Abstract
Background
Since 1965 many ventriculo‐peritoneal shunt systems have been inserted worldwide to treat hydrocephalus. The most frequent indication in adults is normal pressure hydrocephalus (NPH), a condition that can be difficult to diagnose precisely. Surgical intervention with flow‐regulated and differential pressure‐regulated ventriculo‐peritoneal shunts remains controversial. Knowledge about the benefits and harms of these interventions is limited.
Objectives
The objective of this review is to summarize the evidence on benefits and harms of flow‐regulated versus differential pressure‐regulated shunt valves for adult patients with NPH, based on reported findings of randomised clinical trials.
Search methods
The ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialized Register; MEDLINE (from 1950) (Ovid SP); EMBASE (from 1980) (Ovid SP); CINAHL (from 1980) (EBSCOhost); PsycINFO (from 1806) (Ovid SP); LILACS (from 1982 ) (BIREME); ClinicalTrials.gov; Umin Japan Trial Register; WHO portal;The Cochrane Library’s Central Register of Controlled trials (CENTRAL); ISI Web of Knowledge Conference Proceedings; Index to Theses; and Australasian Digital Theses were searched until May 16, 2012.The search terms used were NPH, "normal pressure hydrocephalus," iNPH, idiopathic normal pressure hydrocephalus, sNPH, and "secondary normal pressure hydrocephalus."
Selection criteria
We planned to include randomised clinical trials comparing flow‐regulated versus differential pressure‐regulated shunt valves.
Data collection and analysis
Two authors with expert knowledge within the field independently reviewed studies for eligibility, assessed risk of bias, and extracted data.
Main results
No randomised clinical trials comparing flow‐regulated versus differential pressure‐regulated shunt valves were found.
Authors' conclusions
There is no evidence from randomised clinical trials indicates that flow‐regulated and differential pressure‐regulated shunt valves differ with regard to clinical outcome, shunt failure, or intervention risks. Randomised clinical trials are needed that take into account the large number of VP shunts implanted each year in patients with NPH.
Keywords: Adult; Humans; Hydrocephalus, Normal Pressure; Hydrocephalus, Normal Pressure/surgery; Pressure; Ventriculoperitoneal Shunt; Ventriculoperitoneal Shunt/instrumentation; Ventriculoperitoneal Shunt/methods
Plain language summary
Flow‐regulated versus differential pressure‐regulated shunt valves for adult patients with normal pressure hydrocephalus
Normal pressure hydrocephalus (NPH) is a condition in which the fluid around the brain is not properly absorbed, adversely affecting brain function. It is often treated using a shunt, which drains the extra fluid from the brain into the peritoneal cavity in the abdomen, where the fluid can be absorbed (a ventriculo‐peritoneal shunt). Currently about 5.5 ventriculo‐peritoneal shunt implantations are performed per 100,000 inhabitants in industrial countries per year, even though evidence supporting shunting as treatment for NPH is poor. Approximately 30% to 40% of implanted shunts fail and have to be revised during the first year. To try to reduce the number of complications, many valve and shunt system designs have been developed. These valves can be classified, according to the mechanical design, into two main groups: differential pressure valves and flow‐regulated valves. No randomised clinical trial so far has compared these two types of valve. Thus, there is no high‐quality evidence indicating that flow‐regulated and differential pressure‐regulated shunt valves differ in efficacy or complication rates.
Background
The first valve for implantation into humans to treat hydrocephalus was designed by Spitz and Holter in the 1950s (Nulsen 1951). Initially, the indication was mainly paediatric hydrocephalus. In 1965, the first valve was inserted in an adult patient with normal pressure hydrocephalus (Adams 1965), and since then many ventriculo‐peritoneal shunt systems have been inserted worldwide to treat hydrocephalus in both children and adults. The most frequent indication in adults is normal pressure hydrocephalus (NPH). More recent publications estimate an incidence of VP shunt implantations of 5.5 per 100,000 inhabitants in industrial countries per year (Brean 2008), mainly for patients with NPH (Wu 2007), even though the surgical intervention remains controversial. So far, only one randomised trial has examined the effect of shunt surgery versus no shunt in NPH (Tisell 2011). This trial was very small and entailed a high risk of both type I errors and type II errors (n = 14 participants) and only found a significant difference between the two intervention groups at three months follow‐up regarding psychometric performance in favour of shunt‐treated participants. In practice, there is no consensus about how to identify patients who may benefit from surgery (Klassen 2011).
The probability of the outcome 'shunt failure', defined as requiring surgical revision of the shunting device (as the result of obstruction, disconnection, infection, or overdrainage), is approximately 30% to 40% during the first year after implantation for all types of indications and across patient ages (Reddy 2011; Sotelo 2005). To overcome this high proportion of complications and to improve outcomes, a variety of valve and shunt system designs have evolved over the past 30 years. Valves can be classified, according to mechanical design, into two main groups: differential pressure‐ and flow‐regulated valves. The large number of shunts implanted and the high proportion of associated complications have prompted many publications on the subject of shunt failure. Previous multicentre randomised clinical trials have indicated that the valve design does not alter the outcome or shunt failure rate in paediatric hydrocephalus (Drake 1998).
It seems both necessary and feasible to systematically review the evidence on the relationship between valve mechanism and clinical outcome, including shunt failure rate, in adult patients with NPH.
Description of the condition
The cerebrospinal fluid, which surrounds the brain, is contained within a compartment called the subarachnoid space. The fluid is continually produced by the choroid plexus at a rate of approximately 0.3 ml per minute, with a total production of 450 to 700 ml a day. Approximately 150 ml is present in the subarachnoid space at any one time. From blood vessels lying in the cerebral ventricles (cavities within the brain), the cerebrospinal fluid flows through the ventricles and then over the surface of the brain, where it is finally reabsorbed into the veins through a complex and not fully understandable mechanism that involves tiny projections of the arachnoid into the veins, known as arachnoid granulations, and possibly molecular water channels (aqua porins) (Skjolding 2010a).
NPH is defined by a clinical triad of dementia, gait difficulties, and urinary urge incontinence coexisting with ventriculomegaly on a relevant radiological examination, such as computed tomography or magnetic resonance scan, and demonstrating ventricular enlargement disproportionate to the degree of cerebral atrophy. Other possible causes of dementia must be excluded, including the finding of an alternative neurological or medical condition or the presence of obstructive hydrocephalus (Relkin 2005). No test or imaging modality is pathognomonic for NPH, so the NPH diagnosis is not always easy.
In NPH failure of absorption of cerebrospinal fluid occurs, which has been demonstrated in the superior sagittal sinus of patients with idiopathic NPH (Bateman 2000). When NPH occurs after haemorrhage, infection, or other pathologies in the subarachnoid space, it is defined as secondary NPH. In idiopathic NPH, there is no known underlying cause. Both secondary NPH and idiopathic NPH are characterised by failure of absorption of cerebrospinal fluid. It is thought that this causes a gradual pressure gradient to slowly build up between the ventricles and the brain surface, resulting in a final new steady state in which cerebrospinal fluid formation is diminished, and the pressure is set at a slightly elevated or upper normal level. This resulting condition is thought to cause damage to nerve cells and tracts in the brain.
Description of the intervention
The mechanical function of valves in hydrocephalus shunts is defined on the basis of the flow control or differential pressure working principle.
Flow control valves are designed to keep a constant flow of cerebrospinal fluid throughout the shunt system despite physiological changes in intracranial pressure. This means that a drop in downstream or lower‐end pressure when standing up should not result in increased cerebrospinal fluid flow. Flow should also be constant during physiological increases in intracranial pressure, for example, during Valsalva's manoeuvre or sneezing. The mechanical principle of this type of valve is thus aimed at removing the risk of overdrainage and 'siphoning'. This valve type contains a security overflow mechanism that allows cerebrospinal fluid flow to increase if intracranial pressure increases above acceptable levels, ensuring that intracranial pressure cannot become dangerously high.
Differential pressure valves open when the pressure difference between the front or upper end and the back or lower end of the closing mechanism exceeds its mechanical resistance; this is known as valve opening pressure. Differential pressure valves are designed to keep a constant intracranial pressure. They are manufactured with fixed opening pressures (high, medium, low) or with an adjustable mechanism, which can be set at a range of opening pressures by applying a magnetic adjusting device over the valve mechanism. The valve setting can be adjusted before insertion into the patient and afterwards, by applying the device over the skin covering the valve.
However, because it is not the absolute magnitude of the intracranial pressure but the pressure difference across the valve that makes the mechanism open, a reduction in pressure at the back or lower end of the valve will cause it to open at a lower intracranial pressure. Reduction in downstream or lower‐end pressure typically occurs in an upright body position and in some cases results in too rapid drainage of cerebrospinal fluid through the shunt system when the patient sits or stands. This phenomenon is known as overdrainage or siphoning. To overcome this, additional position‐sensitive mechanisms can be inserted into the shunt system, causing the opening pressure to be higher in the upright position. Such anti‐siphon devices can be an integrated part of the valve, or they can be an additional component that is inserted below the valve itself.
Ideally, differential pressure valves should keep a constant intracranial pressure regardless of the flow rate through the shunt system, and flow control valves should keep a constant flow regardless of intracranial pressure changes. However, all shunts contain tubing with flow resistance, and in all valves a mechanical resistance has to be overcome for the mechanism to open. This means that, in reality, all valve types and all shunt systems have a combination of differential pressure and flow control properties. Differential pressure valves are made so that pressure difference is the major determinant of their function; conversely maintenance of constant flow is the major determinant of function in flow control valves (Sgouros 2010).
How the intervention might work
Surgical intervention for NPH is based on the presumption that provision of a device to divert cerebrospinal fluid from the ventricles will lead to normalisation of the pressure difference, and thereby to stability or improvement in symptoms and signs. The cerebrospinal fluid is drained through a tube (shunt) from the brain ventricles to an absorption site outside the cranial cavity. The system is divided into three functional units. The first unit, the intraventricular catheter, is inserted into the brain ventricles through a burr hole in the skull. This is followed by the second unit, a valve, which is placed subcutaneously on the head; and finally the third unit, which consists of a tube from the valve to the extracranial absorption site. The preferred distal placement for extracranial absorption of cerebrospinal fluid is the peritoneal cavity, in which case the system is termed a ventriculo‐peritoneal, or VP, shunt. Much less frequently, the distal catheter is placed in the right atrium, and rarely in other sites.
Why it is important to do this review
To the best of our knowledge, no systematic review comparing shunt types for NPH has previously been conducted. It is important for the surgeon to choose the shunt valve with the best outcomes, if such a shunt exists, and with this result to explore the cost benefit of shunt implementation in NPH patients. Also if a superior shunt system can be identified, this knowledge may help in understanding the cerebrospinal dynamics of NPH.
Objectives
The objective of this systematic review is to summarise the evidence on benefits and harms of flow‐regulated versus differential pressure‐regulated shunt valves for adult patients with NPH, on the basis of findings of randomised clinical trials.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials comparing flow‐regulated versus differential pressure‐regulated shunt valves.
Types of participants
Surgical NPH patients aged 18 years or older participated.
Patients with both idiopathic and secondary NPH were included.
Types of interventions
Surgical ventriculo‐peritoneal shunt insertion in patients with NPH.
Comparison: flow‐regulated valve type versus differential‐pressure valve type.
Types of outcome measures
Primary outcomes
Death from any cause.
Participants with one or more serious adverse events (SAEs) including and excluding all‐cause mortality, defined according to International Conference of Harmonization of Good Clinical Practice (ICH‐GCP) for devices. Additionally, we will include complications and adverse events specific for hydrocephalus shunt systems, such as (1) clinical and radiological signs of shunt obstruction; (2) computed tomography (CT)‐ or x‐ray-confirmed shunt disconnection; and (3) clinical and radiological signs of overdrainage including subdural hematoma.
Worsening of clinical symptoms of NPH (triad of gait disturbance, urinary incontinence, and subcortical cognitive problems (dementia)).
Quality of life (QOL), measured with any score.
Secondary outcomes
Participants with shunt failure, defined as proportion of shunt re‐interventions (surgical shunt interventions for any reason) within the longest follow‐up in each trial.
Changes in the Evans ratio (radiological ventriculomegaly).
Cost benefit of either intervention.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialized Register. The search terms were NPH, "normal pressure hydrocephalus," iNPH (idiopathic normal pressure hydrocephalus), and sNPH (secondary normal pressure hydrocephalus).
ALOIS is maintained by the Trials Search Co‐ordinator for the Cochrane Dementia and Cognitive Improvement Group and contains planned, ongoing, and completed dementia and cognitive improvement studies identified from the following:
Monthly searches of a number of major healthcare databases: MEDLINE (1950 to date) (Ovid SP), EMBASE (1980 to date) (Ovid SP), CINAHL (1980 to date) (EBSCOhost), PsycINFO (1806 to date) (Ovid SP), and LILACS (1982 to date) (BIREME).
Monthly searches of a number of trial registers: ClinicalTrials.gov; Umin Japan Trial Register; WHO portal (which covers ClinicalTrials.gov; ISRCTN; Chinese Clinical Trials Register; German Clinical trials register; Iranian Registry of Clinical Trials; and the Netherlands National Trials Register, plus others).
Quarterly search of The Cochrane Library’s Central Register of Controlled trials (CENTRAL).
Monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; and Australasian Digital Theses.
To view a list of all sources searched for ALOIS, see About ALOIS on the ALOIS Website.
Additional separate searches were run in many of the above sources plus additional sources (such as the Chinese Biomedical Literature Database and BIOSIS Previews) to ensure that the most up‐to‐date results were retrieved. The search strategies that were used for retrieval of reports of trials from MEDLINE (via the Ovid SP platform) can be seen in Appendix 1.
Searches were performed without language or date restrictions.
Searching other resources
We contacted manufacturers and companies associated with producing the devices or sponsoring the trials where the valves are used, including the following companies: GE Healthcare; Codman and Shurtleff; Transonic Systems Inc.; Johnson & Johnson; Nihon Medi‐Physics Co Ltd.; and Daiichi Pharmaceuticals.
We handsearched the reference list of reviews, randomised and non‐randomised studies, and editorials for additional studies. We contacted the main authors of studies and experts in this field to ask about any missed, unreported, or ongoing trials.
Data collection and analysis
Selection of studies
Two authors (MZ and MJ) independently evaluated all relevant publications for eligibility. There were no disagreements. No eligible studies were identified for inclusion. We provide a detailed description of the excluded articles in the section Characteristics of excluded studies. We also provide a detailed description of our search results.
Data extraction and management
We screened the titles and abstracts to identify studies that are eligible. MZ and MJ independently extracted and collected the data on a standardised paper form (Appendix 2). MZ and MJ were not blinded to the author, institution, or publication source of trials. MZ and MJ resolved disagreements by discussion. We approached all corresponding authors of included trials for additional information relevant to the review's outcomes measures and risk of bias components. For more specific information, please see the section Contributions of authors.
Dealing with missing data
We contacted all first authors and contact persons of trials with missing data to retrieve the relevant data.
Results
Description of studies
Figure 1 reviews the process of how studies were identified for inclusion in the review; also see Characteristics of excluded studies.
1.
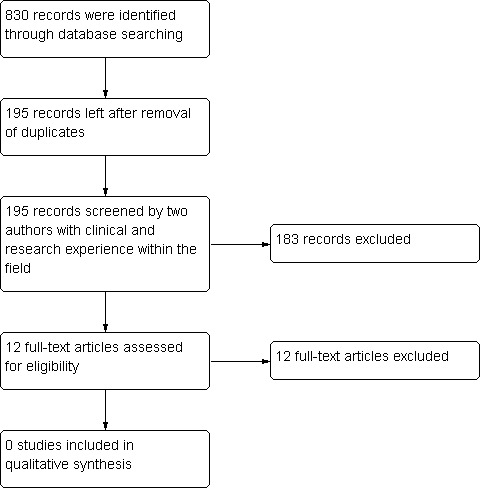
Flow diagram of study selection. For additional details, please see Characteristics of excluded studies.
The studies identified for the review did not include any randomised clinical trials of flow‐regulated versus differential pressure‐regulated shunt valves for adult participants with normal pressure hydrocephalus. No published evidence indicates that such a trial has been performed. Randomised clinical trials related to shunting in adult NPH participants are restricted to those in which differential pressure‐regulated shunt valves with different pressure settings are compared.
Results of the search
The search was performed by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group by the 17th of May 2012.
Included studies
No randomised clinical trials are included in this review. (Please see protocol for intentional approach if studies were found Ziebell 2012)
Excluded studies
See Characteristics of excluded studies. The following studies have been excluded from the review: Boon 1997; Boon 1998; Farahmand 2009; Lund‐Johansen 1994; Meier 2006a; Meier 2006b; Meier 2010; Meier 2011; Pollack 1999; Weiner 1995; Lemcke 2012; Toma 2011.
Risk of bias in included studies
There are no included studies comparing flow‐regulated versus differential pressure‐regulated shunt valves.
Effects of interventions
Presently, no published trials have compared flow‐regulated versus differential pressure‐regulated shunt valves.
We found only one study, by Weiner 1995, with the specified purpose of comparing flow‐regulated versus differential pressure‐regulated shunt valves. This was a retrospective study in which the selection criteria reduced the included number of participants to 37 from the initial unselected number of 1500 shunt participants. In this study, the distribution between shunt types was approximately 50/50, and the authors found no statistically significant difference in shunt survival. Another retrospective study by Lund‐Johansen 1994 used wider clinical inclusion criteria to report on a group of 95 participants, amongst whom 25 participants with NPH were included. In the entire group of 95 participants, 40 had a flow‐regulated and 55 a differential pressure‐regulated valve. Investigators found no statistically significant differences in failure, complications, or time to first revision. Farahmand 2009 also conducted a non‐randomised controlled study on 450 participants with various types of hydrocephalus including NPH. In this retrospectively analysed material, only six flow‐regulated valves were compared with 443 differential pressure‐regulated valves, and no statistically significant difference in revision rate was noted.
Discussion
Shunting as an intervention for normal pressure hydrocephalus remains controversial, and so far only one randomised controlled study of shunt versus no shunt has been reported (Tisell 2011). The trial was very small (n = 14 participants), entailing high risks of both type I and type II errors, and found a significant difference between the two intervention groups only on psychometric test performance at three months' follow‐up, favouring participants in the intervention group who had undergone surgery. No statistically significant difference was noted between the two intervention groups in terms of gait or overall clinical performance (perceptual speed and accuracy, reaction time, manual dexterity, verbal learning and memory, motor speed, speed, response selection, and inhibition). All of these tests have been used in individuals with iNPH and have been shown to be valid and sensitive to postoperative results. We identified one ongoing randomised trial that aims to compare conservative versus surgical management of idiopathic NPH (Toma 2011). Although the trial was planned to end during 2011, results so far are not available.
A few small retrospective studies included participants with either differential pressure‐regulated or flow‐regulated shunts as the intervention (Farahmand 2009; Lund‐Johansen 1994; Weiner 1995), but only one of these studies specifically compared the two valve types (Weiner 1995). None of these studies showed statistically significant differences in shunt failure between the two shunt types. However, comparisons based on small, retrospective, non‐randomised controlled studies should be assessed with utmost caution because observational studies can never take into consideration unmeasured confounding, especially confounding by indication.
Of the remaining studies, we identified none that addressed the review question (see Characteristics of excluded studies).
In general, the number of randomised clinical trials in the field of neurosurgery is limited. There seems to be an obvious reason for the lack of high‐quality evidence regarding optimum treatment of NPH. Diagnosis of NPH is still controversial, and no test or imaging modality is pathognomonic for NPH. Therefore, it is still problematic to identify with a high degree of certainty which patients will benefit from a ventriculo‐peritoneal shunt, and which will not.
Finally (even though it is beyond the scope of this review), it is notable that a few multicenter trials comparing valve designs conducted in paediatric hydrocephalus populations have been performed, which found no difference in outcome when comparing different valve types (Drake 1998; Kestle 2000). However, hydrocephalus in children is a different condition. Child hydrocephalus is extremely seldom described as normal pressure, but rather is described as high‐pressure, hydrocephalus. Additionally, child hydrocephalus requires a slightly different surgical technique, and different kinds of complications are observed because of the level of physical activity, lack of compliance with having a shunt implanted, and 'out‐growing' of the device. Thus, evidence derived from studies of children cannot be applied to adults. However, these publications support the need for similar randomised trials in adult hydrocephalus, and they show how similar randomised trials on NPH can be conducted in adults.
Authors' conclusions
Implications for practice.
There is no evidence from randomised clinical trials exploring the benefits and harms of flow‐regulated versus differential pressure‐regulated shunt valves in adult patients with normal pressure hydrocephalus.
Implications for research.
Randomised clinical trials exploring the benefits and harms of flow‐regulated versus differential pressure‐regulated shunt valves for adult patients with normal pressure hydrocephalus are needed. Even though this is not the topic of this review, it seems to be in line with the lack of evidence comparing shunts versus no shunts. Clearly, additional randomised clinical trials are warranted, preferably undertaken to compare open shunts versus no shunts. Only in this way can we obtain both short‐ and long‐term knowledge about the benefits and harms.
Appendices
Appendix 1. Search strategy: MEDLINE
| Source | Search strategy |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950‐present (Ovid SP) | 1. Hydrocephalus, NormalPressure/ 2. normal pressure hydrocephalus.ti,ab. 3. NPH.ti,ab. 4. sNPH.ti,ab. 5. iNPH.ti,ab. 6. or/1‐5 7. valve*.ti,ab. 8. shunt*.ti,ab. 9. (flow adj2 (control or regulat*)).ti,ab. 10. differential pressure.ti,ab. 11. Ventriculoperitoneal Shunt/ or Peritoneovenous Shunt/ 12. ("pressure sensitive" adj5 shunt*).ti,ab. 13. or/7‐12 14. 6 and 13 15. randomized controlled trial.pt. 16. controlled clinical trial.pt. 17. randomi?ed.ab. 18. placebo.ab. 19. randomly.ab. 20. trial.ab. 21. groups.ab. 22. or/15‐21 23. (animals not (humans and animals)).sh. 24. 22 not 23 25. 14 and 24 |
Appendix 2. Data extraction form
Flow‐regulated versus differential pressure‐regulated shunt valves for adult patients with normal pressure hydrocephalus
Study Selection, Quality Assessment and Data Extraction Form
| First author | Journal/Conference Proceedings etc | Year |
Study eligibility
| RCT | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
*Issue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
| A | The paper listed above | ||
| B | Further papers | ||
| C | |||
| D | |||
| E |
Participants and trial characteristics
| Participant characteristics | |
| Covariate | Further details |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers / %, etc) | |
| Disease status / type, iNPH, sNPH or both | |
Trial characteristics
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Low risk of bias (Random) |
| High risk of bias (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Low risk of bias | |
| High risk of bias | |
| Unclear | |
| Blinding | ||
| Person responsible for participants' care | Yes / No | |
| Participant | Yes / No | |
| Outcome assessor | Yes / No | |
| Other (please specify) | Yes / No | |
| Incomplete outcome data | ||
| Low risk of bias, if the numbers and reasons for dropouts and withdrawals in the intervention groups were described, or if it was specified that there were no dropouts or withdrawals. | Yes / No | |
| High risk of bias, if the number or reasons for dropouts and withdrawals were not described. | Yes / No | |
| Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated. | Yes / No | |
| Selective outcome reporting | ||
| Low risk of bias, if predefined or clinically relevant and reasonably expected outcomes are reported on. | Yes / No | |
| High risk of bias, one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded. | Yes / No | |
| Unclear, not all pre‐defined, or clinically relevant and reasonably expected outcomes are reported on or are not reported fully, or it is unclear whether data on these outcomes were recorded or not. | Yes / No | |
| Baseline imbalance | ||
| Low risk of bias, if there was no baseline imbalance in important characteristics. | Yes / No | |
| High risk of bias, if there was a baseline imbalance due to chance or due to imbalanced exclusion after randomisation. | Yes / No | |
| Unclear, if the baseline characteristics were not reported. | Yes / No | |
| Early stopping | ||
| Low risk of bias, if sample size calculation was reported and the trial was not stopped, or the trial was stopped early by formal stopping rules at a point where the likelihood of observing an extreme intervention effect due to chance was low. | Yes / No | |
| High risk of bias, if the trial was stopped early due to informal stopping rules or the trial was stopped early by a formal stopping rule at a point where the likelihood of observing an extreme intervention effect due to chance was high. | Yes / No | |
| Unclear, if sample size calculation was not reported and it is not clear whether the trial was stopped early or not. | Yes / No | |
| Other bias | ||
| No risk of other bias, the trial appears to be free of other components that could put it at risk of bias. | Yes / No | |
| Risk of other bias, there are other factors in the trial that could put it at risk of bias, e.g., 'for‐profit’ involvement, authors have conducted trials on the same topic, etc. | Yes / No | |
| Unclear, the trial may or may not be free of other components that could put it at risk of bias. | Yes / No | |
|
Modified intention‐to‐treat A modified intention‐to‐treat analysis is one in which all the participants in a trial are operated and analysed according to the intervention to which they were allocated, whether they received it or not. |
||
| All participants entering trial after surgery. | ||
| 15% or fewer excluded. | ||
| More than 15% excluded. | ||
| Not analysed as modified ‘intention‐to‐treat’. | ||
| Unclear. | ||
Were withdrawals described? Yes ? No ? not clear ?
Discuss if appropriate
| Trial characteristics | |
| Further details | |
| Single centre / Multicentre | |
| Country / Countries | |
| How was participant eligibility defined? | |
| How many participants were randomised? | |
| Number of participants in each intervention group | |
| Number of participants who received intended intervention (per protocol population) | |
| Number of participants who were analysed | |
| Duration of surgery in minutes | |
| Antibiotic regime used | |
| Time of antibiotic administration | |
| Administration of oxygen, type of face mask ? | |
| Duration of supplemental oxygen intervention including postoperative period or not ? | |
| Mean temperature and range during anaesthesia | |
| Mean fluid volume and range management during anaesthesia | |
| Fraction of patients with epidural anaesthesia | |
| Fraction of patients administered vasopressors | |
| Median (range) length of follow‐up reported in this paper (state weeks, months, or years or if not stated) | |
| Time‐points within which SSI were diagnosed during the trial ? | |
| Time‐points reported in the trial ? | |
| Time‐points you are using in RevMan | |
| Trial design (e.g. parallel / cross‐over*) | |
| Other | |
* If cross‐over design, please refer to the Cochrane Editorial Office for further advice on how to analyse these data
Other design characteristics of the trial
1. The trial used clinical history criteria to NPH Yes ? No ?
2. The trial used brain imaging criteria Yes ? No ?
3. The trial used physical criteria Yes ? No ?
4. The trial used physiological criteria Yes ? No ?
5. The trial included only sNPH or iNPH or both Yes ? No ?
Data extraction
| Outcomes | Available for the trial |
| 1.1 Overall mortality. We will use the longest follow‐up data from each trial regardless of the period of follow‐up. | Yes/No |
| 1.2 Serious adverse events including and excluding all‐cause mortality defined according to International Conference of Harmonization of Good Clinical Practice (ICH‐GCP) for devices and in addition: Subdural Haematoma, CT/x‐ray or MR confirmed shunt disconnection, clinical signs of over‐drainage, and clinical measure of non‐drainage into abdomen. | Yes/No |
| 1.3 Re‐occurrence of clinical signs to NPH (triad of gait disturbance, urinary incontinence, and sub‐cortical cognitive problems (dementia). | Yes/No |
| 1.4 Quality of life measured with any score. | Yes/No |
| 2.1 Shunt failure defined as proportion of shunt re‐interventions (surgical shunt interventions for any reason) within the longest follow‐up in each trial. | Yes/No |
| 2.2 Changes in the Evans ratio (radiology sign of hydrocephalus). | Yes/No |
| For Continuous data | |||||||
| Code of paper |
Outcomes (rename) |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A etc. | 2.3 Duration of postoperative hospitalisation. | Days | |||||
| A etc. | 2.4 Quality of life | Score (any) | |||||
| For Dichotomous data | |||
| Code of paper | Outcomes | Intervention group E/N E = number of events N = number of participants |
Control group E/N E = number of events n = number of participants |
| A | 1.1 Overall mortality. | ||
| 1.2 Re‐occurrence of clinical signs to NPH (triad of gait disturbance, urinary incontinence, and sub‐cortical cognitive problems (dementia). | |||
| 2.1 Shunt‐failure defined as proportion of shunt re‐interventions (surgical shunt interventions for any reason) within the longest follow‐up in each trial. | |||
| 2.2 Serious adverse events including and excluding all‐cause mortality defined according to International Conference of Harmonization of Good Clinical Practice (ICH‐GCP) for devices and, in addition, Subdural Haematoma, CT/x‐ray or MR confirmed shunt disconnection, clinical signs of over‐drainage, and clinical measure of non‐drainage into abdomen. | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. | ||
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boon 1997 | Randomised clinical trial comparing low‐pressure versus medium‐pressure shunts in 101 normal pressure hydrocephalus participants. Boths systems are differential‐pressure valves. |
| Boon 1998 | Same trial as Boon 1997, although reporting on only 96 participants. |
| Farahmand 2009 | Not a randomised trial. A prospective study on 450 participants with hydrocephalus in any form. Six flow‐regulated valve participants were compared with 443 differential‐pressure valve participants. No statistically significant difference in revision was noted. |
| Lund‐Johansen 1994 | Retrospective study of 95 mixed hydrocephalus participants (25 normal pressure hydrocephalus). 40 flow‐regulated valve participants compared with 55 differential‐pressure valve participants. No statistically significant difference in revision. |
| Meier 2006a | An open prospective trial to determine the optimal opening pressure for pressure‐relief valves used in shunts for normal pressure hydrocephalus. |
| Meier 2006b | Same trial as Meier 2006a. |
| Meier 2010 | Randomised multicentre trial comparing programmable differential‐pressure valves with and without anti‐gravity unit in 152 participants with idiopathic normal pressure hydrocephalus. |
| Meier 2011 | Same trial as Meier 2010, although only 133 participants were described. |
| Pollack 1999 | Randomised clinical trial to assess safety of a specific differential‐pressure programmable shunt (experimental) versus differential‐pressure and flow‐regulated shunt valves (control). Included 377 participants with varying causes of hydrocephalus; separate results for those with NPH not given. No randomisation between differential‐pressure (n = 178) and flow‐regulated shunt valves (n = 5) in control group. |
| Weiner 1995 | Retrospective study of 37 idiopathic normal pressure hydrocephalus participants comparing differential‐pressure programmable shunt versus differential‐pressure and flow‐regulated shunt valves. Not a randomised trial. |
Characteristics of ongoing studies [ordered by study ID]
Lemcke 2012.
| Trial name or title | On the method of a randomised comparison of programmable valves with and without anti‐gravitational units: The SVASONA trial. |
| Methods | Randomised clinical trial. |
| Participants | Normal pressure hydrocephalus. |
| Interventions | Programable shunt with and without gravitational unit. |
| Outcomes | Clinical outcome. |
| Starting date | |
| Contact information | |
| Notes |
Toma 2011.
| Trial name or title | Conservative versus surgical management of idiopathic normal pressure hydrocephalus: a prospective double‐blind randomised clinical trial: trial protocol. |
| Methods | RCT. |
| Participants | Idiopathic normal pressure hydrocephalus. |
| Interventions | Conservative versus surgical management of idiopathic normal pressure hydrocephalus. |
| Outcomes | Primary is clinical outcome (improvement in gait). |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
Conceiving the review: Morten Ziebell (MZ), Magnus Tisell (MT), Jørn Wetterslev (JW), Christian Gluud (CG), Marianne Juhler (MJ). Co‐ordinating the review: MZ. Undertaking manual searches: MZ. Screening search results: MZ, MJ. Organizing retrieval of papers: MZ, MJ. Screening retrieved papers against inclusion criteria: MZ, MJ. Appraising quality of papers: MZ, MJ. Abstracting data from papers: MZ, MJ. Writing to authors of papers for additional information: MZ. Providing additional data about papers: MZ. Obtaining and screening data on unpublished studies: MZ, MJ. Data management for the review: MZ, MJ.
Entering data into Review Manager (RevMan 5.0): MZ, MJ. RevMan statistical data: MZ, MJ, JW. Other statistical analysis not using RevMan (TSA): MZ. Double entry of data: (data entered by person one: MZ; data entered by person two: MJ). Interpretation of data: MZ, MJJW, CM, LNJ, LSR, CG. Statistical analysis: MZ, MJ, JW, CG. Writing the review: MZ, MJ, JW, CG, MT.
Securing funding for the review: MZ, MJ. Performing previous work that was the foundation of the present study: NA. Guarantor for the review (one author): MZ. Person responsible for reading and checking the review before submission: MZ.
Declarations of interest
None known for any author.
New
References
References to studies excluded from this review
Boon 1997 {published data only}
- Boon AJW, Tans JTJ, Delwel EJ, Egeler‐Peerdeman SM, Hanlo PW, Wurzer HAL, et al. Dutch normal‐pressure hydrocephalus study: prediction of outcome after shunting by resistance to outflow of cerebrospinal fluid. Journal of Neurosurgery 1997;87(5):687‐93. [DOI] [PubMed] [Google Scholar]
Boon 1998 {published data only}
- Boon AJW, Tans JTJ, Delwel EJ, Egeler‐Peerdeman SM, Hanlo PW, Wurzer HAL, et al. Dutch normal‐pressure hydrocephalus study: randomized comparison of low‐ and medium‐pressure shunts. Journal of Neurosurgery 1998;88(3):490‐5. [DOI] [PubMed] [Google Scholar]
Farahmand 2009 {published data only}
- Farahmand D, Hilmarsson H, Hogfeldt M, Tisell M. Perioperative risk factors for short term shunt revisions in adult hydrocephalus patients. Journal of Neurology, Neurosurgery, & Psychiatry 2009;80(11):1248‐53. [DOI] [PubMed] [Google Scholar]
Lund‐Johansen 1994 {published data only}
- Lund‐Johansen M, Svendsen F, Wester K, McComb JG, Benzel EC. Shunt failures and complications in adults as related to shunt type, diagnosis, and the experience of the surgeon. Neurosurgery 1994;35(5):839‐44. [DOI] [PubMed] [Google Scholar]
Meier 2006a {published data only}
- Meier U, Kiefer M, Lemcke J. On the optimal opening pressure of hydrostatic valves in cases of idiopathic normal‐pressure hydrocephalus: a prospective randomized study with 122 patients. Neurosurgery Quarterly 2005;15(2):103‐9. [DOI] [PubMed] [Google Scholar]
Meier 2006b {published data only}
- Meier U, Kiefer M, Neumann U, Lemcke J. On the optimal opening pressure of hydrostatic valves in cases of idiopathic normal‐pressure hydrocephalus: a prospective randomized study with 123 patients. Acta Neurochirurgica 2006;Suppl 96:358‐63. [DOI] [PubMed] [Google Scholar]
Meier 2010 {published data only}
- Meier U, Lemcke J, Muller C, Fritsch M, Kiefer M, Eymann R, et al. First results of the interim analysis of the randomized controlled SVASONA trial for idiopathic normal pressure hydrocephalus. Proceedings of the 78th Annual Meeting of the American Association of Neurological Surgeons; 2010; AANS.
Meier 2011 {published data only}
- Meier U, Lemcke J, Muller C, Fritsch M, Kiefer M, Eymann R, et al. Final results of the SVASONA study in idiopathic normal pressure hydrocephalus. Proceedings of the Journal of Neurosurgery Conference; 2011; AANS.
Pollack 1999 {published data only}
- Pollack IF, Albright AL, Adelson PD. A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Neurosurgery 1999;45(6):1399‐408. [DOI] [PubMed] [Google Scholar]
Weiner 1995 {published data only}
- Weiner HL, Constantini S, Cohen H, Wisoff JH. Current treatment of normal‐pressure hydrocephalus - Comparison of flow‐regulated and differential‐pressure shunt valves. Neurosurgery 1995;37(5):877‐84. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Lemcke 2012 {published data only}
- On the method of a randomised comparison of programmable valves with and without anti‐gravitational units: The SVASONA trial.. Ongoing study Starting date of trial not provided. Contact author for more information.
Toma 2011 {published data only}
- Conservative versus surgical management of idiopathic normal pressure hydrocephalus: a prospective double‐blind randomised clinical trial: trial protocol.. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Adams 1965
- Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with "normal" cerebrospinal‐fluid pressure: a treatable syndrome. New England Journal of Medicine 1965;273:117‐26. [PUBMED: 14303656] [DOI] [PubMed] [Google Scholar]
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ (Clinical research ed.) 2003;326(7382):219. [PUBMED: 12543843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 2000
- Bateman GA. Vascular compliance in normal pressure hydrocephalus. AJNR American Journal of Neuroradiology 2000;21(9):1574‐85. [PUBMED: 11039334] [PMC free article] [PubMed] [Google Scholar]
Bekelman 2003
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003;289(4):454‐65. [PUBMED: 12533125] [DOI] [PubMed] [Google Scholar]
Brean 2008
- Brean A, Eide PK. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand 2008;118:48–53. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive -Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Krleza‐Jeric K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ Canadian Medical Association Journal 2004;171(7):735‐40. [PUBMED: 15451835] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickersin 1990
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990;263(10):1385‐9. [PUBMED: 2406472] [PubMed] [Google Scholar]
Drake 1998
- Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J Jr, et al. Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 1998;43(2):294‐303; discussion 303‐5. [PUBMED: 9696082] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research edition) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [PUBMED: 15878471] [DOI] [PubMed] [Google Scholar]
Higgins 2003a
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research edition) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. www.cochrane‐handbook.org. [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ (Clinical research ed.) 1999;319(7211):670‐4. [PUBMED: 10480822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kestle 2000
- Kestle J, Drake J, Milner R, Sainte‐Rose C, Cinalli G, Boop F, et al. Long‐term follow‐up data from the Shunt Design Trial. Pediatric Neurosurgery 2000;33(5):230‐6. [PUBMED: 11155058] [DOI] [PubMed] [Google Scholar]
Keus 2009
- Keus F, Wetterslev J, Gluud C, Gooszen HG, Laarhoven CJ. Robustness assessments are needed to reduce bias in meta‐analyses that include zero‐event randomized trials. American Journal of Gastroenterology 2009;104(3):546‐51. [PUBMED: 19262513] [DOI] [PubMed] [Google Scholar]
Klassen 2011
- Klassen BT, Ahlskog JE. Normal pressure hydrocephalus: how often does the diagnosis hold water?. Neurology 2011;77(12):1119‐25. [PUBMED: 21849644] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659‐63. [Google Scholar]
Nulsen 1951
- Nulsen FE, Spitz EB. Treatment of hydrocephalus by direct shunt from ventricle to jugular vein. Surgical Forum 1951;351:399‐403. [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93; discussion 661‐6. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
Reddy 2011
- Reddy GK, Shi R, Guthikonda B. Obstructive hydrocephalus in adult patients: the Louisiana State University Health Sciences Center‐Shreveport experience with ventriculoperitoneal shunts. World Neurosurgery 2011;76(1‐2):176‐82. [PUBMED: 21839971] [DOI] [PubMed] [Google Scholar]
Relkin 2005
- Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal‐pressure hydrocephalus. Neurosurgery 2005;57 Suppl 3:4‐16; discussion ii‐v. [PUBMED: 16160425] [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [PUBMED: 17592831] [DOI] [PubMed] [Google Scholar]
Sgouros 2010
- Sgouros S, Kombogiorgas D. Cerebrospinal fluid shunts. In: Mallucci C, Sgouros S editor(s). Cerebrospinal Fluid Disorders. New York: Informa Healthcare, USA, 2010. [Google Scholar]
Skjolding 2010a
- Skjolding AD, Rowland IJ, Sogaard LV, Praetorius J, Penkowa M, Juhler M. Hydrocephalus induces dynamic spatiotemporal regulation of aquaporin‐4 expression in the rat brain. Cerebrospinal Fluid Research 2010;7:20. [PUBMED: 21054845] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sotelo 2005
- Sotelo J, Arriada N, Lopez MA. Ventriculoperitoneal shunt of continuous flow vs valvular shunt for treatment of hydrocephalus in adults. Surgical Neurology 2005;63(3):197‐203; discussion 203. [PUBMED: 15734497] [DOI] [PubMed] [Google Scholar]
Sutton 2002
- Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta‐analysis of rare and adverse event data. Expert Review of Pharmacoeconomics & Outcomes Research 2002;2(4):367‐79. [PUBMED: 19807443] [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [PUBMED: 15116347] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
Tisell 2011
- Tisell M, Tullberg M, Hellstrom P, Edsbagge M, Hogfeldt M, Wikkelso C. Shunt surgery in patients with hydrocephalus and white matter changes. Journal of Neurosurgery 2011;114(5):1432‐8. [PUBMED: 21235310] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9(86):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al . Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. British Medical Journal 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2007
- Wu Y, Green NL, Wrensch MR, Zhao S, Gupta N. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery 2007;61(3):557‐62; discussion 562‐3. [PUBMED: 17881969] [DOI] [PubMed] [Google Scholar]
Ziebell 2012
- Ziebell M, Wetterslev J, Tisell M, Gluud C, Juhler M. Flow‐regulated versus differential pressure‐regulated shunt valves for adult patients with normal pressure hydrocephalus. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009706] [DOI] [PMC free article] [PubMed] [Google Scholar]


