Abstract
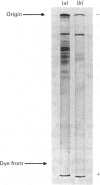
L-3-Glycerophosphate dehydrogenase (EC 1.1.99.5) was purified from pig brain mitochondria by extraction with deoxycholate, ion-exchange chromatography and (NH4)2SO4 fractionation in cholate, and preparative isoelectric focusing in Triton X-100. Sodium dodecyl sulphate/polyacrylamide gel electrophoresis shows that the purified enzyme consists of a single subunit of mol.wt. 75 000. The enzyme contains non-covalently bound FAD and low concentrations of iron and acid labile sulphide. No substrate reducible e.p.r. signals were detected. The conditions of purification, particularly the isoelectric focusing step, lead to considerable loss of FAD and possibly iron-sulphur centres. It is therefore not possible to decide with certainty whether the enzyme is a flavoprotein or a ferroflavoprotein. The enzyme catalyses the oxidation of L-3-glycerophosphate by a variety of electron acceptors, including ubiquinone analogues. A number if compounds known to inhibit ubiquinone oxidoreduction by other enzymes of the respiratory chain failed to inhibit L-3-glycerophosphate dehydrogenase, except at very high concentrations.
Full text
PDF


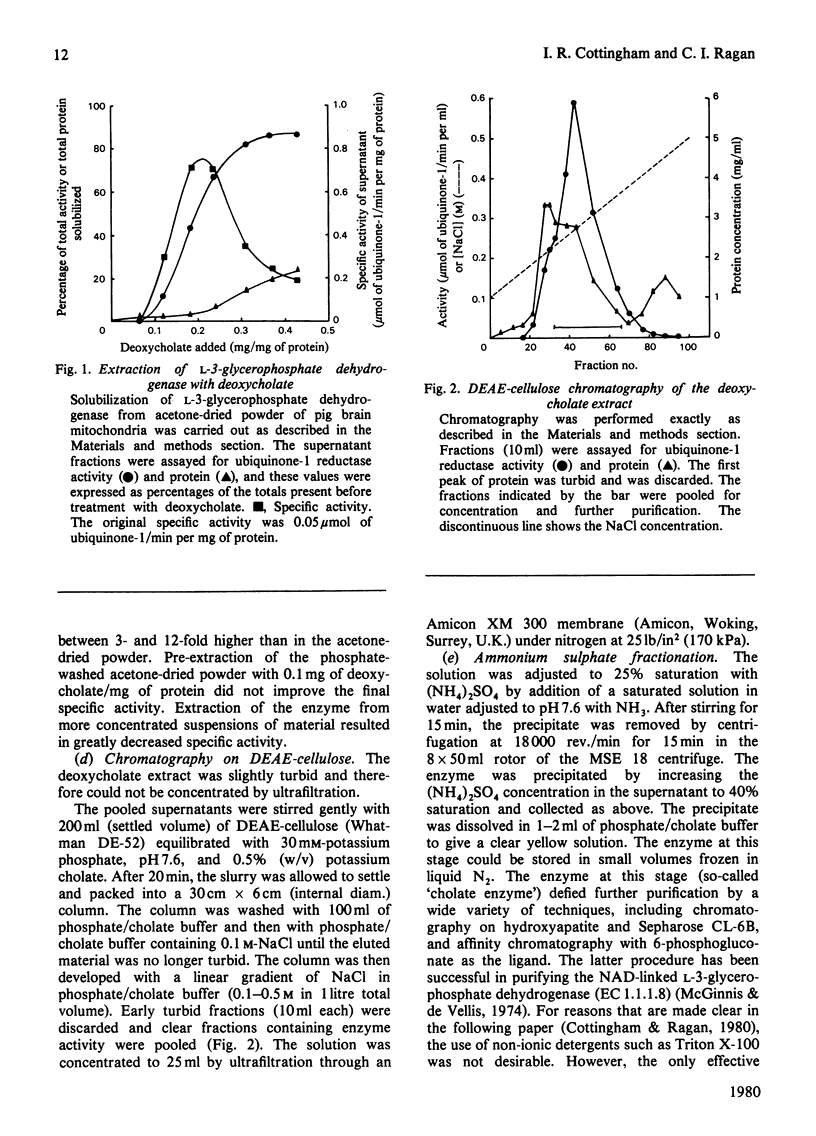

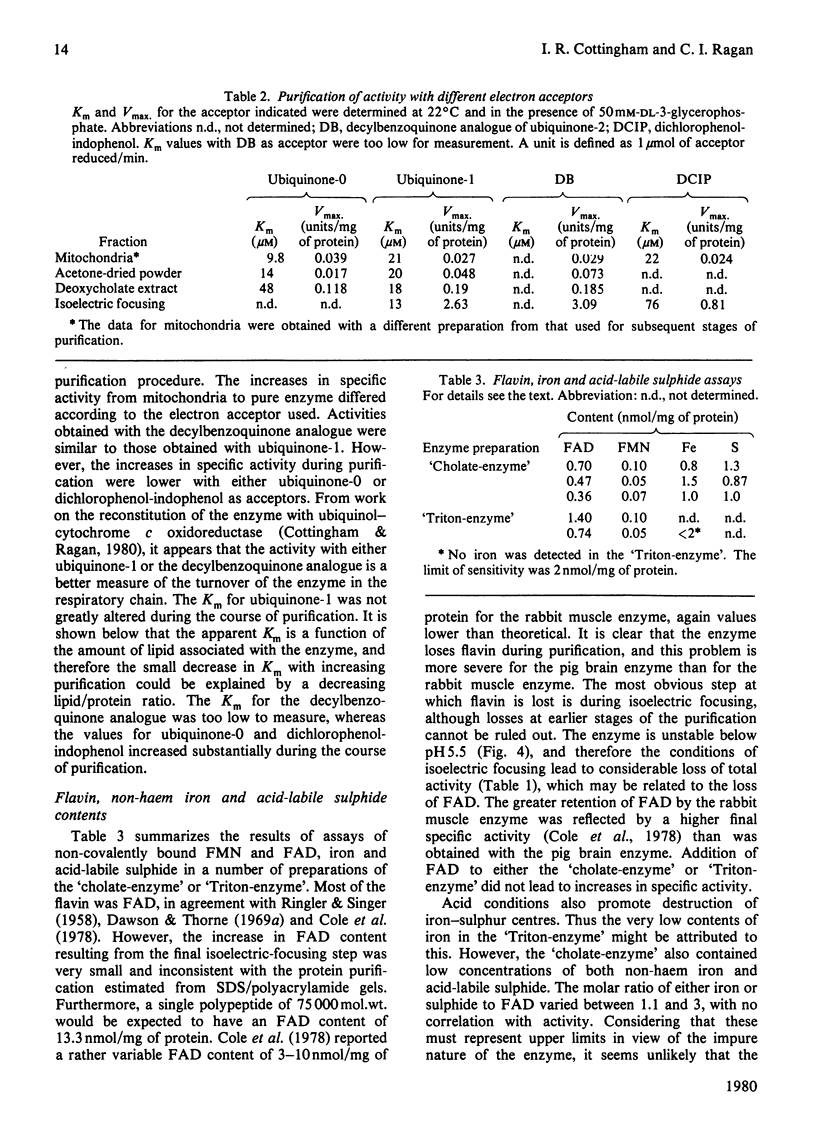
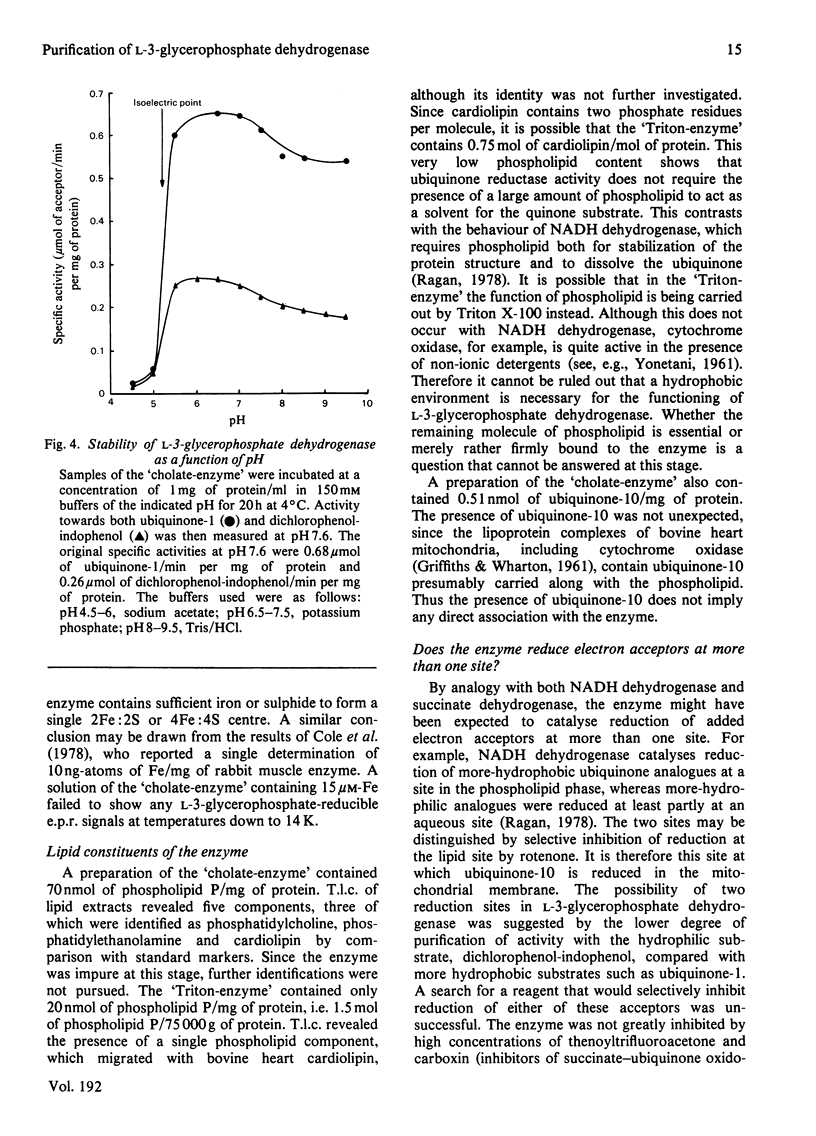

Images in this article
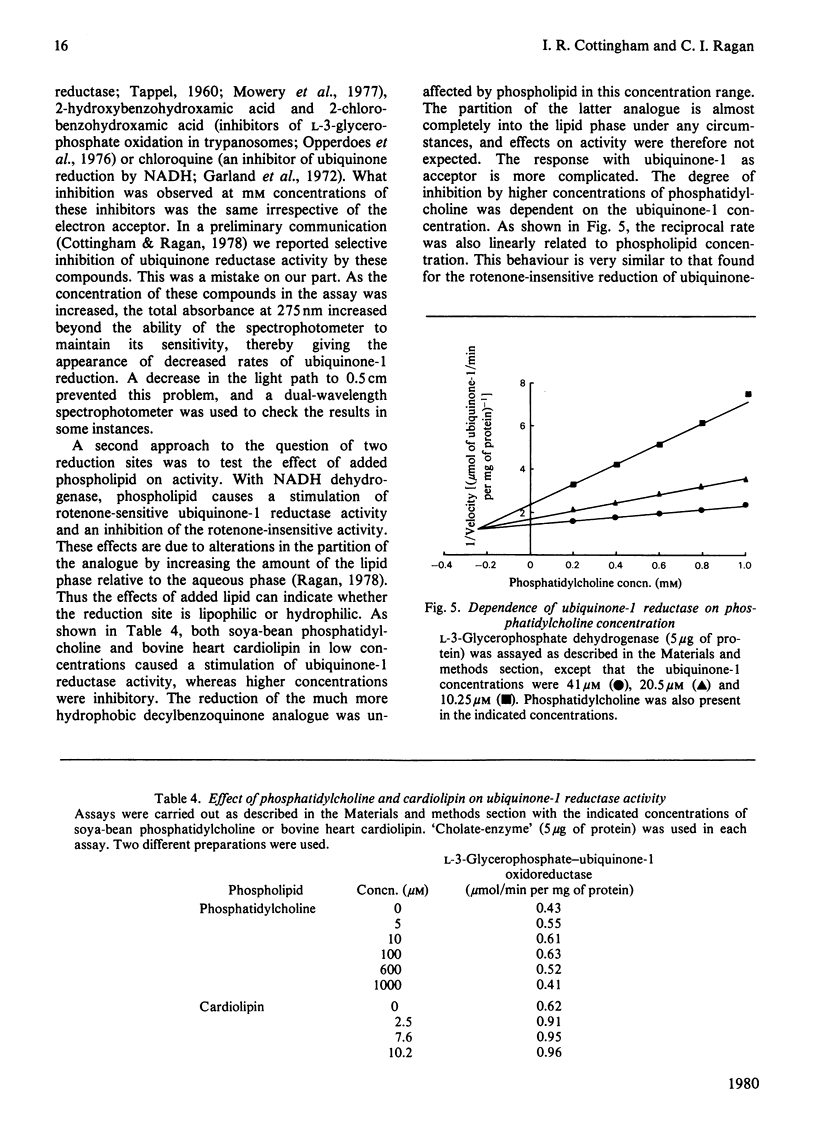
Selected References
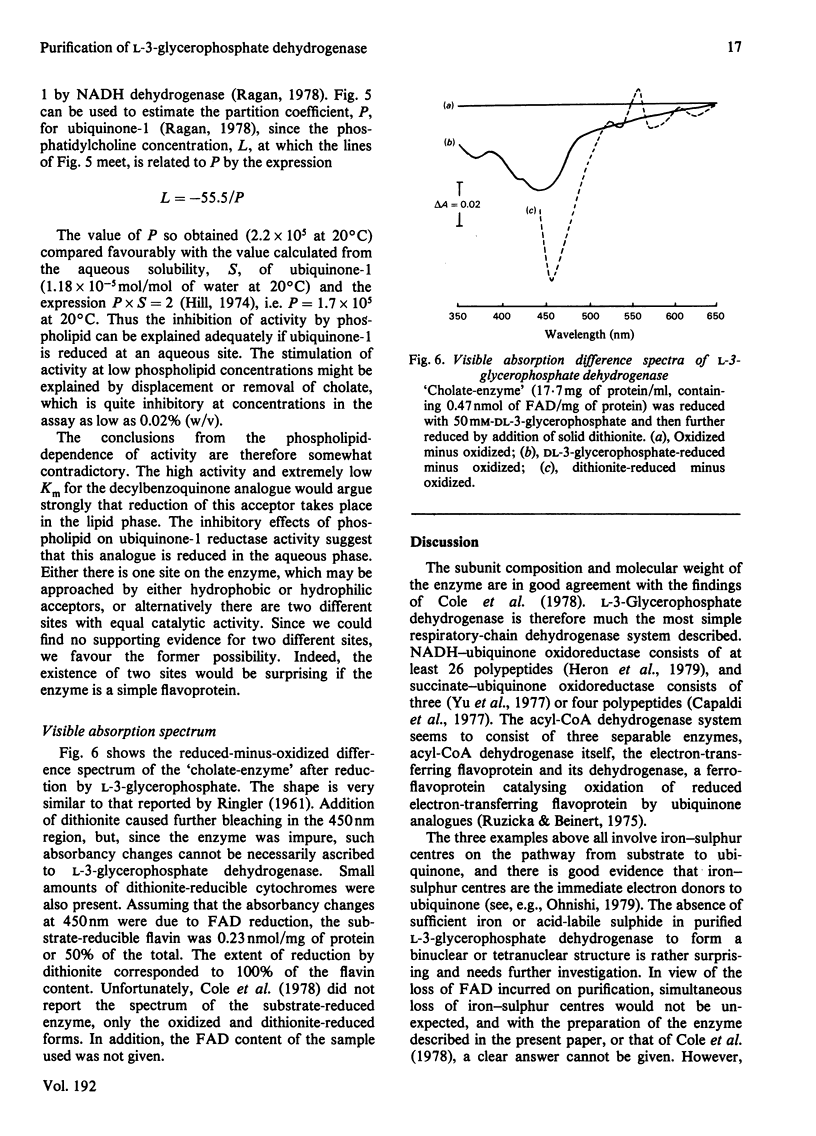
These references are in PubMed. This may not be the complete list of references from this article.
- CERLETTI P., SILIPRANDI N. Pure crystalline flavine adenine dinucleotide. Arch Biochem Biophys. 1958 Jul;76(1):214–224. doi: 10.1016/0003-9861(58)90136-x. [DOI] [PubMed] [Google Scholar]
- Capaldi R. A., Sweetland J., Merli A. Polypeptides in the succinate-coenzyme Q reductase segment of the respiratory chain. Biochemistry. 1977 Dec 27;16(26):5707–5710. doi: 10.1021/bi00645a009. [DOI] [PubMed] [Google Scholar]
- Cole E. S., Lepp C. A., Holohan P. D., Fondy T. P. Isolation and characterization of flavin-linked glycerol-3-phosphate dehydrogenase from rabbit skeletal muscle mitochondria and comparison with the enzyme from rabbit brain. J Biol Chem. 1978 Nov 10;253(21):7952–7959. [PubMed] [Google Scholar]
- Cottingham I. R., Ragan C. I. Purification and properties of mitochondrial L-3-glycerophosphate-ubiquinone oxidoreductase [proceedings]. Biochem Soc Trans. 1978;6(6):1307–1310. doi: 10.1042/bst0061307. [DOI] [PubMed] [Google Scholar]
- Cottingham I. R., Ragan C. I. The reconstitution of L-3-glycerophosphate-cytochrome c oxidoreductase from L-3-glycerophosphate dehydrogenase, ubiquinone-10 and ubiquinol-cytochrome c oxidoreductase. Biochem J. 1980 Oct 15;192(1):19–31. doi: 10.1042/bj1920019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Thorne C. J. Preparation and some properties of L-3-glycerophosphate dehydrogenase from pig brain mitochondria. Biochem J. 1969 Jan;111(1):27–34. doi: 10.1042/bj1110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Thorne C. J. The reaction of mitochondrial L-3-glycerophosphate dehydrogenase with various electron acceptors. Biochem J. 1969 Aug;114(1):35–40. doi: 10.1042/bj1140035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. F., Barker M. D., Wood J., Beechey R. B. Specificity and locale of the L-3-glycerophosphate-flavoprotein oxidoreductase of mitochondria isolated from the flight muscle of Sarcophaga barbata thoms. Biochem J. 1970 Dec;120(3):467–478. doi: 10.1042/bj1200467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS D. E., WHARTON D. C. Studies of the electron transport system. XXXV. Purification and properties of cytochrome oxidase. J Biol Chem. 1961 Jun;236:1850–1856. [PubMed] [Google Scholar]
- Heron C., Smith S., Ragan C. I. An analysis of the polypeptide composition of bovine heart mitochondrial NADH-ubiquinone oxidoreductase by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1979 Aug 1;181(2):435–443. doi: 10.1042/bj1810435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. W. The effect of anaesthetic-like molecules on the phase transition in smectic mesophases of dipalmitoyllecithin. I. The normal alcohol up to C equals 9 and three inhalation anaesthetics. Biochim Biophys Acta. 1974 Jul 12;356(1):117–124. doi: 10.1016/0005-2736(74)90299-5. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. On the role of ubiquinone in mitochondria. II. Redox reactions of ubiquinone under the control of oxidative phosphorylation. Biochem Z. 1966 Jun 7;344(4):317–336. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGinnis J. F., de Vellis J. A novel affinity column for purification of glycerol phosphate dehydrogenase. Biochem Biophys Res Commun. 1974 Sep 9;60(1):186–195. doi: 10.1016/0006-291x(74)90190-9. [DOI] [PubMed] [Google Scholar]
- Mowery P. C., Steenkamp D. J., Ackrell A. C., Singer T. P., White G. A. Inhibition of mammalian succinate dehydrogenase by carboxins. Arch Biochem Biophys. 1977 Jan 30;178(2):495–506. doi: 10.1016/0003-9861(77)90220-x. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P., Fonck K. The potential use of inhibitors of glycerol-3-phosphate oxidase for chemotherapy of African trypanosomiasis. FEBS Lett. 1976 Feb 15;62(2):169–172. doi: 10.1016/0014-5793(76)80045-2. [DOI] [PubMed] [Google Scholar]
- RINGLER R. L., SINGER T. P. Solubilization and assay of the insoluble alpha-glycero-phosphate dehydrogenase of animal tissues. Biochim Biophys Acta. 1958 Sep;29(3):661–662. doi: 10.1016/0006-3002(58)90036-2. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 28. The reconstitution of the first site of energy conservation. J Biol Chem. 1973 Apr 10;248(7):2563–2569. [PubMed] [Google Scholar]
- Ragan C. I. The role of phospholipids in the reduction of ubiquinone analogues by the mitochondrial reduced nicotinamide-adenine dinucleotide-ubiquinone oxidoreductase complex. Biochem J. 1978 Jun 15;172(3):539–547. doi: 10.1042/bj1720539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A new membrane iron-sulfur flavoprotein of the mitochondrial electron transfer system. The entrance point of the fatty acyl dehydrogenation pathway? Biochem Biophys Res Commun. 1975 Sep 16;66(2):622–631. doi: 10.1016/0006-291x(75)90555-0. [DOI] [PubMed] [Google Scholar]
- TAPPEL A. L. Inhibition of electron transport by antimycin A, alkyl hydroxy naphthoquinones and metal coordination compounds. Biochem Pharmacol. 1960 Jul;3:289–296. doi: 10.1016/0006-2952(60)90094-0. [DOI] [PubMed] [Google Scholar]
- Wan Y. P., Williams R. H., Folkers K., Leung K. H., Racker E. Low molecular weight analogs of coenzyme Q as hydrogen acceptors and donors in systems of the respiratory chain. Biochem Biophys Res Commun. 1975 Mar 3;63(1):11–15. doi: 10.1016/s0006-291x(75)80003-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. III. Improved preparation and some properties. J Biol Chem. 1961 Jun;236:1680–1688. [PubMed] [Google Scholar]
- Yu C. A., Yu L., King T. E. The existence of an ubiquinone binding protein in the reconstitutively active cytochrome b-c1 complex. Biochem Biophys Res Commun. 1977 Sep 9;78(1):259–265. doi: 10.1016/0006-291x(77)91248-7. [DOI] [PubMed] [Google Scholar]