Abstract
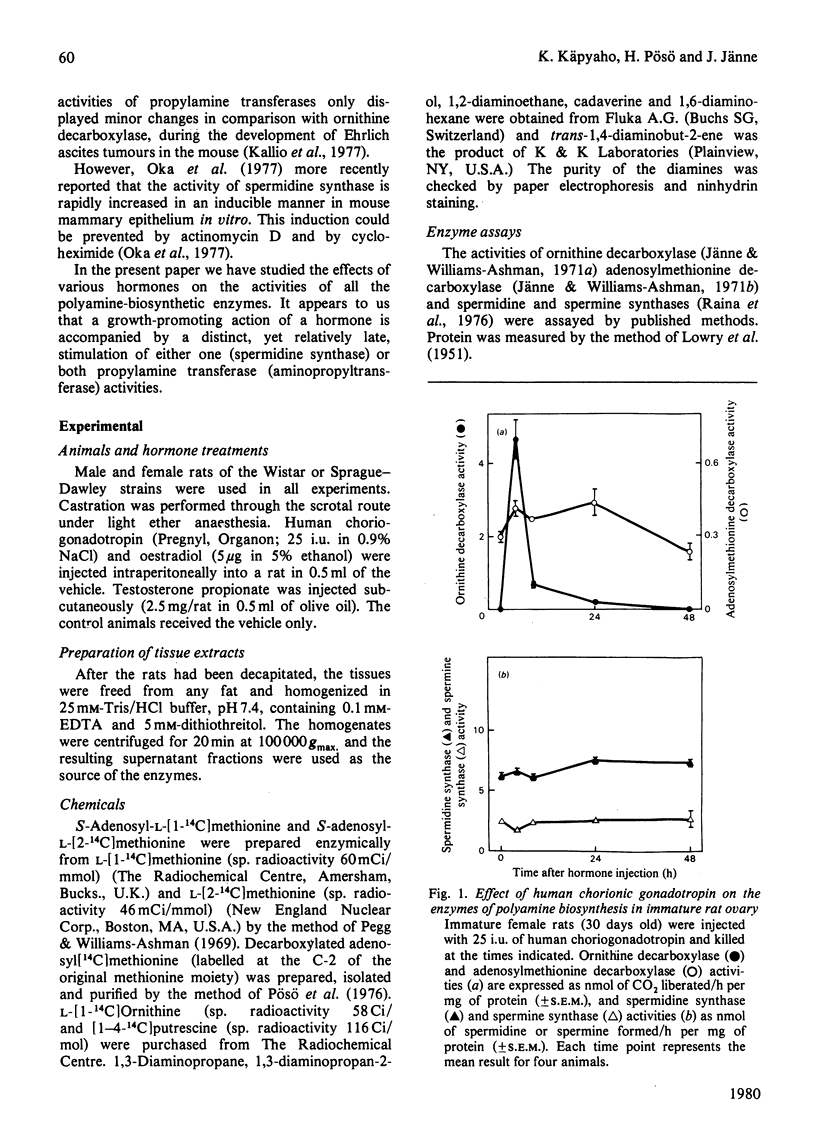
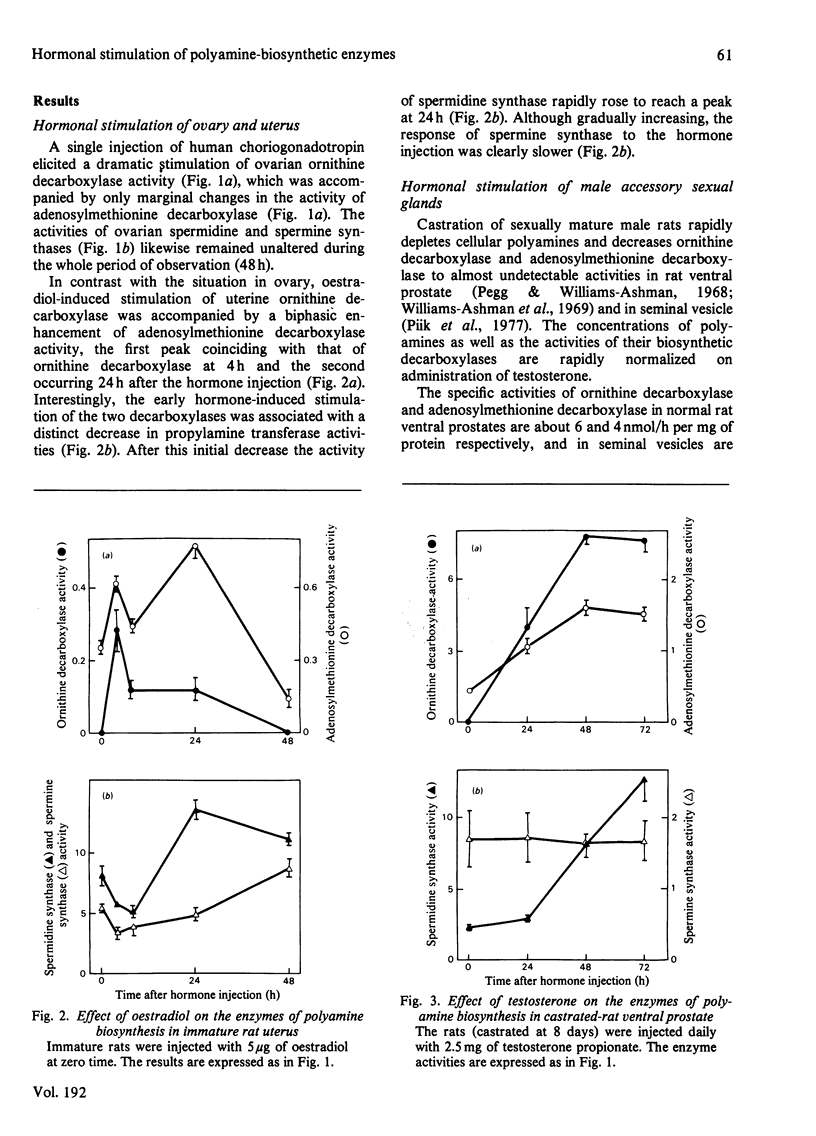
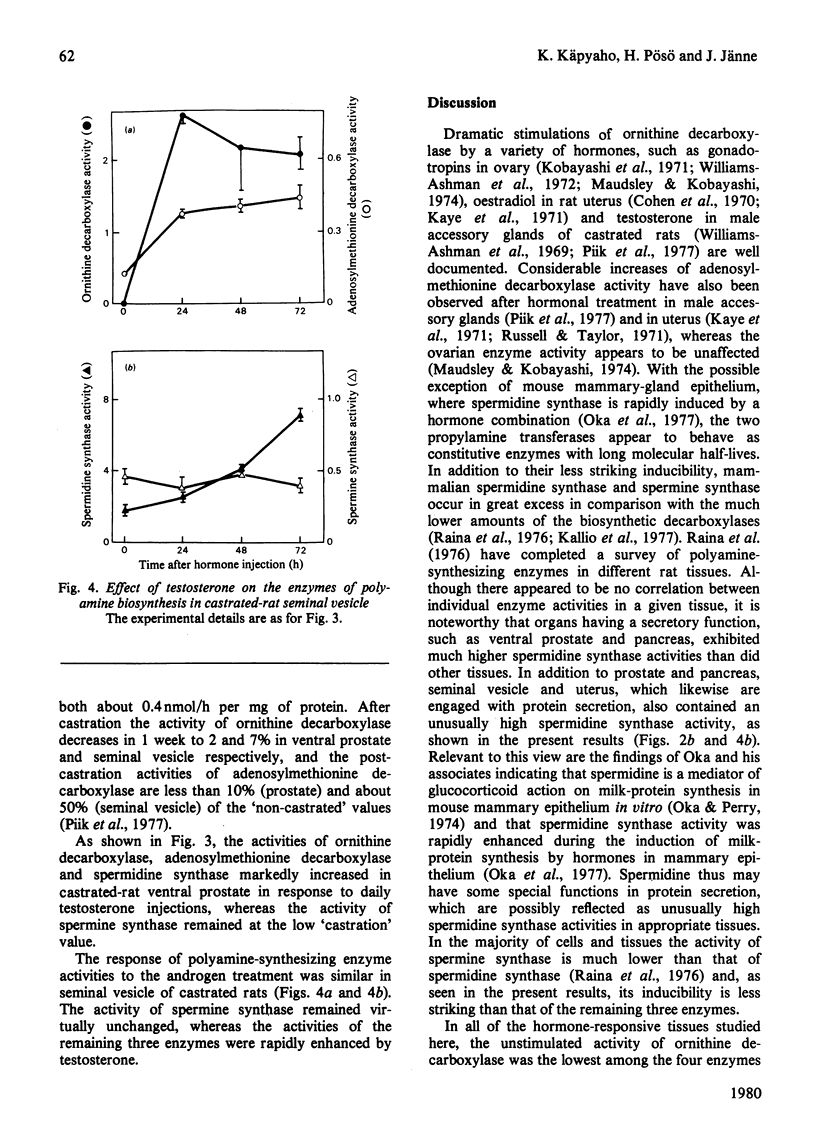
The effect of various hormones on the activities of the four enzymes engaged with the biosynthesis of the polyamines has been investigated in the rat. Human choriogonadotropin induced a dramatic, yet transient, stimulation of l-ornithine decarboxylase (EC 4.1.1.17) activity in rat ovary, with no or only marginal changes in the activities of S-adenosyl-l-methionine decarboxylase (EC 4.1.1.50), spermidine synthase (aminopropyltransferase; EC 2.5.1.16) or spermine synthase. A single injection of oestradiol into immature rats maximally induced uterine ornithine decarboxylase at 4h after the injection. This early stimulation of ornithine decarboxylase activity was accompanied by a distinct enhancement of adenosylmethionine decarboxylase activity and a decrease in the activities of spermidine synthase and spermine synthase. In the seminal vesicle of castrated rats, testosterone treatment elicited a striking and persistent stimulation of ornithine decarboxylase and adenosylmethionine decarboxylase activities. The activity of spermidine synthase likewise rapidly increased between the first and the second day after the commencement of the hormone treatment, whereas the activity of spermine synthase remained virtually unchanged during the whole period of observation. Testosterone-induced changes in polyamine formation in the ventral prostate were comparable with those found in the seminal vesicle, with the possible exception of a more pronounced stimulation of spermidine synthase activity. It thus appears that an enhancement in one or both of the propylamine transferase (aminopropyltransferase) activities in response to hormone administration is an indicator of hormone-dependent growth (uterus and the male accessory sexual glands), and is not necessarily associated with non-proliferative hormonal responses, such as gonadotropin-induced luteinization of the ovarian tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S., O'Malley B. W., Stastny M. Estrogenic induction of ornithine decarboxylase in vivo and in vitro. Science. 1970 Oct 16;170(3955):336–338. doi: 10.1126/science.170.3955.336. [DOI] [PubMed] [Google Scholar]
- Hannonen P., Raina A., Jänne J. Polyamine synthesis in the regenerating rat liver: stimulation of S-adenosyl methionine decarboxylase, and spermidine and spermine synthases after partial hepatectomy. Biochim Biophys Acta. 1972 Jun 26;273(1):84–90. doi: 10.1016/0304-4165(72)90194-8. [DOI] [PubMed] [Google Scholar]
- Hölttä E., Jänne J. Ornithine decarboxylase activity and the accumulation of putrescine at early stages of liver regeneration. FEBS Lett. 1972 Jun 1;23(1):117–121. doi: 10.1016/0014-5793(72)80298-9. [DOI] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Dissociation of putrescine-activated decarboxylation of S-adenosyl-L-methionine from the enzymic synthesis of spermidine and spermine by purified prostatic enzyme preparations. Biochem Biophys Res Commun. 1971 Jan 22;42(2):222–229. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kallio A., Pösö H., Guha S. K., Jänne J. Polyamines and their biosynthetic enzymes in Ehrlich ascites-carcinoma cells. Modification of tumour polyamine pattern by diamines. Biochem J. 1977 Jul 15;166(1):89–94. doi: 10.1042/bj1660089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye A. M., Icekson I., Lindner H. R. Stimulation by estrogens of ornithine and S-adenosylmethionine decarboxylases in the immature rat uterus. Biochim Biophys Acta. 1971 Oct;252(1):150–159. doi: 10.1016/0304-4165(71)90103-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kupelian J., Maudsley D. V. Ornithine decarboxylase stimulation in rat ovary by luteinizing hormone. Science. 1971 Apr 23;172(3981):379–380. doi: 10.1126/science.172.3981.379. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maudsley D. V., Kobayashi Y. Induction of ornithine decarboxylase in rat ovary after administration of luteinizing hormone or human chorionic gonadotrophin. Biochem Pharmacol. 1974 Oct 1;23(19):2697–2703. doi: 10.1016/0006-2952(74)90040-9. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Oka T., Perry J. W., Kano K. Hormonal regulation of spermidine synthase during the development of mouse mammary epithelium in vitro. Biochem Biophys Res Commun. 1977 Dec 7;79(3):979–986. doi: 10.1016/0006-291x(77)91206-2. [DOI] [PubMed] [Google Scholar]
- Oka T., Perry J. W. Spermidine as a possible mediator of glucocorticoid effect on milk protein synthesis in mouse mammary epithelium in vitro. J Biol Chem. 1974 Dec 10;249(23):7647–7652. [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. Biosynthesis of putrescine in the prostate gland of the rat. Biochem J. 1968 Jul;108(4):533–539. doi: 10.1042/bj1080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Piik K., Rajamäki P., Guha S. K., Jänne J. Regulation of L-ornithine decarboxylase and S-adenosyl-L-methionine decarboxylase in rat ventral prostate and seminal vesicle. Biochem J. 1977 Dec 15;168(3):379–385. doi: 10.1042/bj1680379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Raina A., Jänne J., Siimes M. Stimulation of polyamine synthesis in relation to nucleic acids in regenerating rat liver. Biochim Biophys Acta. 1966 Jul 20;123(1):197–201. doi: 10.1016/0005-2787(66)90173-0. [DOI] [PubMed] [Google Scholar]
- Raina A., Pajula R. L., Eloranta T. A rapid assay method for spermidine and spermine synthases. Distribution of polyamine-synthesizing enzymes and methionine adenosyltransferase in rat tissues. FEBS Lett. 1976 Sep 1;67(3):252–255. doi: 10.1016/0014-5793(76)80540-6. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Taylor R. L. Polyamine synthesis and accumulation in the castrated rat uterus after estradiol-17-beta stimulation. Endocrinology. 1971 Jun;88(6):1397–1403. doi: 10.1210/endo-88-6-1397. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Jänne J., Coppoc G. L., Geroch M. E., Schenone A. New aspects of polyamine biosynthesis in eukaryotic organisms. Adv Enzyme Regul. 1972;10:225–245. doi: 10.1016/0065-2571(72)90016-7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Pegg A. E., Lockwood D. H. Mechanisms and regulation of polyamine and putrescine biosynthesis in male genital glands and other tissues of mammals. Adv Enzyme Regul. 1969;7:291–323. doi: 10.1016/0065-2571(69)90024-7. [DOI] [PubMed] [Google Scholar]


