appropriateness of surgical antimicrobial prophylaxis practices in Australia. JAMA Netw Open. 2019;2(11):e1915003. doi: 10.1001/jamanetworkopen.2019.15003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plachouras D, Kärki T, Hansen S, et al. ; Point Prevalence Survey Study Group. Antimicrobial use in European acute care hospitals: results from the second Point Prevalence Survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill. 2018;23(46):1800393. doi: 10.2807/1560-7917.ES.23.46.1800393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puig-Asensio M, Perencevich EN, Livorsi DJ. Prolonged postprocedural antimicrobial use: a survey of the Society for Healthcare Epidemiology of America Research Network. Infect Control Hosp Epidemiol. 2019;40(11):1281–1283. doi: 10.1017/ice.2019.242 [DOI] [PubMed] [Google Scholar]
- 17.Ullah K, Dogar AW, Jan Z, et al. Role of antibiotic prophylaxis on surgical site infection prevention in a low-risk population undergoing laparoscopic cholecystectomy: a randomized controlled study. Ann Med Surg (Lond). 2022;78:103804. doi: 10.1016/j.amsu.2022.103804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins A, London J, Charland S, et al. Prophylactic antibiotics for elective laparoscopic cholecystectomy: are they necessary? Arch Surg. 1999;134(6):611–613. doi: 10.1001/archsurg.134.6.611 [DOI] [PubMed] [Google Scholar]
- 19.Sanabria A, Dominguez LC, Valdivieso E, Gomez G. Antibiotic prophylaxis for patients undergoing elective laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2010;(12):CD005265. doi: 10.1002/14651858.CD005265.pub2 [DOI] [PubMed] [Google Scholar]
- 20.Vohra RS, Hodson J, Pasquali S, Griffiths EA; CholeS Study Group and West Midlands Research Collaborative. Effectiveness of antibiotic prophylaxis in non-emergency cholecystectomy using data from a population-based cohort study. World J Surg. 2017;41(9):2231–2239. doi: 10.1007/s00268-017-4018-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aktas A, Kayaalp C, Gunes O, et al. Surgical site infections after laparoscopic bariatric surgery: is routine antibiotic prophylaxis required? Surg Infect (Larchmt). 2021;22(7):705–712. doi: 10.1089/sur.2020.426 [DOI] [PubMed] [Google Scholar]
- 22.Zheng L, Jiang L, Li D, et al. Antimicrobial prophylaxis in patients undergoing endoscopic mucosal resection for 10- to 20-mm colorectal polyps: a randomized prospective study. Medicine (Baltimore). 2022;101(50):e31440. doi: 10.1097/MD.0000000000031440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polistena A, Prete FP, Avenia S, et al. Effect of antibiotic prophylaxis on surgical site infection in thyroid and parathyroid surgery: a systematic review and meta-analysis. Antibiotics (Basel). 2022;11(3):290. doi: 10.3390/antibiotics11030290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goff DA, Mangino JE, Glassman AH, Goff D, Larsen P, Scheetz R. Review of guidelines for dental antibiotic prophylaxis for prevention of endocarditis and prosthetic joint infections and need for dental stewardship. Clin Infect Dis. 2020;71(2):455–462. doi: 10.1093/cid/ciz1118 [DOI] [PubMed] [Google Scholar]
- 25.Barbieri JS, Etzkorn JR, Margolis DJ. Use of antibiotics for dermatologic procedures from 2008 to 2016. JAMA Dermatol. 2019;155(4):465–470. doi: 10.1001/jamadermatol.2019.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fay A, Nallasamy N, Bernardini F, et al. Multinational comparison of prophylactic antibiotic use for eyelid surgery. JAMA Ophthalmol. 2015;133(7):778–784. doi: 10.1001/jamaophthalmol.2015.0789 [DOI] [PubMed] [Google Scholar]
- 27.Brook N, Khalaf Y, Coomarasamy A, Edgeworth J, Braude P. A randomized controlled trial of prophylactic antibiotics (co-amoxiclav) prior to embryo transfer. Hum Reprod. 2006;21(11):2911–2915. doi: 10.1093/humrep/del263 [DOI] [PubMed] [Google Scholar]
- 28.Savitz SI, Rivlin MM, Savitz MH. The ethics of prophylactic antibiotics for neurosurgical procedures. J Med Ethics. 2002;28(6):358–363. doi: 10.1136/jme.28.6.358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazaki T, Mado K, Masuda H, Shiono M, Tochikura N, Kaburagi M. A randomized trial of antibiotic prophylaxis for the prevention of surgical site infection after open mesh-plug hernia repair. Am J Surg. 2014;207(4):476–484. doi: 10.1016/j.amjsurg.2013.01.047 [DOI] [PubMed] [Google Scholar]
- 30.Chongsomchai C, Lumbiganon P, Thinkhamrop J, Ounchai J, Vudhikamraksa N. Placebo-controlled, double-blind, randomized study of prophylactic antibiotics in elective abdominal hysterectomy. J Hosp Infect. 2002;52(4):302–306. doi: 10.1053/jhin.2002.1312 [DOI] [PubMed] [Google Scholar]
- 31.Zijderveld SA, Smeele LE, Kostense PJ, Tuinzing DB. Preoperative antibiotic prophylaxis in orthognathic surgery: a randomized, double-blind, and placebo-controlled clinical study. J Oral Maxillofac Surg. 1999;57(12):1403–1406. doi: 10.1016/S0278-2391(99)90718-8 [DOI] [PubMed] [Google Scholar]
- 32.Petignat C, Francioli P, Harbarth S, et al. Cefuroxime prophylaxis is effective in noninstrumented spine surgery: a double-blind, placebo-controlled study. Spine (Phila Pa 1976). 2008;33(18):1919–1924. doi: 10.1097/BRS.0b013e31817d97cf [DOI] [PubMed] [Google Scholar]
- 33.Gaillard T, Gilsbach JM. Intra-operative antibiotic prophylaxis in neurosurgery: a prospective, randomized, controlled study on cefotiam. Acta Neurochir (Wien). 1991;113(3–4):103–109. doi: 10.1007/BF01403193 [DOI] [PubMed] [Google Scholar]
- 34.Jacewicz M, Günzel K, Rud E, et al. Antibiotic prophylaxis versus no antibiotic prophylaxis in transperineal prostate biopsies (NORAPP): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. 2022;22(10):1465–1471. doi: 10.1016/S1473-3099(22)00373-5 [DOI] [PubMed] [Google Scholar]
- 35.Halani S, McIntyre M, Vaisman A. The harms of postoperative antibiotic prophylaxis: a teachable moment. JAMA Intern Med. 2022;182(5):545–546. doi: 10.1001/jamainternmed.2022.0413 [DOI] [PubMed] [Google Scholar]
- 36.de Jonge SW, Boldingh QJJ, Solomkin JS, et al. Effect of postoperative continuation of antibiotic prophylaxis on the incidence of surgical site infection: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(10):1182–1192. doi: 10.1016/S1473-3099(20)30084-0 [DOI] [PubMed] [Google Scholar]
- 37.Sattar AK, Masroor T, Martins RS, et al. Impact of postoperative antibiotic prophylaxis on surgical site infections rates after mastectomy with drains but without immediate reconstruction: a multicenter, double-blinded, randomized control superiority trial. Ann Surg Oncol. 2023;30(10):5965–5973. doi: 10.1245/s10434-023-13887-5 [DOI] [PubMed] [Google Scholar]
- 38.He K, Nayak RB, Allori AC, et al. Correlation between postoperative antimicrobial prophylaxis use and surgical site infection in children undergoing nonemergent surgery. JAMA Surg. 2022;157(12):1142–1151. doi: 10.1001/jamasurg.2022.4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirokawa F, Hayashi M, Miyamoto Y, et al. Evaluation of postoperative antibiotic prophylaxis after liver resection: a randomized controlled trial. Am J Surg. 2013;206(1):8–15. doi: 10.1016/j.amjsurg.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 40.Harbarth S, Samore MH, Lichtenberg D, Carmeli Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation. 2000;101(25):2916–2921. doi: 10.1161/01.CIR.101.25.2916 [DOI] [PubMed] [Google Scholar]
- 41.Niederhäuser U, Vogt M, Vogt P, Genoni M, Künzli A, Turina MI. Cardiac surgery in a high-risk group of patients: is prolonged postoperative antibiotic prophylaxis effective? J Thorac Cardiovasc Surg. 1997;114(2):162–168. doi: 10.1016/S0022-5223(97)70140-5 [DOI] [PubMed] [Google Scholar]
- 42.Kabulski GM, Northup A, Wiggins BS. Postoperative antibiotic prophylaxis following cardiac implantable electronic device placement. J Innov Card Rhythm Manag. 2019;10(8):3777–3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghantous Y, Araidy S, Yaffe V, Mirochnik R, El-Raziq MA, El-Naaj IA. The efficiency of extended postoperative antibiotic prophylaxis in orthognathic surgery: a prospective, randomized, double-blind, placebo-controlled clinical trial. J Craniomaxillofac Surg. 2019;47(2):228–232. doi: 10.1016/j.jcms.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 44.Ghert M, Schneider P, Guyatt G, et al. ; Prophylactic Antibiotic Regimens in Tumor Surgery (PARITY) Investigators. Comparison of prophylactic intravenous antibiotic regimens after endoprosthetic reconstruction for lower extremity bone tumors: a randomized clinical trial. JAMA Oncol. 2022;8(3):345–353. doi: 10.1001/jamaoncol.2021.6628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagata K, Yamada K, Shinozaki T, et al. ; OSSI investigators. Effect of antimicrobial prophylaxis duration on health care–associated infections after clean orthopedic surgery: a cluster randomized trial. JAMA Netw Open. 2022;5(4):e226095. doi: 10.1001/jamanetworkopen.2022.6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pivazyan G, Khan Z, Williams JD, et al. Utility of prolonged prophylactic systemic antibiotics for wound drains in posterior spine surgery: a systematic review and meta-analysis. J Neurosurg Spine. 2023;38(5):585–594. doi: 10.3171/2022.12.SPINE221218 [DOI] [PubMed] [Google Scholar]
- 47.Branch-Elliman W, O’Brien W, Strymish J, Itani K, Wyatt C, Gupta K. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019;154(7):590–598. doi: 10.1001/jamasurg.2019.0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calderwood MS, Anderson DJ, Bratzler DW, et al. Strategies to prevent surgical site infections in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol. 2023;44(5):695–720. doi: 10.1017/ice.2023.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kullar R, Goff DA, Schulz LT, Fox BC, Rose WE. The “epic” challenge of optimizing antimicrobial stewardship: the role of electronic medical records and technology. Clin Infect Dis. 2013;57(7):1005–1013. doi: 10.1093/cid/cit318 [DOI] [PubMed] [Google Scholar]
- 50.Shah SD, Cifu AS. From guideline to order set to patient harm. JAMA. 2018;319(12):1207–1208. doi: 10.1001/jama.2018.1666 [DOI] [PubMed] [Google Scholar]
- 51.Park S, Kim S, Kim HB, Youn SW, Ahn S, Kim K. Effects of implementing a clinical pathway on antibiotic prophylaxis for patients who underwent an elective surgery. Sci Rep. 2022;12(1):20176. doi: 10.1038/s41598-022-24145-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dellinger EP. When will the surgical community acknowledge the evidence regarding prophylaxis with oral antibiotics for scheduled colorectal operations? JAMA Netw Open. 2018;1(6):e183257. doi: 10.1001/jamanetworkopen.2018.3257 [DOI] [PubMed] [Google Scholar]
- 53.Rawson TM, Moore LSP, Hatcher JC, Donaldson H, Holmes AH. Plasmid-mediated colistin resistance mechanisms: is it time to revise our approach to selective digestive decontamination? Lancet Infect Dis. 2016;16(2):149–150. doi: 10.1016/S1473-3099(15)00539-3 [DOI] [PubMed] [Google Scholar]
- 54.Raineri EJM, Altulea D, van Dijl JM. Staphylococcal trafficking and infection—from “nose to gut” and back. FEMS Microbiol Rev. 2022;46(1):fuab041. doi: 10.1093/femsre/fuab041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acton DS, Plat-Sinnige MJT, van Wamel W, de Groot N, van Belkum A. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur J Clin Microbiol Infect Dis. 2009;28(2):115–127. doi: 10.1007/s10096-008-0602-7 [DOI] [PubMed] [Google Scholar]
- 56.Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis. 2009;49(6):935–941. doi: 10.1086/605495 [DOI] [PubMed] [Google Scholar]
- 57.Wenzel RP. Surgical site infections and the microbiome: an updated perspective. Infect Control Hosp Epidemiol. 2019;40(5):590–596. doi: 10.1017/ice.2018.363 [DOI] [PubMed] [Google Scholar]
- 58.Seidelman JL, Mantyh CR, Anderson DJ. Surgical site infection prevention: a review. JAMA. 2023;329(3):244–252. doi: 10.1001/jama.2022.24075 [DOI] [PubMed] [Google Scholar]
- 59.Miles-Jay A, Snitkin ES, Lin MY, et al. Longitudinal genomic surveillance of carriage and transmission of Clostridioides difficile in an intensive care unit. Nat Med. 2023;29(10):2526–2534. doi: 10.1038/s41591-023-02549-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dickey SW, Cheung GYC, Otto M. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat Rev Drug Discov. 2017;16(7):457–471. doi: 10.1038/nrd.2017.23 [DOI] [PubMed] [Google Scholar]
- 61.Hyoju SK, Keskey R, Castillo G, et al. A novel nonantibiotic gut-directed strategy to prevent surgical site infections. Ann Surg. 2022;276(3):472–481. doi: 10.1097/SLA.0000000000005547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright TI, Baddour LM, Berbari EF, et al. Antibiotic prophylaxis in dermatologic surgery: advisory statement 2008. J Am Acad Dermatol. 2008;59(3):464–473. doi: 10.1016/j.jaad.2008.04.031 [DOI] [PubMed] [Google Scholar]
- 63.Goh M, Hollewand C, McBride S, Ryan N, van der Werf B, Mathy JA. Effect of microdoses of incisional antibiotics on the rate of surgical site infections in skin cancer surgery: a randomized clinical trial. JAMA Surg. 2023;158(7):718–726. doi: 10.1001/jamasurg.2023.1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Itani KM, Wilson SE, Awad SS, Jensen EH, Finn TS, Abramson MA. Ertapenem versus cefotetan prophylaxis in elective colorectal surgery. N Engl J Med. 2006;355(25):2640–2651. doi: 10.1056/NEJMoa054408 [DOI] [PubMed] [Google Scholar]
- 65.Feldt SL, Keskey R, Krishnan P, Hyman NH, Shogan BD. Is previous postoperative infection an independent risk factor for postoperative infection after second unrelated abdominal operation? J Am Coll Surg. 2022;235(2):285–292. doi: 10.1097/XCS.0000000000000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dubinsky-Pertzov B, Temkin E, Harbarth S, et al. ; R-GNOSIS WP4 Study Group. Carriage of extended-spectrum beta-lactamase–producing enterobacteriaceae and the risk of surgical site infection after colorectal surgery: a prospective cohort study. Clin Infect Dis. 2019;68(10):1699–1704. doi: 10.1093/cid/ciy768 [DOI] [PubMed] [Google Scholar]
- 67.Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi: 10.2146/ajhp120568 [DOI] [PubMed] [Google Scholar]
- 68.Branch-Elliman W, Ripollone JE, O’Brien WJ, et al. Risk of surgical site infection, acute kidney injury, and Clostridium difficile infection following antibiotic prophylaxis with vancomycin plus a beta-lactam versus either drug alone: a national propensity-score–adjusted retrospective cohort study. PloS Med. 2017;14(7):e1002340. doi: 10.1371/journal.pmed.1002340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffman T, Lellouche J, Nutman A, et al. ; Resistance in Gram-Negative Organisms: Studying Intervention Strategies (R-GNOSIS) WP4 Study Group. The effect of prophylaxis with ertapenem versus cefuroxime/metronidazole on intestinal carriage of carbapenem-resistant or third-generation-cephalosporin–resistant Enterobacterales after colorectal surgery. Clin Microbiol Infect. 2021;27(10):1481–1487. doi: 10.1016/j.cmi.2021.02.002 [DOI] [PubMed] [Google Scholar]
- 70.Global guidelines for the prevention of surgical site infection. December 22, 2018. World Health Organization. Accessed May 13, 2024. https://www.who.int/publications/i/item/9789241550475 [PubMed] [Google Scholar]
- 71.Machutta K, Xiao J, Winters CA, et al. Defeating cancel culture in surgical site infection research: a plea to include microbial cultures and antibiotic sensitivity data. Surg Infect (Larchmt). 2022;23(10):902–907. doi: 10.1089/sur.2022.247 [DOI] [PubMed] [Google Scholar]
- 72.Bischoff P, Kubilay NZ, Allegranzi B, Egger M, Gastmeier P. Effect of laminar airflow ventilation on surgical site infections: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(5):553–561. doi: 10.1016/S1473-3099(17)30059-2 [DOI] [PubMed] [Google Scholar]
- 73.Jentzsch T, Kutschke L, Zingg PO, Farshad M. Operating room architecture is not a risk factor for surgical site infections. Sci Rep. 2021;11(1):13391. doi: 10.1038/s41598-021-90574-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wills BW, Smith WR, Arguello AM, McGwin G, Ghanem ES, Ponce BA. Association of surgical jacket and bouffant use with surgical site infection risk. JAMA Surg. 2020;155(4):323–328. doi: 10.1001/jamasurg.2019.6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baker AW, Ilieş I, Benneyan JC, et al. Early recognition and response to increases in surgical site infections using optimised statistical process control charts—the Early 2RIS Trial: a multicentre stepped wedge cluster randomised controlled trial. EClinicalMedicine. 2022;54:101698. doi: 10.1016/j.eclinm.2022.101698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Vries FEE, Gans SL, Solomkin JS, et al. Meta-analysis of lower perioperative blood glucose target levels for reduction of surgical-site infection. Br J Surg. 2017;104(2):e95–e105. doi: 10.1002/bjs.10424 [DOI] [PubMed] [Google Scholar]
- 77.Norman G, Shi C, Goh EL, et al. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database Syst Rev. 2022;4(4):CD009261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Groenen H, Jalalzadeh H, Buis DR, et al. Incisional negative pressure wound therapy for the prevention of surgical site infection: an up-to-date meta-analysis and trial sequential analysis. EClinicalMedicine. 2023;62:102105. doi: 10.1016/j.eclinm.2023.102105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nobuhara H, Matsugu Y, Soutome S, et al. Perioperative oral care can prevent surgical site infection after colorectal cancer surgery: a multicenter, retrospective study of 1,926 cases analyzed by propensity score matching. Surgery. 2022;172(2):530–536. doi: 10.1016/j.surg.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 80.Alverdy JC, Hyman N, Gilbert J. Re-examining causes of surgical site infections following elective surgery in the era of asepsis. Lancet Infect Dis. 2020;20(3):e38–e43. doi: 10.1016/S1473-3099(19)30756-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kok DE, Arron MNN, Huibregtse T, et al. Association of habitual preoperative dietary fiber intake with complications after colorectal cancer surgery. JAMA Surg. 2021;156(9):1–10. doi: 10.1001/jamasurg.2021.2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Keskey R, Papazian E, Lam A, et al. Defining microbiome readiness for surgery: dietary prehabilitation and stool biomarkers as predictive tools to improve outcome. Ann Surg. 2022;276(5):e361–e369. doi: 10.1097/SLA.0000000000004578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chowdhury AH, Adiamah A, Kushairi A, et al. Perioperative probiotics or synbiotics in adults undergoing elective abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. Ann Surg. 2020;271(6):1036–1047. doi: 10.1097/SLA.0000000000003581 [DOI] [PubMed] [Google Scholar]
- 84.Besselink MG, van Santvoort HC, Buskens E, et al. ; Dutch Acute Pancreatitis Study Group. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9613):651–659. doi: 10.1016/S0140-6736(08)60207-X [DOI] [PubMed] [Google Scholar]
- 85.Grant MC, Yang D, Wu CL, Makary MA, Wick EC. Impact of enhanced recovery after surgery and fast track surgery pathways on healthcare-associated infections: results from a systematic review and meta-analysis. Ann Surg. 2017;265(1):68–79. doi: 10.1097/SLA.0000000000001703 [DOI] [PubMed] [Google Scholar]
- 86.Bode LGM, Kluytmans JAJW, Wertheim HFL, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9–17. doi: 10.1056/NEJMoa0808939 [DOI] [PubMed] [Google Scholar]


