Abstract
Rationale
Although previous studies have assessed the clinical or economic value of specific technologies, the economic value of improving sensitivity for malignancy in lung cancer diagnoses broadly across technologies is unclear.
Objectives
To identify the economic value of improving sensitivity of bronchoscopy biopsy for the diagnosis of lung cancer.
Methods
A decision analytic model was developed to quantify the economic value of increased sensitivity for malignancy for bronchoscopy biopsy of peripheral pulmonary lesions. Primary clinical outcomes included time to diagnosis and survival. Economic outcomes included 1) net monetary benefit (NMB), defined as the health benefits measured in quality-adjusted life-years (QALYs) times willingness to pay ($100,000/QALY) net of changes in medical costs; and 2) incremental cost-effectiveness ratio. A decision tree modeling framework with two Markov module branches was developed. The two Markov modules corresponded to patients with cancer who were 1) diagnosed and treated or 2) undiagnosed and remained untreated. Outcomes were measured from a U.S. payer perspective over 30 years.
Results
Improving sensitivity for malignancy by 10 percentage points decreased average time to diagnosis for patients with lung cancer by 0.85 month (4 wk) and increased survival by 0.36 year (19 wk) because of faster treatment initiation. Overall health outcomes improved by 0.20 QALYs per patient. Cost increased by $6,727 per patient primarily through increased treatment costs among those diagnosed with cancer. Increasing sensitivity for malignancy by 10 percentage points improved NMB by $8,729 over 30 years (incremental cost-effectiveness ratio of $34,052), driven largely by improved sensitivity to early-stage cancer (stage-specific NMB, I/II, $19,805; III, $2,101; IV, −$1,438). Forty-two percent of overall NMB ($3,668) accrued within 5 years of biopsy. The relationship between change in sensitivity and NMB was approximately linear (1% vs. 10% sensitivity improvement corresponded to NMB of $885 vs. $8,729). The model was most sensitive to cancer treatment efficacy and follow-up time after a negative result.
Conclusions
Increasing sensitivity of malignancy by 10 percentage points resulted in a $8,729 improvement in net economic value. Health systems can use this information when making decisions regarding the value of new bronchoscopy technologies.
Keywords: bronchoscopy, sensitivity for malignancy, economics, economic value
Early diagnosis is vital for effective lung cancer management. In 2021, the U.S. Preventive Services Task Force revised 2013 lung cancer screening guidelines, lowering age eligibility from 55 to 50 and pack-year history from 30 to 20 and increasing the estimated population eligibility for screening by 53.7%. In addition, over 1.5 million pulmonary lesions are incidentally identified each year (1–4). As a result, the use of minimally invasive biopsy techniques for evaluating pulmonary lesions is expected to increase.
Minimally invasive biopsy approaches for sampling of peripheral lesions vary in sensitivity for malignancy and safety outcomes. Currently, computed tomography (CT)-guided transthoracic needle biopsy, a widely used minimally invasive biopsy method, has a sensitivity for malignancy of approximately 90% (5). However, approximately one-quarter of patients who undergo CT-guided transthoracic needle biopsy experience complications such as pneumothorax (23.3%), hemorrhage (3.6%), and air embolism (0.02%), with some patients requiring additional interventions for management (6). Some lesions may also be inaccessible via a transthoracic approach because of anatomy or patient risk factors.
An alternative methodology for biopsy of peripheral pulmonary lesions is bronchoscopy. Although conventional bronchoscopy has demonstrated diagnostic sensitivity (60–76% sensitivity for malignancy) (7, 8), recent innovation in the bronchoscopy field (e.g., real-time ultrasound, advanced navigation platforms, advanced imaging modalities [9]) now allows targeting peripheral lesions with greater accuracy while preserving patient safety. Furthermore, robotics enhances maneuverability and improves reach into the periphery and stability to increase sensitivity for malignancy (10–13). Bronchoscopy allows mediastinal staging, sampling of multiple lesions, and a reduced risk of complications.
The diagnosis of peripheral pulmonary lesions with bronchoscopy involves a complex decision-making process in which clinicians consider factors such as the likelihood of establishing a diagnosis, complications, diagnosis time, staging, cost, nodal metastasis risk, and mortality. Previous studies evaluated that the most cost-effective diagnostic strategy for lung cancer depends on lesion stage, location, and type of biopsy (14). Other studies have assessed the economic value between bronchoscopy and traditional CT-guided biopsy (15, 16); however, little is known about the economic outcomes of increased test sensitivity when diagnosing patients with suspected lung cancer. The purpose of this study was to quantify the health and economic value of improved sensitivity for malignancy for bronchoscopic procedures targeting peripheral pulmonary lesions to inform health care decision makers on decisions related to diagnostic technologies for health systems.
Methods
Model Overview and Decision Context
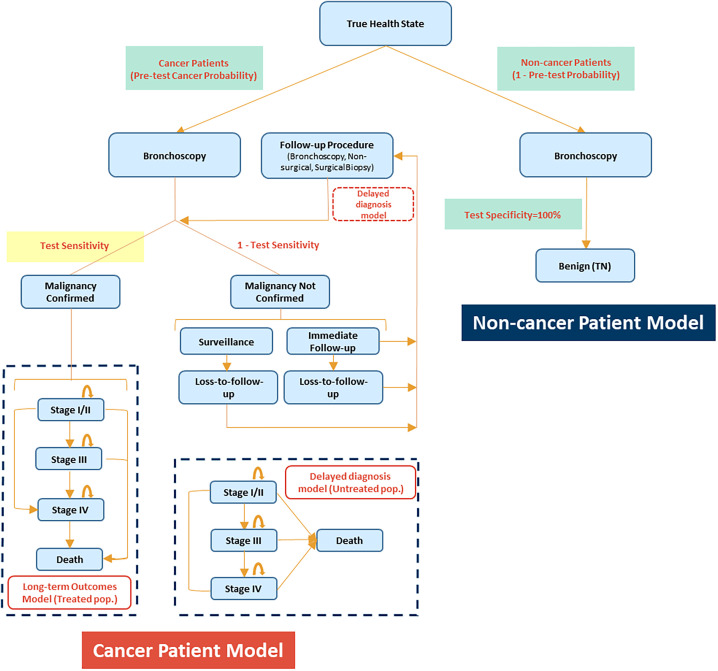
A decision-tree model of patients who undergo bronchoscopy as the initial procedure for lung cancer diagnosis (Figure 1) was developed. The target population for the model consisted of a hypothetical cohort of 1,000 patients with peripheral pulmonary lesions suspected of being malignant who required a biopsy and were deemed candidates for a bronchoscopic procedure following previous studies (14). The population excludes patients who received a rapid on-site diagnosis from lymph node sampling that precludes the need for a peripheral biopsy. The model population had a 67% prevalence of cancer and incorporated lesion factors such as size, location, and patient history, consistent with large-scale bronchoscopy studies (17, 18).
Figure 1.
Model structure.
The simulated model contained two submodels: one for patients with lesions that are not malignant (noncancer submodel) and another for patients whose lesions are malignant (cancer submodel). The intervention was a hypothetical increase of sensitivity for malignancy of 10%, hereinafter referred to as the high-sensitivity group. Baseline sensitivity for malignancy was assumed to be 70%, hereinafter referred to as the low-sensitivity group. This model did not consider changes in bronchoscopy cost as sensitivity increases, because the aim of this study was to quantify the economic value of improved sensitivity for malignancy, regardless of any specific bronchoscopy technology. As such, the utility of this model is to support the evaluation of future bronchoscopy technologies. The final model was conducted in Microsoft Excel 365 and followed Consolidated Health Economic Evaluation Reporting Standards guidelines (19).
At index, bronchoscopy results were either malignant or nonmalignant on the basis of test sensitivity. Patients diagnosed with malignancy (true positive) in the model had their disease progress based on a first-order Markov chain with a 1-month cycle length, which is defined by three severity stages of lung cancer—stage I/II (localized), stage III (regional), and stage IV (distant)—and death and the transition probabilities between stages, hereafter referred to as the long-term outcomes model.
Patients with nonmalignant histopathologic results were classified as either true negative and exited the model or false negative and entered the delayed-diagnosis model. The model assumes that improvements in sensitivity for malignancy did not affect detection of other (i.e., nonmalignant) diseases, thus keeping costs and outcomes for true-negative lung cancer patients unchanged. Patients with a false-negative result (undiagnosed) were either sent to immediate follow-up biopsy or surveillance until diagnosis. Specificity was assumed to be 100%. Undiagnosed patients with lung cancer remained untreated, and their lung cancer progressed until diagnosed through a follow-up biopsy, hereafter referred to as the delayed-diagnosis model. Delays in follow-up biopsy within the immediate follow-up, surveillance, and lost-to-follow-up arms were 1, 6, and 24 months, respectively, from index bronchoscopy. Subsequent procedures included both nonsurgical (bronchoscopy or transthoracic needle aspiration) and surgical biopsies (video-assisted thoracic surgery or thoracotomy; see Table E1 in the data supplement) (20, 21).
Outcomes were estimated from a U.S. payer perspective over 30 years. Clinical outcomes assessed included 1) time until a biopsy correctly confirms malignancy (i.e., time to diagnosis) and 2) distribution of cancer stage at diagnosis and patient survival (i.e., life-years gained). Health gains were quantified on the basis of quality-adjusted life-years (QALYs) and monetized assuming a willingness to pay (WTP) of $100,000 per QALY (22). Costs included initial bronchoscopy procedure (assumed to be equal between both sensitivity groups), follow-up diagnostic procedures, related adverse events, cancer treatment costs, and all other lifetime medical costs. Treatment value was assessed using net monetary benefit (NMB) and incremental cost-effectiveness ratio (ICER). NMB is a summary statistic that measures the value of an intervention in monetary terms at a specified WTP threshold for a unit of benefit (e.g., QALY). More precisely, NMB is defined as the incremental health benefit times the WTP, net of incremental costs. NMB measures the difference between alternative interventions, so a positive NMB indicates that an intervention is cost-effective compared with the alternative at the given WTP threshold, whereas a negative NMB indicates that costs exceed benefits. Conceptually, ICER is similar to NMB, but it is a ratio rather than additive. The discount rate used was 3% (23).
Stage-specific NMB was determined by calculating NMB when 100% of the population had stage I/II, III, or IV lung cancer. With this information, one can calculate the NMB of increased sensitivity in other populations using the following equation: (Economic value stage I/II × Cancer prevalence × % stage I/II) + (Economic value stage III × Cancer prevalence × % stage III) + (Economic value stage IV × Cancer prevalence × % stage IV).
Model Inputs
Model inputs were composed of 1) clinical inputs including the efficacy, safety, and use of procedures and treatments; 2) transition probabilities for long-term and delayed-diagnosis models; 3) utility inputs for health-related quality-of-life measurements; and 4) financial inputs, including costs of procedures and treatments (Tables 1 and E1).
Table 1.
Table of model inputs
| Category | Parameter | Value | Source |
|---|---|---|---|
| Clinical input | Pretest prevalence of lung cancer | 67% | Folch et al. (25) |
| Test sensitivity | |||
| Initial biopsy | |||
| Baseline (low sensitivity) | 70% | Assumption | |
| Intervention (high sensitivity) | 80% | Assumption | |
| Follow-up biopsy | |||
| Bronchoscopy | |||
| Baseline | 70% | Assumption | |
| Intervention | 80% | Assumption | |
| TTNA | 90% | DiBardino et al. (5) | |
| Surgical biopsy | 100% | Feller-Kopman et al. (21) | |
| Noncancer disease | 100% | Assumption | |
| Biopsy use | |||
| First biopsy | |||
| Bronchoscopy | 100% | Assumption | |
| TTNA | 0% | Assumption | |
| Follow-up biopsy | |||
| Bronchoscopy | 25% | Zhang et al. (20) | |
| TTNA | 43% | Zhang et al. (20) | |
| Surgery | 32% | Zhang et al. (20) | |
| Thoracotomy | 58% | Feller-Kopman et al. (21) | |
| VATS | 42% | Feller-Kopman et al. (21) | |
| Distribution of follow-up conditional on false negatives | |||
| Surveillance (benign) | 50% | Assumption | |
| Loss to follow-up conditional on surveillance (benign) | 10% | Assumption | |
| Immediate follow-up (nondiagnostic) | 50% | Assumption | |
| Loss to follow-up conditional on immediate follow-up (nondiagnostic) | 10% | Assumption | |
| Distribution of lung cancer stages at diagnosis | |||
| Stage I/II | 0.6527 | Folch et al. (25) | |
| Stage III | 0.1702 | Folch et al. (25) | |
| Stage IV | 0.1772 | Folch et al. (25) | |
| Monthly survival rates | |||
| 1–12 mo | |||
| Stage I/II | 0.889 | Author’s calculations based on NIH SEER (2019) | |
| Stage III | 0.771 | Author’s calculations based on NIH SEER (2019) | |
| Stage IV | 0.381 | Author’s calculations based on NIH SEER (2019) | |
| 13–36 mo | |||
| Stage I/II | 0.952 | Author’s calculations based on NIH SEER (2019) | |
| Stage III | 0.816 | Author’s calculations based on NIH SEER (2019) | |
| Stage IV | 0.660 | Author’s calculations based on NIH SEER (2019) | |
| 37–60 mo | |||
| Stage I/II | 0.952 | Author’s calculations based on NIH SEER (2019) | |
| Stage III | 0.905 | Author’s calculations based on NIH SEER (2019) | |
| Stage IV | 0.829 | Author’s calculations based on NIH SEER (2019) | |
| 61–360 mo | |||
| Stage I/II | 0.964 | Author’s calculations based on NIH SEER (2019) | |
| Stage III | 0.937 | Author’s calculations based on NIH SEER (2019) | |
| Stage IV | 0.907 | Author’s calculations based on NIH SEER (2019) | |
| Monthly transition probability (delay-period model) | |||
| Stage I/II to stage III | 5.08% | Hofer et al. (27) | |
| Stage I/II to stage IV | 3.74% | Hofer et al. (27) | |
| Stage I/II to stage death | 4.86% | Hofer et al. (27) | |
| Stage III to stage IV | 5.11% | Hofer et al. (27) | |
| Stage III to death | 5.37% | Hofer et al. (27) | |
| Stage IV to death | 11.12% | Hofer et al. (27) | |
| Utility | Quality of life without cancer | 0.867 | Szende et al. (30) |
| Baseline quality of life by lung cancer stage | |||
| Stage I/II | 0.825 | Sturza (29) | |
| Stage III | 0.772 | Sturza (29) | |
| Stage IV | 0.573 | Sturza (29) | |
| Disutility by indeterminate test results | −0.0033 | Toumazis et al. (37) | |
| Cost | Biopsy (including adverse event costs) | ||
| Bronchoscopy | $6,684 | Calculated using Chiu et al. (24) | |
| TTNA | $2,756 | Calculated using Chiu et al. (24) | |
| Surgery | $32,247 | Calculated using Chiu et al. (24) | |
| Physician visits (including screening counseling) | $173 | CMS, ACR | |
| Monthly treatment cost for patients with lung cancer | |||
| Long-term outcomes model (diagnosed patients) | |||
| Stage I/II | $1,771 | Calculated using Sheehan et al. (40) | |
| Stage III | $3,740 | Calculated using Sheehan et al. (40) | |
| Stage IV | $5,880 | Calculated using Sheehan et al. (40) | |
| Delayed-period model (undiagnosed patients) | |||
| Stage I/II | $896 | Calculated using Sheehan et al. (40) | |
| Stage III | $630 | Calculated using Sheehan et al. (40) | |
| Stage IV | $760 | Calculated using Sheehan et al. (40) |
Definition of abbreviations: NIH SEER = National Institute of Health Surveillance, Epidemiology, and End Results Program; TTNA = transthoracic needle aspiration; VATS = video-assisted thoracoscopic surgery.
Clinical Inputs
The test sensitivities for follow-up bronchoscopies were 70% and 80% for the baseline (low sensitivity) and intervention (high sensitivity) groups, respectively; test sensitivities for follow-up transthoracic needle aspiration and surgical biopsies were 90% and 100%, respectively (21). Among those requiring a follow-up biopsy after bronchoscopy, the model assumed 25.0% received another bronchoscopy, 43.0% transthoracic needle aspiration, 18.4% thoracotomy, and 13.6% video-assisted thoracic surgery (20, 21). Diagnostic procedure safety was identified by reported occurrence rates of adverse events (i.e., pneumothorax, hemorrhage, and mechanical ventilation for nonsurgical procedures and pneumonia and empyema additionally for surgical procedures) (24).
Transition Probabilities
In the long-term outcomes model, the distribution for the stage at diagnosis was parameterized from the largest prospective study on bronchoscopy (25). For this model, we used the 1-, 3-, 5-, and 10-year lung cancer survival rates for localized disease (stage I/II), regional (stage III), and distant (for stage IV) from 2022 Surveillance, Epidemiology, and End Results Program data (26). Assuming that transitioning to later stage or death followed a Poisson process for each stage, we analytically derived survival for 1–12, 13–36, 37–60, and 61–360 months on the basis of 1-, 3-, 5-, and 10-year survival rates, respectively. A numerical approach was used to derive transition probabilities from a lower cancer stage to a higher stage or all-cause mortality. Survival functions of patients in stages I/II, III, and IV are shown in Figure E1.
For the delayed-diagnosis model, monthly transition probabilities of the undiagnosed/untreated patients with lung cancer were derived from literature that calibrated the quarterly hazard rate of natural progression for untreated lung cancer, assuming exponential survival (27). These transition probabilities approximate the accelerated progression of more advanced stages of lung cancer from lack of treatment. To validate the accuracy of progression and mortality rates for the delay period, we compared the model’s estimated effects of early diagnosis on mortality with the efficacy estimates of low-dose CT screening on the long-term mortality rate of lung cancer from the NLST (National Lung Screening Trial) (Appendix E1) (28).
Utility Inputs for Health-related Quality-of-Life Measurements
Health-related quality-of-life estimates were measured using two components: quality of life by cancer stage and adverse events. Quality-of-life estimates by cancer stage were drawn from a meta-analysis of lung cancer utility (29, 30). Additive impacts from adverse events were based on disutilities of empyema, hemorrhage, mechanical ventilation, pneumonia, and pneumothorax (15, 24, 29, 31–37).
Financial Inputs
Healthcare costs were assumed to be impacted by changes in sensitivity for malignancy through two pathways. The first comprised costs increased from additional biopsies and surveillance needed when the initial biopsy did not confirm malignancy, which included costs for CT scans, physician office visits (38, 39), biopsy, and related adverse events (24). Biopsy-related and adverse event costs were identified by calculating the weighted average of median costs for each biopsy modality between inpatient and outpatient settings (24). The second comprised costs increased resulting from delayed diagnosis: If cancer diagnosis was delayed, patients were more likely to be diagnosed at a later stage of lung cancer when cancer treatment costs are higher. Monthly medical costs of treated patients at each stage were derived using lung cancer stage-specific time to death calculated from Surveillance, Epidemiology, and End Results data and total medical cost of patients with lung cancer measured by stage at diagnosis (26, 40). Monthly medical costs of undiagnosed/untreated patients were calculated by total medical costs of patients with lung cancer net of lung cancer treatment costs (40). Costs were inflated to 2022 U.S. dollars (41).
Sensitivity and Scenario Analyses
One-way sensitivity analysis (i.e., adjusted model parameters by ±10%) was performed to evaluate model robustness. Scenario analyses were conducted to consider the interaction between sensitivity for malignancy with follow-up time, different distribution of immediate follow-up, surveillance, loss to follow-up, and changes in surgery use for any follow-up biopsies. This study also calculated the economic impacts at WTP thresholds of $50,000, $100,000 (baseline), $150,000, and $200,000 per QALY (22). Last, we conducted a societal perspective analysis based on nonmedical out of cost, caregiver burden, and productivity loss (Table E6). Two-way sensitivity analyses were developed on the three most impactful parameters from the one-way sensitivity analysis (Appendix E2).
Results
Base Case Analysis Results
Increasing sensitivity for malignancy of bronchoscopy from 70% to 80% reduced time to diagnosis by 0.85 months (4 wk) compared with the low-sensitivity group (1.61 vs. 2.46 mo or 7 vs. 11 wk; Table 2). This resulted in a higher proportion of patients diagnosed at an earlier cancer stage for the high-sensitivity group relative to the low-sensitivity group (62.96% vs. 61.68% in stages I/II, 18.08% vs. 18.68% in stage III, and 18.95% vs. 19.65% in stage IV) (Table 2).
Table 2.
Base case model results
| Model Output | Low-Sensitivity Group | High-Sensitivity Group |
|---|---|---|
| Time to diagnosis, mo | 2.47 | 1.61 |
| Distribution of cancer stage at diagnosis | ||
| Stage I/II | 61.68% | 62.96% |
| Stage III | 18.68% | 18.08% |
| Stage IV | 19.65% | 18.95% |
| Health outcomes among lung cancer patients | ||
| Years that a patient receives treatment | 10.42 | 10.82 |
| Years that a patient remained untreated | 0.11 | 0.07 |
| Life-years | 10.53 | 10.89 |
| Number of biopsies per patient with cancer | 1.24 | 1.16 |
| QALYs per lung cancer patients | ||
| QALYs accrued after lung cancer diagnosis | 5.72 | 5.94 |
| QALYs before lung cancer diagnosis | 0.08 | 0.05 |
| Biopsy-associated QALYs | −0.0022 | −0.0018 |
| Total | 5.79 | 5.99 |
| Costs per patient with lung cancer | ||
| Medical costs accrued after lung cancer diagnosis | $274,299 | $282,505 |
| Biopsy-associated costs | $9,878 | $8,765 |
| Medical cost before lung cancer | $1,060 | $694 |
| Total | $285,237 | $291,964 |
| Net monetary benefit | $8,729 | |
| Incremental cost-effectiveness ratio | $34,052 | |
Definition of abbreviation: QALY = quality-adjusted life-year.
Because of earlier diagnosis and treatment, patients in the high-sensitivity group had higher rates of lung cancer diagnosis and reduced mortality relative to those in the low-sensitivity group (Figure E2). Life-year gains of the high-sensitivity group were 0.36 year (10.89 vs. 10.53 for the high- and low-sensitivity groups, respectively), or 19 weeks (Table 2). Patients with cancer in the high-sensitivity group also spent more time in treatment than those in the low-sensitivity group: 0.40 year (10.82 vs. 10.42), or 20 weeks. Moreover, the high-sensitivity group spent 0.04 fewer years (0.07 vs. 0.11, or 2 wk) remaining untreated than the low-sensitivity group. A reduction of approximately one unnecessary diagnostic procedure per 12 patients was achieved (1.16 vs. 1.24 procedures per patient).
When considering survival, quality of life, and adverse events, higher sensitivity for malignancy improved health outcomes. Specifically, total discounted QALYs of patients with cancer were higher in the high-sensitivity group (5.99) than in the low-sensitivity group (5.79) by 0.20 QALYs (Table 2). The largest health gains were due to an increased share of patients receiving cancer treatment earlier (5.94 vs. 5.72; difference = 0.22). A negligible proportion of the health improvements was explained by a reduced number of biopsy-related adverse events: <0.01 increase in QALY. Conversely, the high-sensitivity group obtained 0.03 lower QALYs than the low-sensitivity group before cancer diagnosis (0.08 vs. 0.05). The discounted QALY gains when including all patients with and without cancer was 0.13 QALYs (4.01 vs. 3.88).
Although earlier diagnosis of cancer from increased sensitivity for malignancy decreased average monthly cost among patients with cancer ($2,235 vs. $2,257 for the high- and low-sensitivity groups, respectively), total lifetime costs were higher in the high-sensitivity group than in the low-sensitivity group by $6,727 ($291,964 vs. $285,237) because patients lived longer and received treatment longer (Table 2). Increased cost due to longer duration of lung cancer treatment—largely due to increased life expectancy—explained more than 90% of the total costs for both groups ($282,505 vs. $274,299; difference of $8,206). These costs were partly offset by reduced biopsy costs ($8,765 vs. $9,878; difference of −$1,113) and reduced medical costs for undiagnosed patients with lung cancer ($694 vs. $1,060; difference of −$366). For all patients who did and did not have cancer, total discounted costs were $4,507 higher for the high-sensitivity group than for the low-sensitivity group ($195,616 vs. $191,109).
Increasing test sensitivity for malignancy from 70% to 80% generated a positive economic value, NMB of $8,729, corresponding to the ICER of $34,052 per QALY (Tables 2 and E2). About 40% of the value (NMB of $3,668) was realized within 5 years of the initial bronchoscopy (Figure E3). Increasing test sensitivity from 70% to 71% led to an NMB of $885, or approximately one-tenth of increasing sensitivity for malignancy by 10% (Tables E2 and E3).
The stage-specific NMB values of increasing test sensitivity from 70% to 80% when 100% of patients had stage I/II, III, or IV disease were $19,805, $2,101, and −$1,438, respectively (Table 3). The overall NMB of the base case model, $8,729, was obtained by taking a weighted average of the stage-specific NMBs using the prevalence (67%) and cancer stage distribution as weights (i.e., [$19,805 × 67% × 65.27%] + [$2,101 × 67% × 17.02%] + [−$1,438 × 67% × 17.72%]). The NMB for any population can be calculated, provided that the prevalence of cancer and the stage distribution is known, using the stage-specific NMB as shown.
Table 3.
Stage-specific economic value when all patients have malignancy and all are at same stage
| All Patients Begin in Stage | Stage-Specific NMB (A) | Prevalence of Lung Cancer (B) | Stage-Specific NMB at Given Prevalence (C = A × B) | Lung Cancer Distribution (D) | NMB of Base Case Population (A) × (B) × (D) |
|---|---|---|---|---|---|
| I/II | $19,805 | 67% | $13,269 | 65.27% | $8,729* |
| III | $2,101 | 67% | $1,408 | 17.02% | |
| IV | −$1,438 | 67% | −$963 | 17.72% |
Definition of abbreviation: NMB = net monetary benefit.
For a population with a prevalence of cancer (B) and a stage distribution (D), we can calculate the NMB as shown. The stage-specific NMB (A) is the NMB if all patients have the stage of disease shown on the left. If the prevalence of cancer was 67%, as shown in (B), but that was the only stage of cancer present, then the NMB is shown in column (C). In column (D) is the stage distribution used in the base case scenario. Multiplying column (A) × (B) × (D) for each stage and summing the three different stages provides the NMB for the entire population. In the base case that is shown above, that is $8,729. The stage-specific NMB can be used to calculate the NMB for any population, provided the prevalence of cancer is known and the stage distribution of the cancers is specified.
Cross product of columns (C) and (D) do not match NMB exactly because of rounding. Column (D) does not add up to 100% exactly because of rounding.
Sensitivity and Scenario Analysis Results
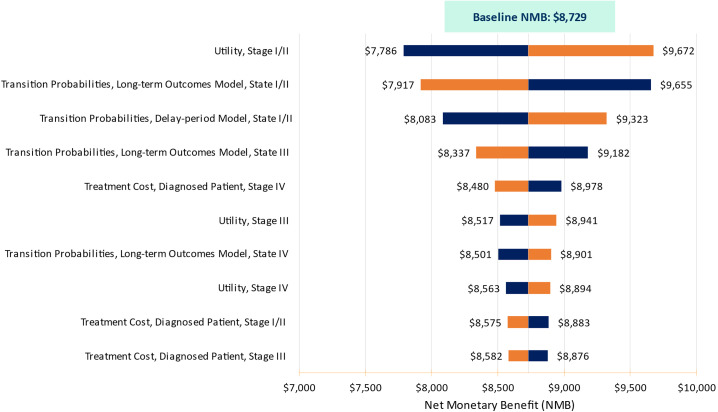
Our results were robust through a variety of sensitivity and scenario analyses. The economic value results were most sensitive to variations in the utility of stage I/II lung cancer (NMB of [$7,786, $9,672]), the stage I/II transition probabilities of the long-term outcomes model ([$7,917, $9,655]), and those of the delay-period model ([$8,083, $9,323]) (Figure 2).
Figure 2.
Sensitivity analysis tornado diagram. NMB = net monetary benefit.
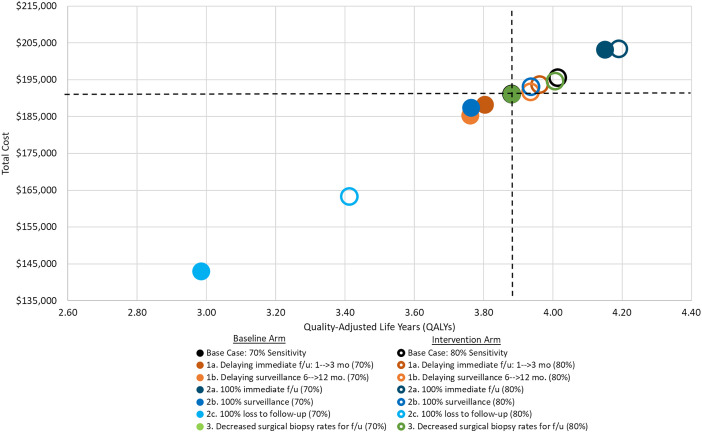
Among the three scenario types, longer delays in follow-up made the value of improved sensitivity for malignancy higher (Table E4 and Figure 3). Delaying immediate follow-up from 1 to 3 months or surveillance from 6 to 12 months increased the value of higher sensitivity for malignancy (NMB of $10,288 and $10,717 for scenarios 1a and 1b). Higher sensitivity was particularly valuable when patients were likely to be lost to follow-up after a false-negative index bronchoscopy (scenario 2c, $22,242) but less valuable when all patients had an immediate follow-up biopsy within 1 month (scenario 2a, $3,635).
Figure 3.
Total cost and quality-adjusted life-years (QALYs) for each scenario. The intersecting dashed black lines outline the cost-effectiveness quadrants relative to the base case scenario with 70% sensitivity. Points in the upper left quadrant relative to this are more costly and less effective. Points in the lower left quadrant are less costly but less effective. Points in the lower right quadrant are less costly and more effective. Points in the upper right quadrant are more costly but also more effective. Relative to the base case scenario with 70% sensitivity, all scenarios in this analysis fall into either the upper right quadrant (more costly but more effective) or in the lower left quadrant (less costly but less effective). Overall, the single best scenario in terms of QALYs is scenario 2a, with 100% immediate follow-up and 80% sensitivity (darker blue hollow dot). Note that compared with the base cases scenario, most of the improvement in QALYs is due to the 100% immediate follow-up. If sensitivity remains at 70% but we change to 100% immediate follow-up, QALYs improve from 3.88 per patient screened in the base case to 4.15, whereas costs increase from $191,109 to $203,060 (incremental cost-effectiveness ratio = $44,359).
Increased sensitivity for malignancy continued to be a cost-effective solution, even after adjusting the WTP threshold. The 30-year NMB values when the WTP threshold was changed to $50,000, $150,000, and $200,000 per QALY were $2,111, $15,347, and $21,965, respectively (Table E5). The base case model showed that increased sensitivity continues to provide economic benefit at the societal perspective (NMB = $9,344; Table E6) (42, 43).
Discussion
This study quantified the economic value of increasing sensitivity for malignancy for bronchoscopy biopsy procedures in diagnosing lung cancer. The model estimated that patients with lung cancer benefit from increases in sensitivity for malignancy by reducing time to treatment by 0.85 month (4 wk) and increasing the proportion of patients diagnosed at stage I/II by 1.28%. Increasing sensitivity for malignancy improved health outcomes (5.99 vs. 5.79 QALYs for the high- and low-sensitivity groups, respectively) but increased cost ($291,964 vs. $285,237) in large part due to patients with lung cancer being diagnosed and treated more rapidly and living longer. As a result, when sensitivity for malignancy increased by 10 percentage points, the NMB was $8,729, assuming a WTP of $100,000 per QALY, and the ICER was $34,052. In other words, the value of health benefits from a 10–percentage point increase in sensitivity for malignancy was $8,729 higher per patient than the costs.
Although this study estimated the economic value of increased sensitivity of malignancy using the distribution of cancer stage from the NAVIGATE (Clinical Evaluation of superDimension Navigation System for Electromagnetic Navigation Bronchoscopy) trial, the stage-specific NMB results from this study can be used to calculate the NMB of increased sensitivity in other populations, provided the prevalence and stage distribution of lung cancer is known (Table 3). Appendix E3 provides sample calculations using the NLST study population (28).
The scenario analysis revealed that the value of improved sensitivity for malignancy varies, depending on patient follow-up protocols. When physician discretion, budget limitations, or social determinants of health lead to less frequent follow-up or cause patients not to return for follow-up visits (20), the value of improved sensitivity for malignancy for lung cancer diagnosis increases. Conversely, the value of improved sensitivity for malignancy declines with more rapid follow-up, because false-negative or nondiagnostic results can be identified more rapidly. Pairing advanced diagnostic technologies with prompt outreach for follow-up visits would improve health and economic outcomes.
Limitations
This study has several limitations. Although the model aimed to quantify the benefit of increased sensitivity for malignancy in bronchoscopy for patients with peripheral lesions, if applied to a similar population that received endobronchial ultrasound without rapid on-site evaluation (38% among patients with lung cancer undergoing endobronchial ultrasound) (44), the model would overestimate the NMB, because some patients receiving a nondiagnostic peripheral biopsy would be diagnosed by lymph node biopsy, and no treatment delay would ensue. Second, the model assumed immediate treatment upon diagnosis, whereas in practice, there may be delays between diagnosis and treatment (45). Third, the model assumes the population is similar to the NAVIGATE population and that increased sensitivity for malignancy did not change stage distribution or prevalence among those who underwent a bronchoscopy. This might not be true in the future. However, using stage-specific NMBs calculated in Table 3 can address this, provided that prevalence and stage distribution are accurately specified. Fourth, the model does not consider potential correlations between cancer stage and the probability of immediate follow-up biopsies versus surveillance after a negative test result. For instance, negative results would be suspicious to physicians among patients with larger lesions; surveillance would only be likely for small lesions (e.g., <1 cm) more likely to be stage I if malignant. The model assumes surveillance would be equally likely in patients with across stages. Moreover, loss to follow-up was assumed to be equally likely among groups, but it is probable that patients who are asymptomatic with earlier stage disease would be much more likely to be lost to follow-up than patients who are symptomatic with stage III/IV disease. Fifth, we do not account for benefits from new therapies. Although external validation was performed comparing the proportional effect with the effect observed in the NLST study (Appendix E1) (28), in practice, the benefits of improved sensitivity for malignancy may vary because new immunotherapy and other agents have improved lung cancer survival in recent years (46). Sixth, this model does not account for overdiagnosis of lung cancer. If there is more overdiagnosis due to lung cancer screening, then the impact of improved sensitivity would be mitigated for those patients (47). A specificity of 100% was also assumed due to bronchoscopy having nearly 100% specificity and previously published models assumed 100% specificity (17, 21, 48, 49). Next, because of the limited evidence from the literature, follow-up time intervals were estimated from clinical experience of three pulmonologists from different U.S. medical institutions. Moreover, this study does not explicitly consider lesion size. However, lesion size is implicitly addressed through cancer stage because lesion size and stage are closely associated with each other. Last, this study does not discuss the economic benefit of improving accuracy in nonmalignant diseases; in practice, however, more advanced bronchoscopy technologies that improve sensitivity for malignancy are also likely to improve diagnosis of certain other diseases, and thus this model likely provides a conservative estimate of economic value.
Conclusions
In summary, increasing sensitivity for malignancy resulted in meaningful health economic benefits. Specifically, $8,729 of health economic value is generated when increasing sensitivity for malignancy from 70% to 80%. Improved sensitivity for malignancy through advanced bronchoscopy technologies coupled with interventions to stratify the risk of patients with nondiagnostic bronchoscopy results and expedite follow-up biopsy time can maximize patient outcomes and generate economic value for payers and health systems.
Supplemental Materials
Footnotes
Supported by Johnson & Johnson. The views expressed in this article do not communicate an official position of FTI Consulting, Johnson & Johnson, the University of Texas MD Anderson Cancer Center, or the University of Pennsylvania.
Author Contributions: D.E.O., F.M., J.S., I.K., T.B.A., D.S.H., and A.V. conceptualized the topic of this article. J.K., M.A.M., and J.S. built the economic model. J.K. and M.A.M. conducted the data analysis to parameterize the model. D.E.O., F.M., T.A., D.S.H., and A.V. provided oversight on the model parameters, quality assurance, and model external validity.
This article has a data supplement, which is accessible at the Supplements tab.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Gould MK, Tang T, Liu I-LA, Lee J, Zheng C, Danforth KN, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med . 2015;192:1208–1214. doi: 10.1164/rccm.201505-0990OC. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Preventive Services Task Force. Lung cancer: screening. 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
- 3.U.S. Preventive Services Task Force. Lung cancer: screening. 2013. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening-december-2013
- 4. Ritzwoller DP, Meza R, Carroll NM, Blum-Barnett E, Burnett-Hartman AN, Greenlee RT, et al. Evaluation of population-level changes associated with the 2021 US Preventive Services Task Force lung cancer screening recommendations in community-based health care systems. JAMA Netw Open . 2021;4:e2128176. doi: 10.1001/jamanetworkopen.2021.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis . 2015;7(Suppl 4):S304–S316. doi: 10.3978/j.issn.2072-1439.2015.12.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vachani A, Zhou M, Ghosh S, Zhang S, Szapary P, Gaurav D, et al. Complications after transthoracic needle biopsy of pulmonary nodules: a population-level retrospective cohort analysis. J Am Coll Radiol . 2022;19:1121–1129. doi: 10.1016/j.jacr.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 7. Ost D, Shah R, Anasco E, Lusardi L, Doyle J, Austin C, et al. A randomized trial of CT fluoroscopic-guided bronchoscopy vs conventional bronchoscopy in patients with suspected lung cancer. Chest . 2008;134:507–513. doi: 10.1378/chest.08-0160. [DOI] [PubMed] [Google Scholar]
- 8. Goto T, Hirotsu Y, Nakagomi T, Shikata D, Yokoyama Y, Amemiya K, et al. Detection of tumor-derived DNA dispersed in the airway improves the diagnostic accuracy of bronchoscopy for lung cancer. Oncotarget . 2017;8:79404–79413. doi: 10.18632/oncotarget.18159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kent AJ, Byrnes KA, Chang SH. State of the art: robotic bronchoscopy. Semin Thorac Cardiovasc Surg . 2020;32:1030–1035. doi: 10.1053/j.semtcvs.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 10. Lu M, Nath S, Semaan RW. A review of robotic-assisted bronchoscopy platforms in the sampling of peripheral pulmonary lesions. J Clin Med . 2021;10:5678. doi: 10.3390/jcm10235678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yu Lee‐Mateus A, Reisenauer J, Garcia‐Saucedo JC, Abia‐Trujillo D, Buckarma EH, Edell ES, et al. Robotic‐assisted bronchoscopy versus CT‐guided transthoracic biopsy for diagnosis of pulmonary nodules. Respirology . 2023;28:66–73. doi: 10.1111/resp.14368. [DOI] [PubMed] [Google Scholar]
- 12. Kalchiem-Dekel O, Connolly JG, Lin I-H, Husta BC, Adusumilli PS, Beattie JA, et al. Shape-sensing robotic-assisted bronchoscopy in the diagnosis of pulmonary parenchymal lesions. Chest . 2022;161:572–582. doi: 10.1016/j.chest.2021.07.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pyarali FF, Hakami-Majd N, Sabbahi W, Chaux G. Robotic-assisted navigation bronchoscopy: a meta-analysis of diagnostic yield and complications. J Bronchology Interv Pulmonol . 2023;31:70–81. doi: 10.1097/LBR.0000000000000942. [DOI] [PubMed] [Google Scholar]
- 14. Vakil E, Jackson N, Sainz-Zuñega PV, Molina S, Martinez-Zayas G, Cantor SB, et al. Optimizing diagnostic and staging pathways for suspected lung cancer: a decision analysis. Chest . 2021;160:2304–2323. doi: 10.1016/j.chest.2021.06.065. [DOI] [PubMed] [Google Scholar]
- 15. Rickets W, Lau KKW, Pollit V, Mealing S, Leonard C, Mallender P, et al. Exploratory cost-effectiveness model of electromagnetic navigation bronchoscopy (ENB) compared with CT-guided biopsy (TTNA) for diagnosis of malignant indeterminate peripheral pulmonary nodules. BMJ Open Respir Res . 2020;7:e000595. doi: 10.1136/bmjresp-2020-000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kops SE, Verhoeven RL, Vermeulen RJ, Rovers MM, van der Heijden EH, Govers TM. Cone beam CT-guided navigation bronchoscopy: a cost-effective alternative to CT-guided transthoracic biopsy for diagnosis of peripheral pulmonary nodules. BMJ Open Resp Res . 2022;9:e001280. [Google Scholar]
- 17. Folch E, Bowling M, Nead M, Pritchett M, LeMense G, Gildea T, et al. Diagnostic yield of electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study [abstract] Am J Respir Crit Care Med . 2018;197:A7784. [Google Scholar]
- 18. Gex G, Pralong JA, Combescure C, Seijo L, Rochat T, Soccal PM. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: a systematic review and meta-analysis. Respiration . 2014;87:165–176. doi: 10.1159/000355710. [DOI] [PubMed] [Google Scholar]
- 19. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. CHEERS 2022 ISPOR Good Research Practices Task Force Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care . 2022;38:e13. doi: 10.1017/S0266462321001732. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Simoff MJ, Ost D, Wagner OJ, Lavin J, Nauman B, et al. Understanding the patient journey to diagnosis of lung cancer. BMC Cancer . 2021;21:402–411. doi: 10.1186/s12885-021-08067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feller-Kopman D, Liu S, Geisler BP, DeCamp MM, Pietzsch JB. Cost-effectiveness of a bronchial genomic classifier for the diagnostic evaluation of lung cancer. J Thorac Oncol . 2017;12:1223–1232. doi: 10.1016/j.jtho.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 22.ICER. 2020-2023 value assessment framework. Boston, MA: Institute for Clinical and Economic Review (ICER); 2020. [Google Scholar]
- 23.Weinstein MC, Russell LB, Gold MR, Siegel JE. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 24. Chiu Y-W, Kao Y-H, Simoff MJ, Ost DE, Wagner O, Lavin J, et al. Costs of biopsy and complications in patients with lung cancer. Clinicoecon Outcomes Res . 2021;13:191–200. doi: 10.2147/CEOR.S295494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Folch EE, Pritchett MA, Nead MA, Bowling MR, Murgu SD, Krimsky WS, et al. NAVIGATE Study Investigators Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol . 2019;14:445–458. doi: 10.1016/j.jtho.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Welcome to the Cancer Statistics Explorer Network. 2024. https://seer.cancer.gov/statistics-network/
- 27. Hofer F, Kauczor H-U, Stargardt T. Cost-utility analysis of a potential lung cancer screening program for a high-risk population in Germany: a modelling approach. Lung Cancer . 2018;124:189–198. doi: 10.1016/j.lungcan.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 28. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med . 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sturza J. A review and meta-analysis of utility values for lung cancer. Med Decis Making . 2010;30:685–693. doi: 10.1177/0272989X10369004. [DOI] [PubMed] [Google Scholar]
- 30.Szende A, Janssen B, Cabases J. Self-reported population health: an international perspective based on EQ-5D. Dordrecht, the Netherlands: Springer; 2014. [PubMed] [Google Scholar]
- 31. Laursen LØ, Petersen RH, Hansen HJ, Jensen TK, Ravn J, Konge L. Video-assisted thoracoscopic surgery lobectomy for lung cancer is associated with a lower 30-day morbidity compared with lobectomy by thoracotomy. Eur J Cardiothorac Surg . 2016;49:870–875. doi: 10.1093/ejcts/ezv205. [DOI] [PubMed] [Google Scholar]
- 32. Agostini P, Lugg ST, Adams K, Vartsaba N, Kalkat MS, Rajesh PB, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: a propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomy. Interact Cardiovasc Thorac Surg . 2017;24:931–937. doi: 10.1093/icvts/ivx002. [DOI] [PubMed] [Google Scholar]
- 33.Society for Medical Decision Making. Development of new harm-based weighting approach to composite indicators: the Agency for Healthcare Research and Quality Patient Safety for Selected Indicators (AHRQ PSI-90) 2015. https://smdm.confex.com/smdm/2015mo/webprogram/Paper9360.html
- 34. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol . 2016;17:836–844. doi: 10.1016/S1470-2045(16)00173-X. [DOI] [PubMed] [Google Scholar]
- 35. Shipe ME, Maiga AW, Deppen SA, Haddad DN, Gillaspie EA, Maldonado F, et al. Cost-effectiveness analysis of fibrinolysis vs thoracoscopic decortication for early empyema. Ann Thorac Surg . 2021;112:1632–1638. doi: 10.1016/j.athoracsur.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simon MS, Sfeir MM, Calfee DP, Satlin MJ. Cost-effectiveness of ceftazidime-avibactam for treatment of carbapenem-resistant Enterobacteriaceae bacteremia and pneumonia. Antimicrob Agents Chemother . 2019;63:e00897-00819. doi: 10.1128/AAC.00897-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Toumazis I, Erdogan SA, Bastani M, Leung A, Plevritis SK. A cost-effectiveness analysis of lung cancer screening with low-dose computed tomography and a diagnostic biomarker. JNCI Cancer Spectr . 2021;5:pkab081. doi: 10.1093/jncics/pkab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Medicare and Medicaid Services (CMS) Baltimore, MD: CMS; 2021. Billing and coding: IDTFs and low dose CT scan for lung cancer screening for CPT code 71271.https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=58641#:~:text=71271%20%2D%20Computed%20tomography%2C%20thorax%2C,%2C%20without%20contrast%20material(s) [Google Scholar]
- 39.American College of Radiology. Reston, VA: American College of Radiology; Low-dose CT lung cancer screening FAQ.https://www.acr.org/Clinical-Resources/Lung-Cancer-Screening-Resources/FAQ [Google Scholar]
- 40. Sheehan DF, Criss SD, Chen Y, Eckel A, Palazzo L, Tramontano AC, et al. Lung cancer costs by treatment strategy and phase of care among patients enrolled in Medicare. Cancer Med . 2019;8:94–103. doi: 10.1002/cam4.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. Bureau of Labor Statistics. St. Louis, MO: Federal Reserve Bank of St. Louis; Consumer Price Index for all urban consumers: medical care services in US city average [CUSR0000SAM2]https://fred.stlouisfed.org/series/CUSR0000SAM2 [Google Scholar]
- 42. Van Houtven CH, Ramsey SD, Hornbrook MC, Atienza AA, Ryn M. Economic burden for informal caregivers of lung and colorectal cancer patients. Oncologist . 2010;15:883–893. doi: 10.1634/theoncologist.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Veenstra CM, Regenbogen SE, Hawley ST, Abrahamse P, Banerjee M, Morris AM. Association of paid sick leave with job retention and financial burden among working patients with colorectal cancer. JAMA . 2015;314:2688–2690. doi: 10.1001/jama.2015.12383. [DOI] [PubMed] [Google Scholar]
- 44. Zhao X, Boothe P, Naqvi SMH, Henderson-Jackson E, Mela N, Centeno BA, et al. Assessing ROSE for adequacy of EBUS-TBNA compared with a direct-to-cell block approach as a response to the COVID-19 pandemic. J Am Soc Cytopathol . 2022;11:368–374. doi: 10.1016/j.jasc.2022.07.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yorio JT, Xie Y, Yan J, Gerber DE. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol . 2009;4:1322–1330. doi: 10.1097/JTO.0b013e3181bbb130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Cancer Society. Cancer facts & figures 2022. Atlanta, GA: American Cancer Society; 2022. [Google Scholar]
- 47. Brodersen J, Schwartz LM, Heneghan C, O’Sullivan JW, Aronson JK, Woloshin S. Overdiagnosis: what it is and what it isn’t. BMJ Evid Based Med . 2018;23:1–3. doi: 10.1136/ebmed-2017-110886. [DOI] [PubMed] [Google Scholar]
- 48. Folch EE, Labarca G, Ospina-Delgado D, Kheir F, Majid A, Khandhar SJ, et al. Sensitivity and safety of electromagnetic navigation bronchoscopy for lung cancer diagnosis: systematic review and meta-analysis. Chest . 2020;158:1753–1769. doi: 10.1016/j.chest.2020.05.534. [DOI] [PubMed] [Google Scholar]
- 49. Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, et al. AEGIS Study Team A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med . 2015;373:243–251. doi: 10.1056/NEJMoa1504601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.