Abstract
Background:
APOE-e4 is the strongest genetic risk factor for Alzheimer’s disease. However, the influence of APOE-e4 on dietary fat intake and cognition has not been investigated.
Objective:
We aim to examine the association of types of dietary fat and their association to cognitive decline among those with and without the APOE-e4 allele.
Methods:
The study included 3,360 Chicago Health and Aging Project (CHAP) participants from four Southside Chicago communities. Global cognition was assessed using a composite score of episodic memory, perceptual speed, MMSE, and diet using a 144-item food frequency questionnaire. APOE genotype was assessed by the hME Sequenom mass-array platform. Longitudinal mixed-effect regression models were used to examine the association of dietary fat and the APOE-e4 allele with cognitive decline, adjusted for age, sex, education, smoking status, and calorie intake.
Results
The present study involved 3,360 participants with a mean age of 74 at baseline, 62% African Americans, 63% females, and a mean follow-up of 7.8 years. Among participants with the APOE-e4 risk allele, higher intakes of total and saturated fat (SFA) were associated with a faster decline in global cognition. Among individuals with the APOE-e4 risk allele, a 5% increase in calories from SFA was associated with a 21% faster decline (β = −0.0197, p = 0.0038). However, a higher intake of long-chain n-3 polyunsaturated fatty acids (LC-n3 PUFA) was associated with a slower rate of decline in global cognition among APOE-e4 carriers. Specifically, for every 1% energy increment from LC-n3 PUFA, the annual rate of global cognitive decline was slower by 0.024 standardized unit (SD 0.010, P=0.023), about 30.4% slower annual cognitive decline. Higher SFA or other types of dietary fat were not associated with cognitive decline among APOE-e4 non-carriers.
Conclusions:
Our study found a significant association between SFA and faster cognitive decline, LC-n3 PUFA and slower cognitive decline among those with the APOE-e4 allele. Our findings suggested that higher intake of SFA might contribute faster cognitive decline in combination with APOE-e4 whereas LC-n3 PUFA might compensate the adverse effects of APOE-e4. The interaction between intakes of different types of dietary fat and APOE-e4 on cognitive function warrants further research.
Keywords: Cognition, dietary fat, APOE, cohort, saturated fat, nutrition, long-chain fatty acid
Introduction
Alzheimer’s disease and related dementia (ADRD) is a devastating neurodegenerative condition that significantly affects the quality of life in older adults. Primary prevention through modifiable lifestyle factors, including diet, has become a high public health priority. Previous evidence demonstrated that adherence to specific dietary patterns, i.e., MedDiet, MIND diet, and DASH diet, was associated with slower cognitive decline in older adults across various racial groups1. However, in a recent randomized controlled trial the MIND diet had minimal impact on rates of cognitive decline 2. The heterogeneity among previous findings might be partially attributed to the participant’s apolipoprotein E polymorphisms (APOE-e4 risk allele carriers versus non-carriers). Currently, there is a lack of long-term human studies to determine the practical nutritional approach for preventing AD in APOE-e4 carriers, considering the mechanisms through which this genetic variation confers an increased risk of AD.
Emerging evidence from animal models and human studies demonstrated that ApoE4 impacts blood brain barriers (BBB) integrity 3, microglial and inflammation pathways 4, astrocytes lipid metabolism 5,6, and energy utilization in the brain 7,8. Our previous investigation showed that higher consumption of saturated fatty acids was associated with an increased risk of AD and a faster rate of decline in global cognition 9,10. However, the impact of APOE-e4 phenotypes on the association of dietary fat and cognitive function is not fully understood. The present study further examines the association between intakes of different fat types and the influence of the APOE-e4 risk allele on global cognition among older adults.
Material and Methods
Study population
The analytic sample was drawn from the Chicago Healthy Aging Project (CHAP), a longitudinal, bi-racial, population-based study of AD dementia 11. The CHAP study recruited and enrolled participants 65 years or older from neighborhoods of south side Chicago. Details of the study design were published previously11. In 1993, 6158 individuals participated in an in-home interview. Information on demographic variables, health status, and current functioning was collected. Physical and cognitive performance were assessed as part of the in-home interview. The interview was conducted in three-year intervals up to six cycles. 62% of CHAP participants were self-identified as African Americans (AA).
Cognitive assessment
During the in-home interview at each cycle, we used four functioning tests to assess cognitive performance, including two measures of episodic memory: immediate and delayed recall in the East Boston Story 12,13, one measure of perceptual speed: the oral form of the Symbol Digit Modalities Test 14, and the Mini-Mental State Examination 15. The raw score of each test was transformed to z scores using the mean and standard deviations from the total population at baseline. We created a composite z score to assess global cognition by averaging z scores from all four tests, with positive scores suggesting better performance 16. Combining all four tests creates a global measure based on our previous principal components analysis, with all four tests having loadings of 0.79 or higher on a single factor, accounting for 74% of the variance17. This approach reduces the potential effects of skewness and floor or ceiling effects that might come from individual tests.
Dietary assessment
We used a validated semi-quantitative food frequency questionnaire (FFQ) with over 144 items to measure the usual intake of foods, vitamins, and minerals 18. The use of modified FFQ in older Chicago residents was validated and reported in our previous publication 18. Participants were asked about their average consumption frequency of each food with pre-determined portions over the preceding year. USDA and Harvard food composition databases were used to calculate the nutrient intake. The average fat intake was calculated by multiplying the consumption of each food by its fat content and summing the fat intake across all food items. The intake of varying types of fat was expressed as a percent of total energy intake. Supplements of LC n-3 fatty acids were not included in these analyses.
Apolipoprotein E e4-allele
Two single nucleotide polymorphisms (SNPs), rs7412 and rs429358, were used to determine APOE-e4 genotypes. We used the hME Sequenom MassARRAY platform (Sequenom, Inc., San Diego, CA) to measure these SNPs at the Broad Institute at Harvard University (Cambridge, Massachusetts) 19. For SNP rs7412 and SNP rs429358, genotyping call rates were 100% and 99.8%, respectively. An indicator variable was created based on the two SNPs for participants with one or more copies of the APOE-e4 risk allele.
Assessment of Covariates
Participants’ social and demographic characteristics, for instance, age (years), sex (male/female), education (number of years in school), smoking status (never smoker versus current or former smoker), and ethnicity, were collected using the 1990 US Census questionnaire during the in-home interview 17. Weight (kg) and height (meters) were also measured and later calculated to BMI (kg/meter2). The cognitive activity score was derived from a composite measure of the frequency of participation in 7 cognitive activities 17. The history of myocardial infarction or use of digitalis determined heart disease. Stroke history was self-reported. Hypertension was determined by self-reported or measured systolic blood pressure of 160 mm Hg or higher or diastolic blood pressure of 95 mm Hg or higher. Diabetes mellitus was either self-reported or the use of antidiabetic medication.
Statistics
The primary outcome was the decline rate in global cognition. We used the dietary fat intake from the first available FFQ for the primary analysis. We included participants who responded to the FFQ, had APOE phenotype assessment, and had more than two cognitive assessments. We excluded participants with implausible energy intake (<600 or >3500 kcal/d for women or <800 or >4200 kcal/d for men) or with BMI > 55 or MMSE < 10. The final analyses included 3360 participants (Supplemental Figure 1).
Each dietary fat variable was modeled as continuous variables expressed as a percentage of total calorie intake and categorical variables in quintiles based on the present data distributions. Data are presented as mean and standard deviations (SDs) for continuous variables and frequency (%) for categorical variables. Dietary fat variable was first modeled as a continuous variable.The β coefficient represents the rate of global cognitive decline during the follow-up period. We then modeled the dietary fat variable as categorical variables, the β coefficient represents the differences in the decline rate in global cognition compared to the reference group. A higher β coefficient represents a slower rate of decline in global cognition.
We used linear mixed models to examine the association between each type of dietary fat exposure and the longitudinal change in global cognition. The mixed model allows for within-individual and between-individual variations. The basic model (Model 1) of longitudinal change in cognition included age (years), sex (F/M), education (years), calorie (kcal), smoking status, race, time, and their respective interactions with time. Time was defined as years of follow-up since baseline (time of first available FFQ). We also adjusted models for cardiovascular comorbidities, including history of hypertension, diabetes, myocardial infarction, stroke, and their respective interactions with time. SAS version 9.4 was used for data analysis with a type 1 error rate for significance at 0.05, and all tests were 2-sided.
Results
The mean (SD) age of participants was 74.1 (6.3) years for those with APOE-e4 risk allele carriers and 73.3 (6.0) years for the non-carriers (Table 1), with a higher proportion of AA participants (55.1%) among the risk allele carriers. The mean consumption of total fat, animal fat, and saturated fat, as percent energy (SD), was 30.7% (5.5), 14.5% (4.4), and 9.9% (2.4) for APOE-e4 carriers which was marginally higher compared to non-carriers (Supplemental Figure 2).
Table 1.
Baseline characteristics of CHAP study participants with or without APOE-ɛ4 risk allele
| All (N=3360) | APOE-e4 non-carrier (N=2263) | APOE-e4 carrier (N = 1097) | ||
|---|---|---|---|---|
|
| ||||
| Age, y | 73.9 (6.2) | 74.1 (6.3) | 73.3 (6.0) | <0.001*** |
| Education, y | 13.0 (3.4) | 13.0 (3.4) | 13.0 (3.4) | 0.79 |
| Former smoker, No. (%) | 1355 (40) | 908 (40) | 0447 (41) | 0.74 |
| Body mass index | 27.9 (5.6) | 27.9 (5.6) | 28.1 (5.5) | 0.32 |
| Calories, kcal | 1714 (592) | 1716 (589) | 1709 (596) | 0.74 |
| Sex, No. (% F) | 2146 (64) | 1469 (65%) | 677 (62%) | 0.07 |
| Race, No. (% black) | 1972 (59) | 1242 (55%) | 730 (67%) | <0.001*** |
| Total Fat (%) | 30.5 (5.4) | 30.7 (5.5) | 30.2 (5.4) | 0.015** |
| Animal fat (%) | 14.3 (4.4) | 14.5 (4.4) | 14.1 (4.4) | 0.015** |
| Vegetable fat (%) | 16.2 (4.0) | 16.2 (4.1) | 16.1 (3.9) | 0.53 |
| Saturated fat (%) | 9.8 (2.4) | 9.9 (2.4) | 9.6 (2.3) | <0.001*** |
| Monounsaturated fat (%) | 11.3 (2.2) | 11.4 (2.2) | 11.2 (2.2) | 0.05 |
| Polyunsaturated fat (%) | 6.5 (1.5) | 6.5 (1.5) | 6.5 (1.5) | 0.85 |
| Long chain n-3 fatty acid (%) | 0.08 (0.05) | 0.08 (0.05) | 0.08 (0.05) | 0.78 |
| Trans fat (%) | 1.9 (0.6) | 1.9 (0.6) | 1.8 (0.6) | 0.59 |
Abbreviation: APOE, apolipoprotein E.
Data are mean (SD) for continuous variables, n (%) for categorical variables.
Fat intakes are expressed as % energy.
P-values were calculated using
Welch Two Sample t-test; Wilcoxon rank sum test; Pearson’s Chi-squared test
Higher intakes of total fat and SFA were associated with a faster decline of global cognition among participants with the APOE-e4 risk allele. Among these high-risk participants, an increase of 5% calories from total fat and SFA was associated with a faster decline in global cognitive score by −0.006 SDU (SD 0.003, P=0.039) and −0.02 SDU (SD 0.007, P = 0.004), respectively (Table 2). For individuals with APOE-e4 allele, those in the highest quintile of saturated fat consumption had a more rapid decline in global cognitive score by −0.037 SDU (SD 0.011) than those in the lowest quintile (Table 3).
Table 2.
Association of intakes of total fat and varying types of fat (%) and annual cognitive function change in participants with or without APOE-ɛ4 allele
| Fat intake | Participants with APOE-e4 allele† | P-value | Participants without APOE-e4 allele† | P-value | |
|---|---|---|---|---|---|
|
| |||||
| Total fat (5%) | Model 1 | −0.007 (0.003) | 0.022 | 0.000 (0.002) | 0.778 |
| Model 2 | −0.006 (0.003) | 0.039 | −0.001 (0.001) | 0.621 | |
| Saturated fat (5%) | Model 1 | −0.020 (0.007) | 0.004 | −0.001 (0.004) | 0.731 |
| Model 2 | −0.020 (0.007) | 0.004 | −0.002 (0.004) | 0.575 | |
| Monounsaturated fat (5%) | Model 1 | −0.011 (0.007) | 0.110 | 0.000 (0.004) | 0.994 |
| Model 2 | −0.010 (0.007) | 0.159 | −0.001 (0.004) | 0.799 | |
| Polyunsaturated fat (5%) | Model 1 | −0.009 (0.011) | 0.401 | −0.001 (0.005) | 0.860 |
| Model 2 | −0.005 (0.010) | 0.622 | −0.001 (0.005) | 0.859 | |
| Long-chain n-3 fatty acid (1%)* | Model 1 | 0.021 (0.010) | 0.044 | −0.003 (0.006) | 0.621 |
| Model 2 | 0.024 (0.010) | 0.023 | −0.002 (0.006) | 0.719 | |
| Trans fat (1%) | Model 1 | −0.010 (0.007) | 0.147 | 0.001 (0.003) | 0.758 |
| Model 2 | −0.009 (0.007) | 0.212 | 0.002 (0.003) | 0.548 | |
| Animal fat (5%) | Model 1 | −0.005 (0.004) | 0.138 | −0.002 (0.002) | 0.286 |
| Model 2 | −0.005 (0.004) | 0.203 | −0.003 (0.002) | 0.159 | |
| Vegetable fat (5%) | Model 1 | −0.006 (0.004) | 0.116 | 0.002 (0.002) | 0.434 |
| Model 2 | −0.006 (0.004) | 0.137 | 0.002 (0.002) | 0.390 | |
: Data are expressed as β (SD) representing the additional decline due to a 5% or 1% increase in intakes of varying types of fat.
Long-chain n-3 fatty acid from food sources only without supplementation
Abbreviation: APOE, Apolipoprotein E
Model 1 was adjusted for age (years), sex (F/M), education (years), calorie (kcal), smoking status, race, and their respective interactions with time.
Model 2 was Model 1 further accounted for numbers of cardiovascular disease comorbidities
Table 3.
Association of intakes of total fat and varying types of fat in quintiles and annual cognitive function change in participants with or without APOE-ɛ4 allele
| Q1 (n=762) | Q2† (n=763) | Q3† (n=763) | Q4† (n=763) | Q5† (n=762) | |
|---|---|---|---|---|---|
|
| |||||
| Total fat (%) | |||||
| Median (min, max) | 23.6 (8.7, 26.2) | 28.1 (26.2, 29.5) | 30.9 (29.5, 32.2) | 33.5 (32.2, 35.0) | 37.3 (35.0, 54.9) |
| with APOE-e4 | Ref. | −0.004 (0.009) | −0.008 (0.010) | −0.019 (0.010) | −0.015 (0.010) |
| without APOE-e4 | Ref. | −0.007 (0.005) | −0.003 (0.005) | −0.005 (0.005) | −0.004 (0.005) |
| Saturated fat (%) | |||||
| Median (min, max) | 7.0 (1.9, 8.0) | 8.7 (8.0, 9.3) | 9.8 (9.3, 10.3) | 10.9 (10.3, 11.7) | 12.9 (11.7, 20.4) |
| with APOE-e4 | Ref. | 0.002 (0.009) | −0.016 (0.009) | −0.001 (0.009) | −0.037 (0.011) |
| without APOE-e4 | Ref. | 0.000 (0.005) | 0.001 (0.005) | 0.001 (0.005) | −0.004 (0.005) |
| Monounsaturated fat (%) | |||||
| Median (min, max) | 8.5 (2.7, 9.6) | 10.3 (9.6, 10.9) | 11.4 (10.9, 12.0) | 12.5 (12.0, 13.1 ) | 14.1 (13.1, 24.5) |
| with APOE-e4 | Ref. | −0.003 (0.010) | −0.001 (0.009) | −0.019 (0.010) | −0.009 (0.010) |
| without APOE-e4 | Ref. | −0.007 (0.005) | −0.006 (0.005) | −0.002 (0.005) | −0.003 (0.005) |
| Polyunsaturated fat (%) | |||||
| Median (min, max) | 4.7 (1.5, 5.3) | 5.7 (5.3, 6.1) | 6.4 (6.1, 6.7) | 7.1 (6.7, 7.7) | 8.4 (7.7, 15.2) |
| with APOE-e4 | Ref. | −0.006 (0.010) | 0.001 (0.010) | −0.002 (0.010) | −0.010 (0.010) |
| without APOE-e4 | Ref. | 0.005 (0.005) | 0.003 (0.005) | −0.002 (0.005) | −0.001 (0.005) |
| Long-chain n-3 fatty acid* (%) | |||||
| Median (min, max) | 0.02 (0.0, 0.03) | 0.04 (0.03, 0.05) | 0.06 (0.05, 0.07) | 0.09 (0.07, 0.10) | 0.14 (0.10, 0.45) |
| with APOE-e4 | Ref. | 0.017 (0.014) | 0.034 (0.014) | 0.022 (0.015) | 0.036 (0.016) |
| without APOE-e4 | Ref. | −0.003 (0.008) | −0.003 (0.009) | −0.005 (0.009) | 0.003 (0.010) |
| Trans fat (%) | |||||
| Median (min, max) | 1.2 (0.5, 1.3) | 1.5 (1.4, 1.6) | 1.8 (1.6, 1.9) | 2.1 (1.9, 2.3) | 2.7 (2.3, 5.4) |
| with APOE-e4 | Ref. | 0.004 (0.012) | 0.004 (0.012) | −0.004 (0.012) | −0.020 (0.013) |
| without APOE-e4 | Ref. | 0.004 (0.006) | 0.004 (0.006) | 0.004 (0.006) | 0.001 (0.006) |
| Animal fat (%) | |||||
| Median (min, max) | 9.2 (1.0, 10.8) | 12.1 (10.8, 13.1) | 14.1 (13.1, 15.2) | 16.4 (15.3, 17.8) | 20.2 (17.8, 34.0) |
| with APOE-e4 | Ref. | 0.018 (0.009) | −0.013 (0.010) | 0.008 (0.009) | −0.014 (0.010) |
| without APOE-e4 | Ref. | −0.006 (0.005) | 0.000 (0.005) | −0.006 (0.005) | −0.004 (0.005) |
| Vegetable fat (%) | |||||
| Median (min, max) | 11.3 (3.8, 12.8) | 14.0 (12.9, 15.0) | 16.1 (15.0, 17.1) | 18.1 (17.1, 19.4) | 21.3 (19.4, 37.9) |
| with APOE-e4 | Ref. | −0.002 (0.010) | −0.009 (0.010) | −0.009 (0.010) | −0.014 (0.010) |
| without APOE-e4 | Ref. | 0.006 (0.005) | 0.003 (0.005) | 0.007 (0.005) | 0.001 (0.005) |
: Data are expressed as β (SD) representing the additional change to the rate of annual cognitive function change compared to Q1.
Long-chain n-3 fatty acid from food sources only without supplementation Abbreviation: APOE, Apolipoprotein E
Model adjusted for age (years), sex (F/M), education (years), calorie (kcal), smoking status, race, numbers of cardiovascular disease comorbidities, and their respective interactions with time.
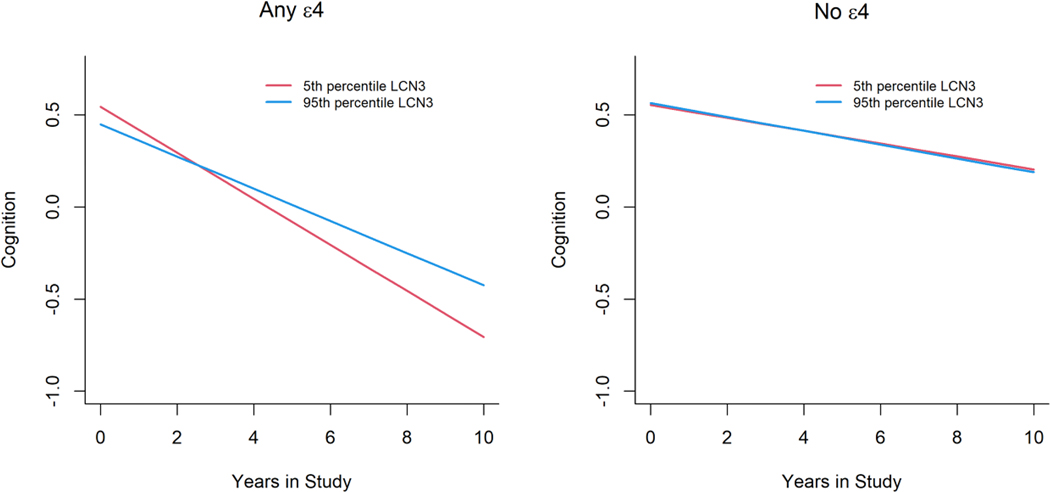
In contrast, higher intakes of dietary LC n-3 fatty acid were associated with a significantly slower cognitive decline in individuals with the APOE-e4 risk allele. Every 1%-energy increment from LC n-3 fatty acid was associated with a slower global cognitive decline by 0.024 SDU (SD 0.010, P=0.023, Table 2). Among APOE-e4 carriers, individuals in the highest quintile of LC n-3 fatty acid intake had a significantly slower global cognitive score decline by 0.036 SDU (SD 0.016) compared with those in the lowest quintile (Table 3).
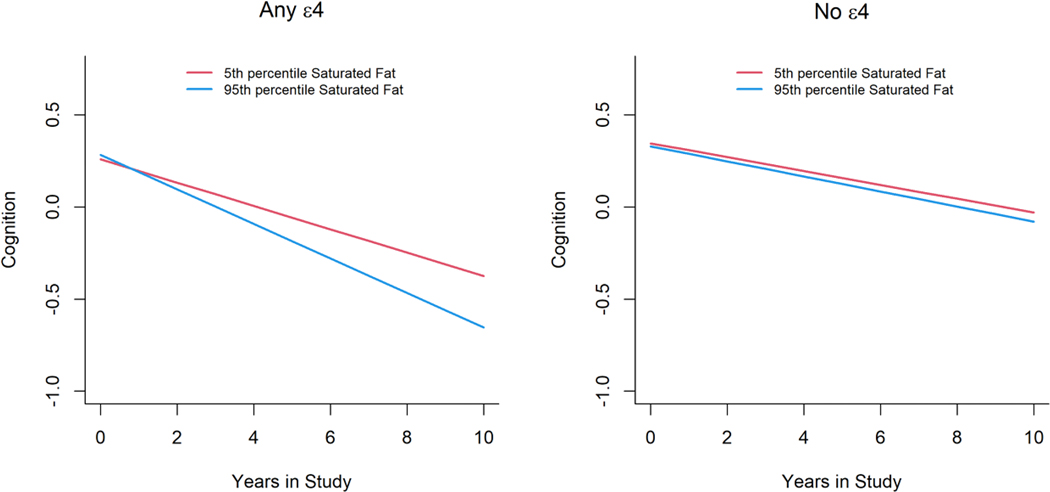
Among APOE-e4 carriers, a person at the 95th percentile of SFA intake (14.2% energy) has a 33% faster decline (Figure 1) compared to a person at the 5th quantile (6.3% energy). In contrast, the effects on cognition for a person at the 95th percentile of LC n-3 fatty acids from food sources (0.15% energy, equivalent to 248 mg)) had a 30.4% slower decline in global cognition compared to a person at the 5th percentile (0.01% energy, equivalent to 19 mg) (Figure 2). There was no association between intakes of different types of fat and global cognition among APOE-e4 non-carriers.
Figure 1.
Intakes of saturated fat (%) and annual cognitive function change among participants with or without APOE-ɛ4 allele (n=3360).
Intakes of saturated fat (%) were modeled and accounted for age (years), sex (F/M), education (years), calorie (kcal), smoking status, race, numbers of cardiovascular disease comorbidities, and their respective interactions with time.
Red line represents participants with a saturated fat intake at the 5th percentile.
Blue line represents participants with a saturated fat intake at the 95th percentile.
Figure 2.
Intakes of long-chain n-3 fatty acid (%) and annual cognitive function change among participants with or without APOE-ɛ4 allele (n=3360).
Intakes of long-chain n-3 fatty acid (%) were modeled and accounted for age (years), sex (F/M), education (years), calorie (kcal), smoking status, race, numbers of cardiovascular disease comorbidities and their respective interactions with time.
Red line represents participants with long-chain n-3 fatty acid intake at the 5th percentile.
Blue line represents participants with long-chain n-3 fatty acid intake at the 95th percentile.
Discussion
In the present study, we observed that a higher intake of SFA was associated with a significantly faster decline in global cognition among individuals with a genetic predisposition to AD. In contrast, higher intakes of LC n-3 fatty acid were associated with slower decline among those with the APOE-e4 risk allele. In the present study, the mean total and SFA intake was 30.6% and 9.9 % at baseline year. The average intake of LC n-3 fatty acid was 0.08% (130 mg) per day. The consumption levels were compatible with intakes of total fat and SFA reported in the Nurses’ Health Study and Health Professional Follow-Up Study 20. Our findings highlight the critical roles of dietary fats in preventing cognitive decline in older adults with a genetic predisposition to AD.
Higher intakes of SFA were associated with an increased risk of AD 9. Two studies examined the relationship between SFA and dementia risk and reported a more profound risk in APOE-e4 carriers 21, with one study limited to mid-age cognitively healthy adults 22. APOE plays a critical role in lipid transport. In the brain, APOE-e4 has effects on astrocytes and lipid metabolism 7. Evidence from the transgenic animal model demonstrated that APOE-e4 increased brain inflammation through upregulated expression of NF-kappaB-regulated genes in response to inflammatory insults 23. Diets high in SFA induced inflammation 24–26. Given the suggested increased brain inflammation associated with APOE-e4, a diet high in SFA might partially exacerbate the adverse effects of inflammation. APOE-e4 also affects brain lipid metabolism. A recent investigation demonstrated that APOE-e4 has detrimental effects on brain fatty acid metabolism and homeostasis, which accelerated lipid dysregulation and energy deficits, increasing AD risk among APOE-e4 carriers 6. Higher intake of SFA might contribute to an unfavorable fatty acid profile in the brain, leading to adverse effects in combination with APOE-e4.
Previous evidence showed that higher fish consumption was associated with slower cognitive decline and risk of AD10,27. Fatty fish are rich sources of LC n-3 fatty acids, including DHA and EPA. DHA is essential for brain function. Previous evidence suggested that the fatty acid composition of AD brains was modified 28 compared to the non-AD brains with an increase in SFA component and a decrease in long-chain PUFA 29. Emerging evidence supports that fatty acid homeostasis is different between APOE-e4 carriers versus non-carriers. One study with a small sample size demonstrated that APOE-e4 carriers utilized DHA at a higher rate through beta-oxidation compared to non-carriers 30, and a higher rate of incorporating DHA in several brain regions was reported in younger adults using PET imaging 31. Hence, we hypothesize that a higher dietary intake of LC n-3 fatty acid, including DHA, may quench the higher metabolic demand among APOE-e4 carriers, thus preserving global cognition and preventing cognitive decline. In addition, evidence from animal models suggested other targeted pathways involved in DHA improved AD pathologies and preserved microglia function 7. APOE-e4 carriers are prone to exhibit BBB dysfunction, which can impair DHA transportation to the brain 32. Therefore, a higher dose of DHA may be needed to compensate for the genetic disadvantage. In the present study, we observed that individuals at the highest consumption levels among the carriers were associated with a significantly slower rate of decline in global cognition. Our findings and previous evidence suggest compensatory mechanisms for an increased metabolism and utilization of LC-n3 PUFAs compared to non-carriers.
It is worth noting that macronutrients are not consumed in isolation but in the matrix of foods and as part of dietary patterns. Epidemiological evidence suggests various dietary patterns, i.e., the Meddiet, MIND, and DASH, were preventive for cognitive decline. However, the recently published randomized controlled trial on the MIND diet on cognitive decline showed minimal effects. It is plausible that the dietary intervention might be associated with and specific to APOE-e4 carriers.
The present study has several strengths, including its prospective design in a biracial community, the in-person evaluation of global cognitive function, and a large sample size of participants with APOE-e4 genotype assessment. There are a few limitations to be considered. First, the dietary fat intake was self-reported FFQ, although previous studies demonstrated the validity of FFQ using objective biomarkers in older adults 18,33. In the present study, we examined the association of dietary data and cognitive decline using first available FFQ; therefore, we were unable to capture causal associations of changes of diet and cognitive function over time. We excluded participants with MMSE less than 10 to address potential recall errors related to cognitive decline. Given the observational study design, residual confounding from other factors is possible. We must caution against a causal interpretation of findings. Future studies are warranted to validate our findings using objective blood markers and further investigate the mechanisms of dietary fat on cognition.
Conclusions
In the present study, the association of intakes of different dietary fat and decline in global cognition was specific to APOE-e4 carriers. Among those APOE-e4 carriers, higher intakes of unhealthy fat were associated with a faster decline in global cognition, whereas a higher intake of LC-n3 PUFAs was associated with a slower decline. The mechanisms targeted on fatty acids metabolism and utilization in the brain among high-risk populations warrant further research. Precision nutrition targeting metabolic pathways altered by APOE4 might provide a tool for preventing the disease.
Supplementary Material
Acknowledgements
We thank the participants of the CHAP study and their families for their dedication to research. We appreciate all research staff’s efforts in collecting data; their tireless efforts truly keep the research going.
Funding sources
The present work was supported by Alzheimer’s Association Research Grant AARG-22-928311, National Institutes of Health grants R01AG058679, and R01AG073627.
Footnotes
Author disclosures
The authors report no conflicts of interest.
Xiaoran Liu and Todd Beck completed the statistical analysis.
References:
- 1.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes LL, Dhana K, Liu X, et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N Engl J Med. 2023;389(7):602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedberg JS, Aytan N, Cherry JD, et al. Associations between brain inflammatory profiles and human neuropathology are altered based on apolipoprotein E ε4 genotype. Sci Rep. 2020;10(1):2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–112. [DOI] [PubMed] [Google Scholar]
- 6.Qi G, Mi Y, Shi X, Gu H, Brinton RD, Yin F. ApoE4 Impairs Neuron-Astrocyte Coupling of Fatty Acid Metabolism. Cell Rep. 2021;34(1):108572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norwitz NG, Saif N, Ariza IE, Isaacson RS. Precision Nutrition for Alzheimer’s Prevention in ApoE4 Carriers. Nutrients. 2021;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. [DOI] [PubMed] [Google Scholar]
- 9.Morris MC, Evans DA, Bienias JL, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60(2):194–200. [DOI] [PubMed] [Google Scholar]
- 10.Morris MC, Evans DA, Bienias JL, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60(7):940–946. [DOI] [PubMed] [Google Scholar]
- 11.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis. 2003;5(5):349–355. [DOI] [PubMed] [Google Scholar]
- 12.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci. 1991;57(3–4):167–178. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 14.Smith A Symbol Digit Modalities Test (SDMT): Manual. Los Angeles,CA:Western Psychological;. 1982. [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 16.Wilson RS, Beckett LA, Bennett DA, Albert MS, Evans DA. Change in cognitive function in older persons from a community population: relation to age and Alzheimer disease. Arch Neurol. 1999;56(10):1274–1279. [DOI] [PubMed] [Google Scholar]
- 17.Wilson RS, Bennett DA, Beckett LA, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54(3):P155–160. [DOI] [PubMed] [Google Scholar]
- 18.Morris MC, Tangney CC, Bienias JL, Evans DA, Wilson RS. Validity and reproducibility of a food frequency questionnaire by cognition in an older biracial sample. Am J Epidemiol. 2003;158(12):1213–1217. [DOI] [PubMed] [Google Scholar]
- 19.Rajan KB, McAninch EA, Wilson RS, Weuve J, Barnes LL, Evans DA. Race, APOEɛ4, and Long-Term Cognitive Trajectories in a Biracial Population Sample. J Alzheimers Dis. 2019;72(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Li Y, Tobias DK, et al. Changes in Types of Dietary Fats Influence Long-term Weight Change in US Women and Men. J Nutr. 2018;148(11):1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laitinen MH, Ngandu T, Rovio S, et al. Fat intake at midlife and risk of dementia and Alzheimer’s disease: a population-based study. Dement Geriatr Cogn Disord. 2006;22(1):99–107. [DOI] [PubMed] [Google Scholar]
- 22.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002;59(8):1258–1263. [DOI] [PubMed] [Google Scholar]
- 23.Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-kappaB signaling cascade. Neurobiol Dis. 2005;20(3):709–718. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC. Dietary fats and coronary heart disease. J Intern Med. 2012;272(1):13–24. [DOI] [PubMed] [Google Scholar]
- 25.Han SN, Leka LS, Lichtenstein AH, Ausman LM, Schaefer EJ, Meydani SN. Effect of hydrogenated and saturated, relative to polyunsaturated, fat on immune and inflammatory responses of adults with moderate hypercholesterolemia. J Lipid Res. 2002;43(3):445–452. [PubMed] [Google Scholar]
- 26.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79(6):969–973. [DOI] [PubMed] [Google Scholar]
- 27.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Fish consumption and cognitive decline with age in a large community study. Arch Neurol. 2005;62(12):1849–1853. [DOI] [PubMed] [Google Scholar]
- 28.Prasad MR, Lovell MA, Yatin M, Dhillon H, Markesbery WR. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem Res. 1998;23(1):81–88. [DOI] [PubMed] [Google Scholar]
- 29.Söderberg M, Edlund C, Kristensson K, Dallner G. Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids. 1991;26(6):421–425. [DOI] [PubMed] [Google Scholar]
- 30.Chouinard-Watkins R, Plourde M. Fatty acid metabolism in carriers of apolipoprotein E epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients. 2014;6(10):4452–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yassine HN, Croteau E, Rawat V, et al. DHA brain uptake and APOE4 status: a PET study with [1–11C]-DHA. Alzheimer’s Research & Therapy. 2017;9(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rawat V, Wang S, Sima J, et al. ApoE4 Alters ABCA1 Membrane Trafficking in Astrocytes. J Neurosci. 2019;39(48):9611–9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tangney CC, Bienias JL, Evans DA, Morris MC. Reasonable estimates of serum vitamin E, vitamin C, and beta-cryptoxanthin are obtained with a food frequency questionnaire in older black and white adults. J Nutr. 2004;134(4):927–934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.