Abstract
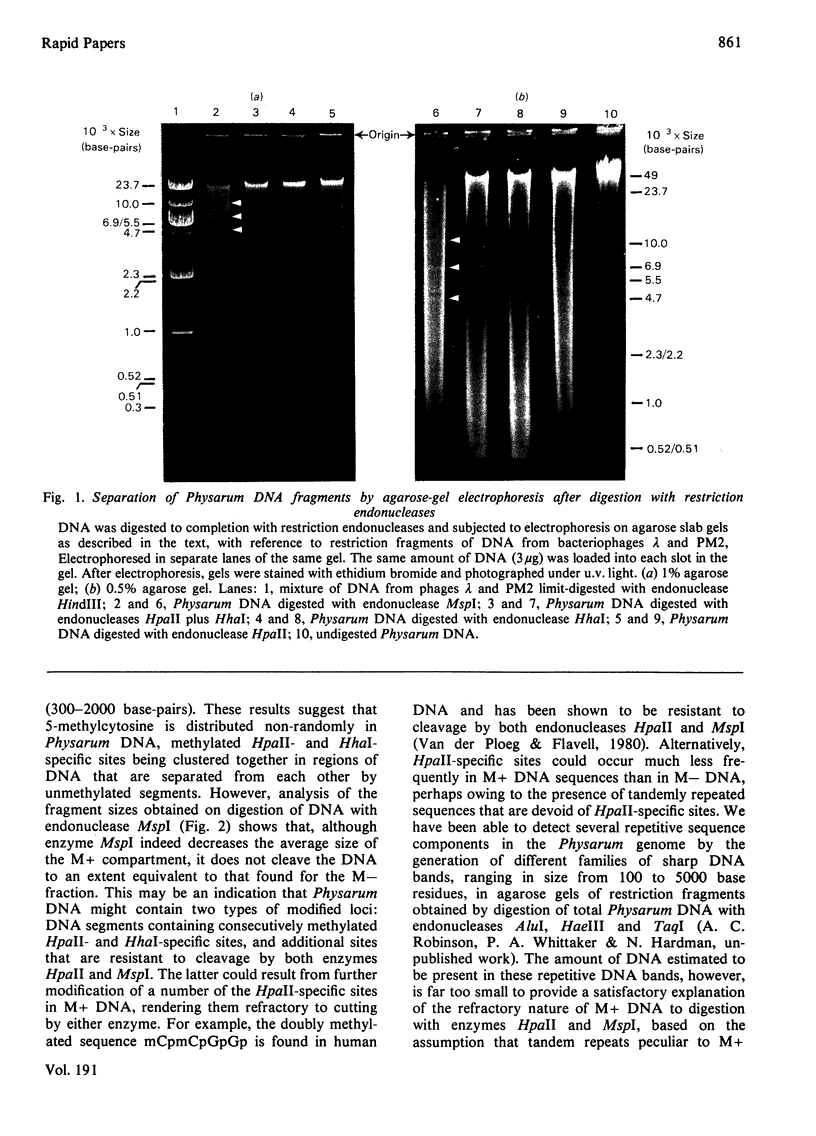
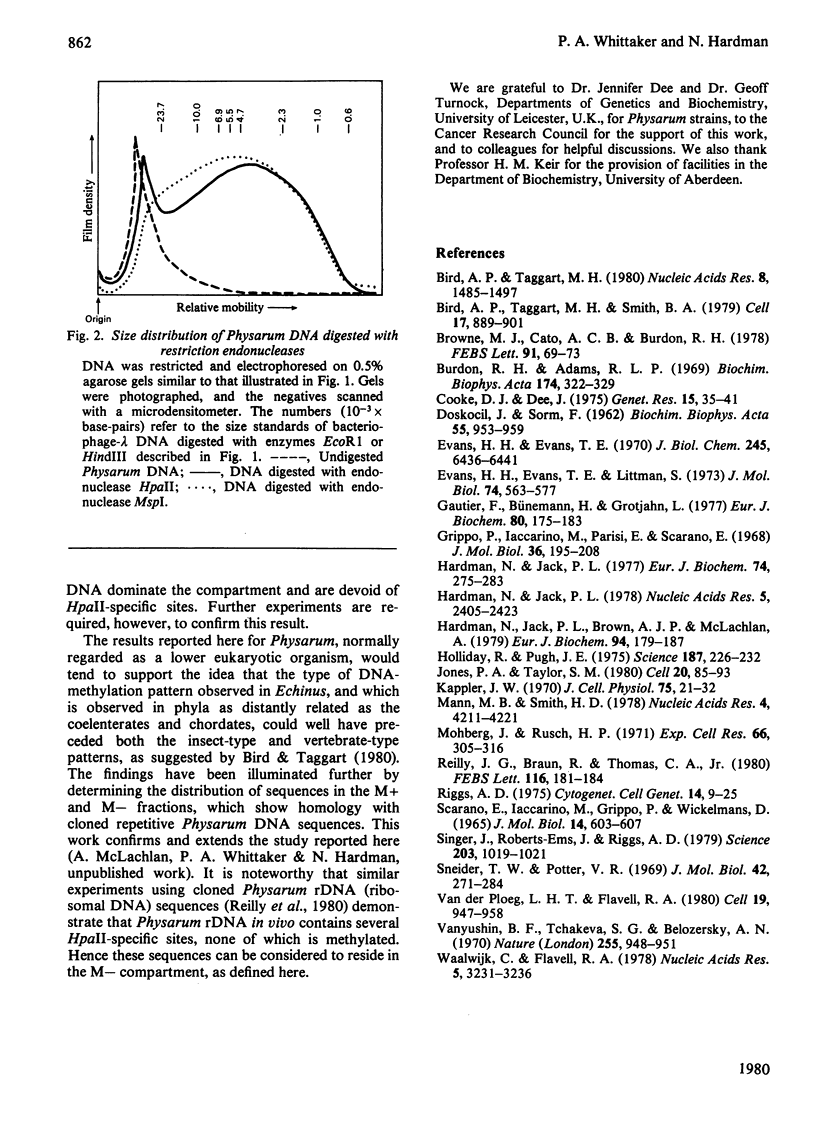
The restriction endonucleases HpaII and HhaI, whose action is inhibited by the presence of methylated base analogues at the recognition sequences in the DNA substrate, were used to investigate the distribution of 5-methylcytosine in nuclear DNA from Physarum polycephalum. Physarum DNA is digested into two fractions by these enzymes: a low-molecular-weight (M--) compartment comprising 80% of the DNA, and a high-molecular-weight (M+) compartment containing 20% of the DNA. The DNA fraction showing resistance to digestion by restriction endonuclease HpaII is cleaved by its isoschizomer MspI, indicating that methylated endonuclease-HpaII-specific sites are present in M + DNA. Additional properties of sequences in the M+ compartment were investigated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. P., Taggart M. H., Smith B. A. Methylated and unmethylated DNA compartments in the sea urchin genome. Cell. 1979 Aug;17(4):889–901. doi: 10.1016/0092-8674(79)90329-5. [DOI] [PubMed] [Google Scholar]
- Bird A. P., Taggart M. H. Variable patterns of total DNA and rDNA methylation in animals. Nucleic Acids Res. 1980 Apr 11;8(7):1485–1497. doi: 10.1093/nar/8.7.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne M. J., Cato A. C., Burdon R. H. The distribution of modified and non-modified C-G doublets in BHK-21 cell DNA. FEBS Lett. 1978 Jul 1;91(1):69–73. doi: 10.1016/0014-5793(78)80019-2. [DOI] [PubMed] [Google Scholar]
- Burdon R. H., Adams R. L. The in vivo methylation of DNA in mouse fibroblasts. Biochim Biophys Acta. 1969 Jan 21;174(1):322–329. doi: 10.1016/0005-2787(69)90257-3. [DOI] [PubMed] [Google Scholar]
- DOSKOCIL J., SORM F. Distribution of 5-methylcytosine in pyrimidine sequences of deoxyribonucleic acids. Biochim Biophys Acta. 1962 Jun 11;55:953–959. doi: 10.1016/0006-3002(62)90909-5. [DOI] [PubMed] [Google Scholar]
- Evans H. H., Evans T. E., Littman S. Methylation of parental and progeny DNA strands in Physarum polycephalum. J Mol Biol. 1973 Mar 15;74(4):563–572. doi: 10.1016/0022-2836(73)90047-8. [DOI] [PubMed] [Google Scholar]
- Evans H. H., Evans T. E. Methylation of the deoxyribonucleic acid of Physarum polycephalum at various periods during the mitotic cycle. J Biol Chem. 1970 Dec 10;245(23):6436–6441. [PubMed] [Google Scholar]
- Gautier F., Bünemann H., Grotjahn L. Analysis of calf-thymus satellite DNA: evidence for specific methylation of cytosine in C-G sequences. Eur J Biochem. 1977 Oct 17;80(1):175–183. doi: 10.1111/j.1432-1033.1977.tb11869.x. [DOI] [PubMed] [Google Scholar]
- Grippo P., Iaccarino M., Parisi E., Scarano E. Methylation of DNA in developing sea urchin embryos. J Mol Biol. 1968 Sep 14;36(2):195–208. doi: 10.1016/0022-2836(68)90375-6. [DOI] [PubMed] [Google Scholar]
- Hardman N., Jack P. L., Brown A. J., McLachlan A. Distribution of inverted repeat sequences in nuclear DNA from Physarum polycephalum. Eur J Biochem. 1979 Feb 15;94(1):179–187. doi: 10.1111/j.1432-1033.1979.tb12884.x. [DOI] [PubMed] [Google Scholar]
- Hardman N., Jack P. L. Characterization of foldback sequences in Physarum polycephalum nuclear DNA using the electron microscope. Eur J Biochem. 1977 Apr 1;74(2):275–283. doi: 10.1111/j.1432-1033.1977.tb11391.x. [DOI] [PubMed] [Google Scholar]
- Hardman N., Jack P. L. Periodic organisation of foldback sequences in Physarum polycephalum nuclear DNA. Nucleic Acids Res. 1978 Jul;5(7):2405–2423. doi: 10.1093/nar/5.7.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Kappler J. W. The kinetics of DNA methylation in cultures of a mouse adrenal cell line. J Cell Physiol. 1970 Feb;75(1):21–31. doi: 10.1002/jcp.1040750104. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation and DNA content of nuclei of Physarum polycephalum. Exp Cell Res. 1971 Jun;66(2):305–316. doi: 10.1016/0014-4827(71)90682-3. [DOI] [PubMed] [Google Scholar]
- Reilly J. G., Braun R., Thomas C. A., Jr Methjylation in Physarum DNA. FEBS Lett. 1980 Jul 28;116(2):181–184. doi: 10.1016/0014-5793(80)80638-7. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Scarano E., Iaccarino M., Grippo P., Winckelmans D. On methylation of DNA during development of the sea urchin embryo. J Mol Biol. 1965 Dec;14(2):603–607. doi: 10.1016/s0022-2836(65)80211-x. [DOI] [PubMed] [Google Scholar]
- Singer J., Roberts-Ems J., Riggs A. D. Methylation of mouse liver DNA studied by means of the restriction enzymes msp I and hpa II. Science. 1979 Mar 9;203(4384):1019–1021. doi: 10.1126/science.424726. [DOI] [PubMed] [Google Scholar]
- Sneider T. W., Potter V. R. Methylation of mammalian DNA: studies on Novikoff hepatoma cells in tissue culture. J Mol Biol. 1969 Jun 14;42(2):271–284. doi: 10.1016/0022-2836(69)90043-6. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Tkacheva S. G., Belozersky A. N. Rare bases in animal DNA. Nature. 1970 Mar 7;225(5236):948–949. doi: 10.1038/225948a0. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]



