Abstract
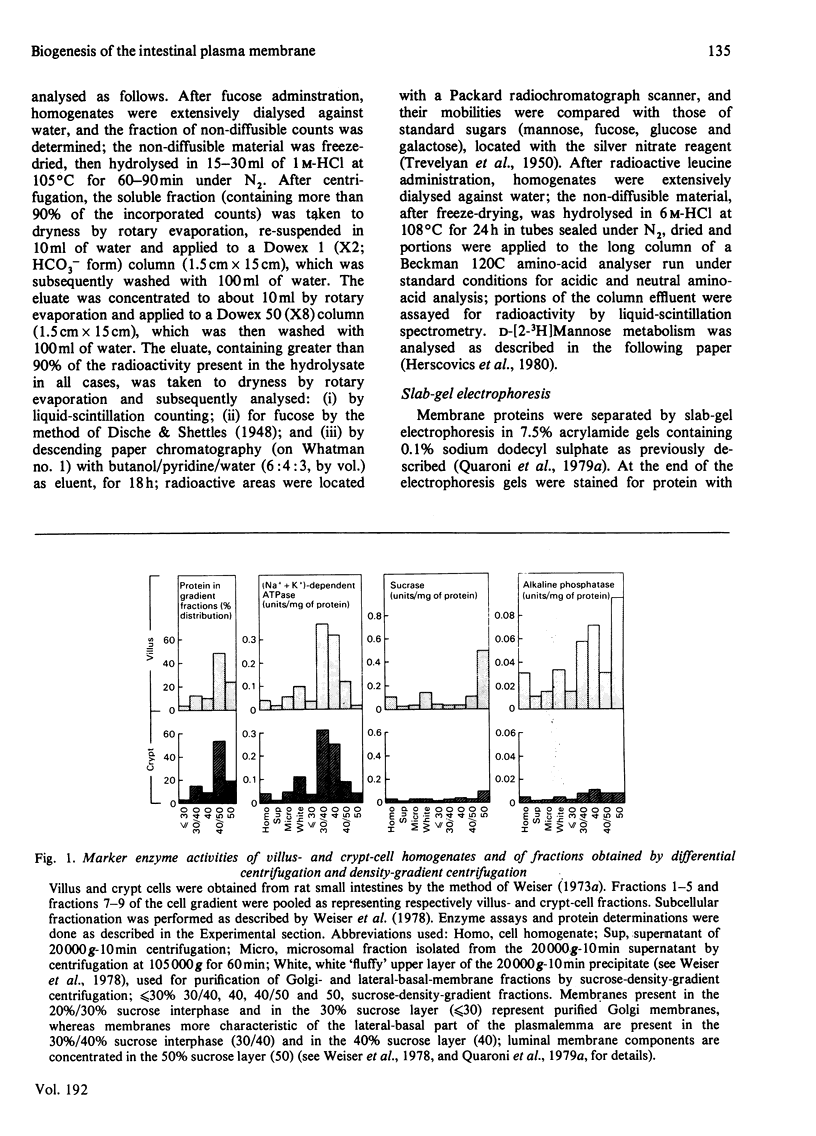
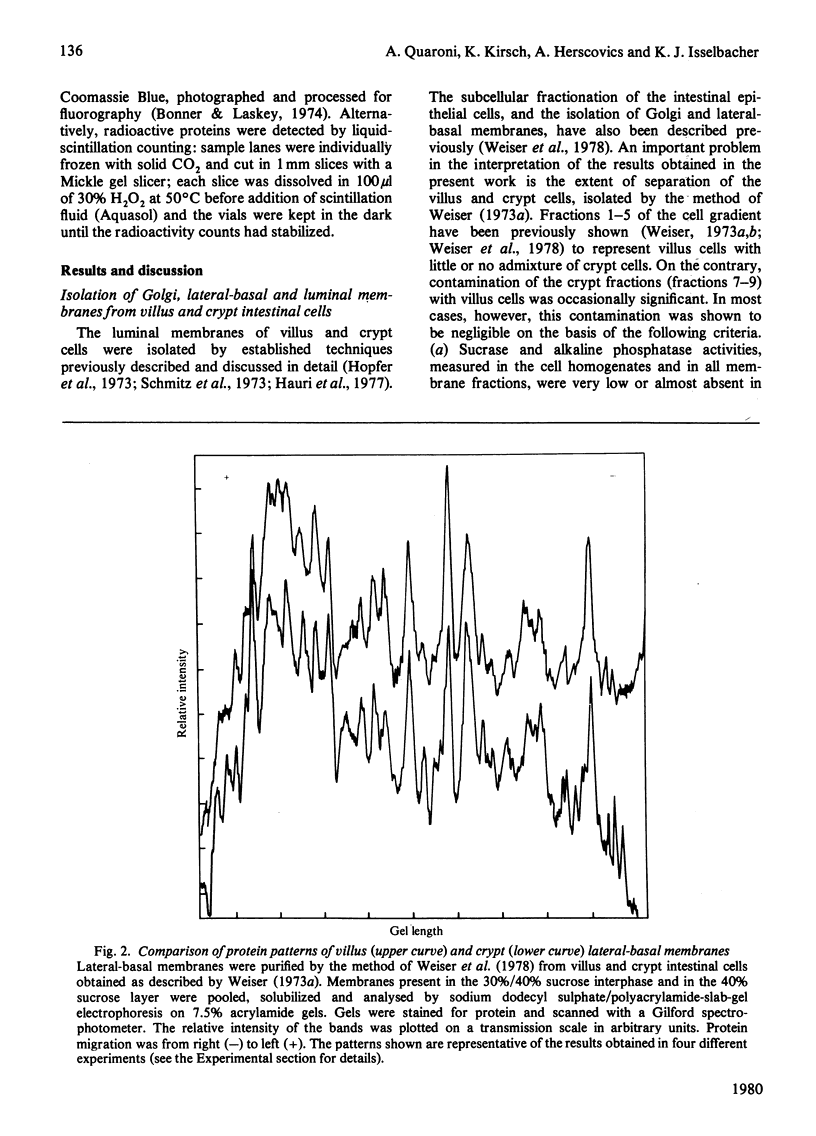
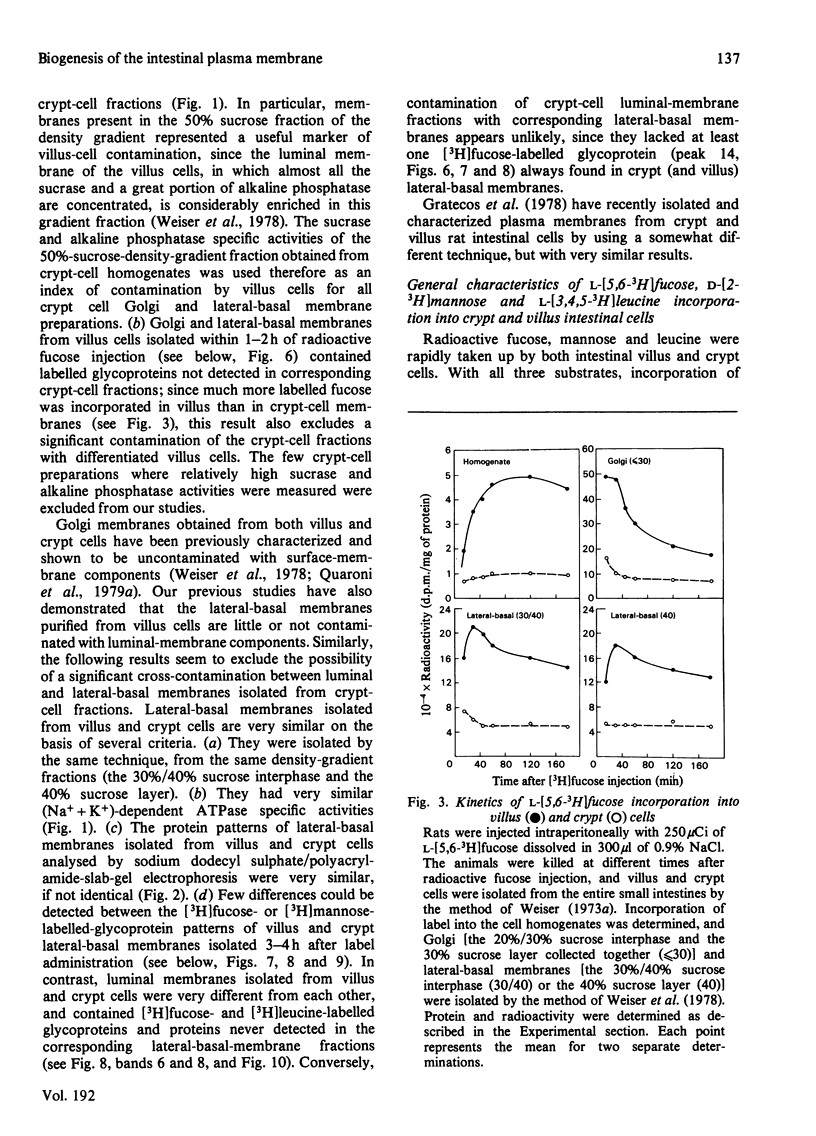
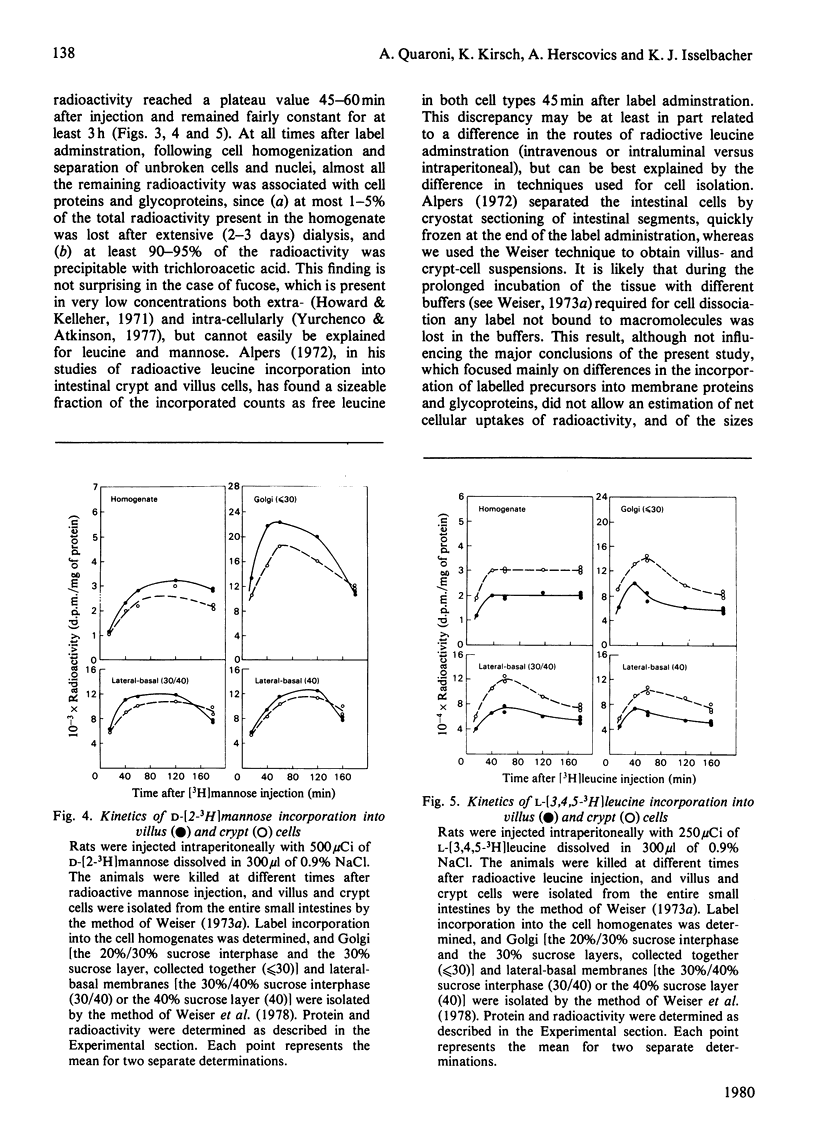
The biosynthesis of membrane proteins and glycoproteins has been studied in rat intestinal crypt and villus cells by measuring the incorporation of L-[5,6-3H] fucose, D-[2-3H] mannose and L-[3,4,5-3H] leucine, given intraperitoneally, into Golgi, lateral-basal and luminal membranes. Incorporation of leucine and mannose was approximately equal in crypt and villus cells, whereas fucose incorporation was markedly higher (3-4 times) in the differentiated villus cells. As previously reported [Quaroni, Kirsch & Weiser (1979) Biochem J. 182. 203-212] most of the fucosylated glyco-proteins synthesized in the villus cells and initially present in the Golgi and lateral-basal membranes were found re-distributed, within 3-4h of label administration, in the luminal membrane. A similar process appeared to occur in the crypt cells, where, however, only few fucose-labelled glycoproteins were identified. In contrast, most of the leucine-labelled and many mannose-labelled membrane components found in the lateral-basal membrane of both crypt and villus cells did not seen to undergo a similar re-distribution process. The fucosylated glycoproteins of the intestinal epithelial cells represent, therefore, a special class of membrane components, most of which appear with differentiation, that are selectively localized in the luminal portion of the plasmalemma. In contrast with the marked differences in protein and glycoprotein patterns between the luminal membrane of villus and crypt cells, only minor differences were found between their lateral-basal membrane components: their protein patterns on sodium dodecyl sulphate/polyacrylamide slab gels, and the patterns of fucose-, mannose- and leucine-labelled components (analysed 3-4h after label administration) were very similar. Although the minor differences detected may be of importance, it appears that most of the surface-membrane changes accompanying cell differentiation in the intestinal epithelial cells are localized in the luminal portion of their surface membrane.
Full text
PDF

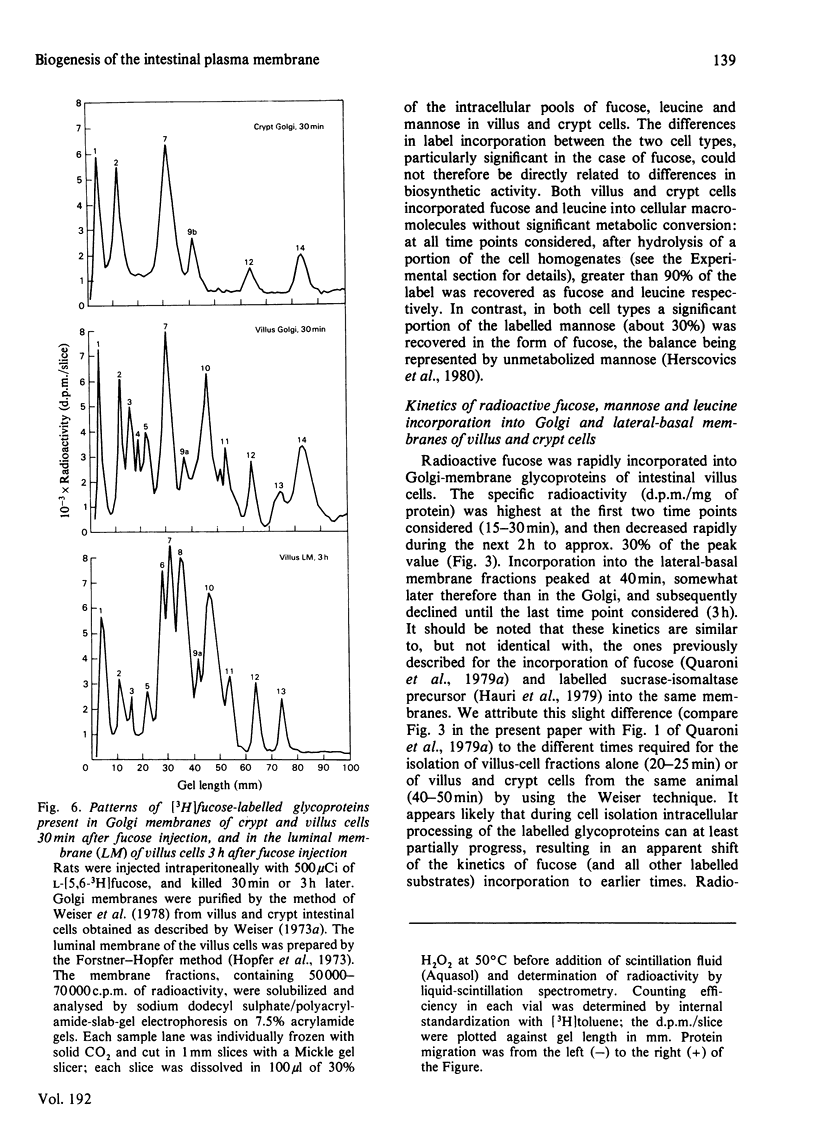
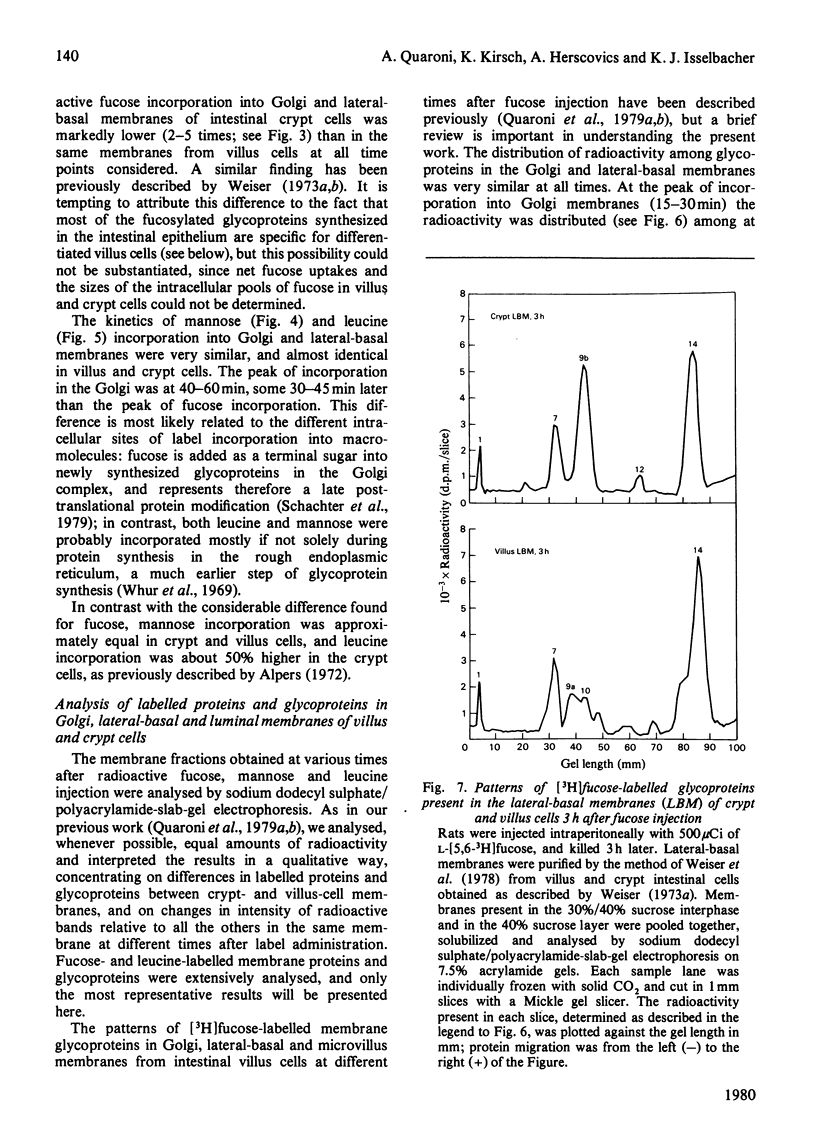
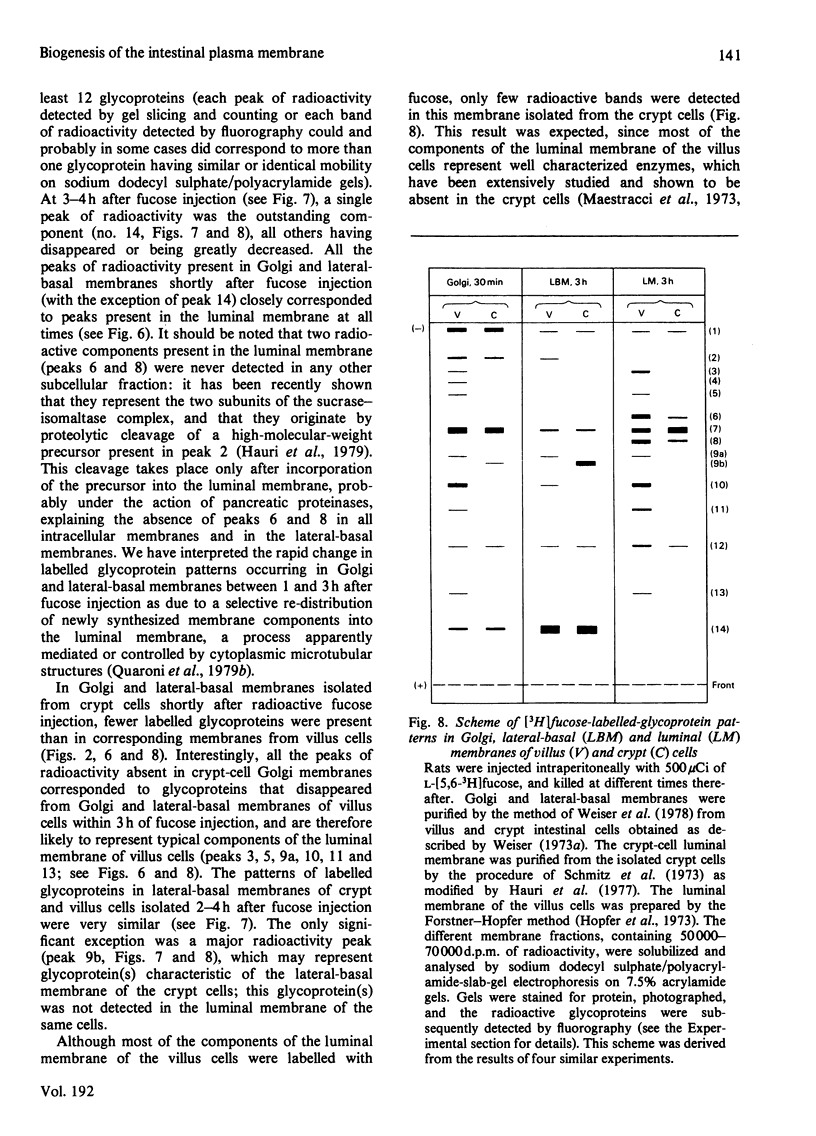
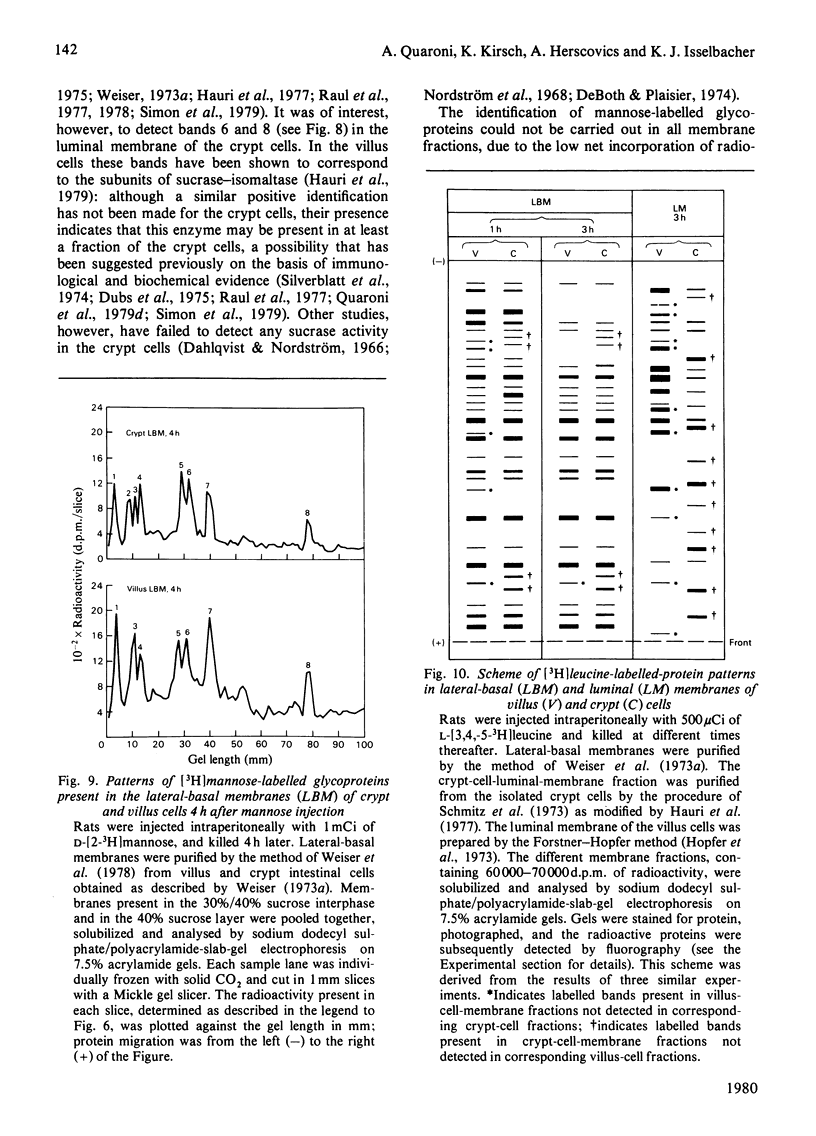


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H. Protein synthesis in intestinal mucosa: the effect of route of administration of precursor amino acids. J Clin Invest. 1972 Jan;51(1):167–173. doi: 10.1172/JCI106788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H. Protein turnover in intestinal mucosal villus and crypt brush border membranes. Biochem Biophys Res Commun. 1977 Mar 7;75(1):130–135. doi: 10.1016/0006-291x(77)91299-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bouhours J. F., Glickman R. M. Rat intestinal glycolipids. II. Distribution and biosynthesis of glycolipids and ceramide in villus and crypt cells. Biochim Biophys Acta. 1976 Jul 20;441(1):123–133. doi: 10.1016/0005-2760(76)90287-3. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. Mucosal architecture and epithelial cell production rate in the small intestine of the albino rat. J Anat. 1970 Nov;107(Pt 3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A., Nordström C. The distribution of disaccharidase activities in the villi and crypts of the small-intestinal mucosa. Biochim Biophys Acta. 1966 Mar 7;113(3):624–626. doi: 10.1016/s0926-6593(66)80024-3. [DOI] [PubMed] [Google Scholar]
- Devik F. Intestinal cell kinetics in irradiated mice. A quantitative investigation of the acute reaction to whole body roentgen irradiation. Acta Radiol Ther Phys Biol. 1971 Feb;10(1):129–149. doi: 10.3109/02841867109129750. [DOI] [PubMed] [Google Scholar]
- Dubs R., Gitzelmann R., Steinmann B., Lindenmann J. Catalytically inactive sucrase antigen of rabbit small intestine: the enzyme precursor. Helv Paediatr Acta. 1975 May;30(1):89–102. [PubMed] [Google Scholar]
- Etzler M. E., Branstrator M. L. Differential localization of cell surface and secretory components in rat intestinal epithelium by use of lectins. J Cell Biol. 1974 Aug;62(2):329–343. doi: 10.1083/jcb.62.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjaard H., Bootsma D. The regulation of cell proliferation and differentiation in intestinal epithelium. II. A quantitative histochemical and autoradiographic study after low doses of x-irradiation. Exp Cell Res. 1969 Nov;58(1):79–92. doi: 10.1016/0014-4827(69)90118-9. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Bouhours J. F. Characterization, distribution and biosynthesis of the major ganglioside of rat intestinal mucosa. Biochim Biophys Acta. 1976 Jan 22;424(1):17–25. doi: 10.1016/0005-2760(76)90045-x. [DOI] [PubMed] [Google Scholar]
- Gratecos D., Knibiehler M., Benoit V., Sémériva M. Plasma membranes from rat intestinal epithelial cells at different stages of maturation. I. Preparation and characterization of plasma membrane subfractions originating from crypt cells and from villous cells. Biochim Biophys Acta. 1978 Oct 4;512(3):508–524. doi: 10.1016/0005-2736(78)90161-x. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Kedinger M., Haffen K., Freiburghaus A., Grenier J. F., Hadorn B. Biosynthesis of brush border glycoproteins by human small intestinal mucosa in organ culture. Biochim Biophys Acta. 1977 Jun 16;467(3):327–339. doi: 10.1016/0005-2736(77)90310-8. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst J. J., Koldovský O. Cell migration and cortisone induction of sucrase activity in jejunum and ileum. Biochem J. 1972 Feb;126(3):471–476. doi: 10.1042/bj1260471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovics A., Bugge B., Quaroni A., Kirsch K. Characterization of glycopeptides labelled from D-[2-3H]mannose and L-[6-3H]fucose in intestinal epithelial cell membranes during differentiation. Biochem J. 1980 Oct 15;192(1):145–153. doi: 10.1042/bj1920145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Howard C. B., Kelleher P. C. Plasma fucose and sialic acid concentrations during oral glucose tolerance tests in normal and diabetic (mellitus) humans. Clin Chim Acta. 1971 Jan;31(1):75–80. doi: 10.1016/0009-8981(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Imondi A. R., Balis M. E., Lipkin M. Changes in enzyme levels accompanying differentiation of intestinal epithelial cells. Exp Cell Res. 1969 Dec;58(2):323–330. doi: 10.1016/0014-4827(69)90512-6. [DOI] [PubMed] [Google Scholar]
- Lesher S. Compensatory reactions in intestinal crypt cells after 300 Roentgens of cobalt-60 gamma irradiation. Radiat Res. 1967 Nov;32(3):510–519. [PubMed] [Google Scholar]
- MESSIER B., LEBLOND C. P. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960 May;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- Maestracci D., Preiser H., Hedges T., Schmitz J., Crane R. K. Enzymes of the human intestinal brush border membrane. Identification after gel electrophoretic separation. Biochim Biophys Acta. 1975 Mar 13;382(2):147–156. doi: 10.1016/0005-2736(75)90173-x. [DOI] [PubMed] [Google Scholar]
- Maestracci D., Schmitz J., Preiser H., Crane R. K. Proteins and glycoproteins of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):113–124. doi: 10.1016/0005-2736(73)90435-5. [DOI] [PubMed] [Google Scholar]
- Messer M., Dahlqvist A. A one-step ultramicro method for the assay of intestinal disaccharidases. Anal Biochem. 1966 Mar;14(3):376–392. doi: 10.1016/0003-2697(66)90280-6. [DOI] [PubMed] [Google Scholar]
- Nordström C., Dahlqvist A., Josefsson L. Quantitative determination of enzymes in different parts of the villi and crypts of rat small intestine. Comparison of alkaline phosphatase, disaccharidases and dipepeptidases. J Histochem Cytochem. 1967 Dec;15(12):713–721. doi: 10.1177/15.12.713. [DOI] [PubMed] [Google Scholar]
- QUASTLER H. The nature of intestinal radiation death. Radiat Res. 1956 Apr;4(4):303–320. [PubMed] [Google Scholar]
- Quaroni A., Kirsch K., Weiser M. M. Synthesis of membrane glycoproteins in rat small-intestinal villus cells. Effect of colchicine on the redistribution of L-[1,5,6-3H]fucose-labelled membrane glycoproteins among Golgi, lateral basal and microvillus membranes. Biochem J. 1979 Jul 15;182(1):213–221. doi: 10.1042/bj1820213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Kirsch K., Weiser M. M. Synthesis of membrane glycoproteins in rat small-intestinal villus cells. Redistribution of L-[1,5,6-3H]fucose-labelled membrane glycoproteins among Golgi, lateral basal and microvillus membranes in vivo. Biochem J. 1979 Jul 15;182(1):203–212. doi: 10.1042/bj1820203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raul F., Simon P. M., Kedinger M., Grenier J. F., Haffen K. Separation and characterization of intestinal brush border enzymes in adult rats and in suckling rats under normal conditions and after hydrocortisone injections. Enzyme. 1978;23(2):89–97. doi: 10.1159/000458557. [DOI] [PubMed] [Google Scholar]
- Raul F., Simon P., Kedinger M., Haffen K. Intestinal enzymes activities in isolated villus and crypt cells during postnatal development of the rat. Cell Tissue Res. 1977 Jan 12;176(2):167–178. doi: 10.1007/BF00229460. [DOI] [PubMed] [Google Scholar]
- Rijke R. P., Hanson W. R., Plaisier H. M., Osborne J. W. The effect of ischemic villus cell damage on crypt cell proliferation in the small intestine: evidence for a feedback control mechanism. Gastroenterology. 1976 Nov;71(5):786–792. [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Simon P. M., Kedinger M., Raul F., Grenier J. F., Haffen K. Developmental pattern of rat intestinal brush-border enzymic proteins along the villus--crypt axis. Biochem J. 1979 Feb 15;178(2):407–413. doi: 10.1042/bj1780407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Tashima Y. Removal of protein interference in the Fiske-Subbarow method by sodium dodecyl sulfate. Anal Biochem. 1975 Dec;69(2):410–414. doi: 10.1016/0003-2697(75)90143-8. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. B., Jr, TOAL J. N., WHITE J., CARPENTER H. M. Effect of total-body x radiation from near-threshold to tissue-lethal doses on small-bowel epithelium of the rat. I. Changes in morphology and rate of cell division in relation to time and dose. J Natl Cancer Inst. 1958 Jul;21(1):17–61. [PubMed] [Google Scholar]
- Webster H. L., Harrison D. D. Enzymic activities during the transformation of crypt to columnar intestinal cells. Exp Cell Res. 1969 Aug;56(2):245–253. doi: 10.1016/0014-4827(69)90009-3. [DOI] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. II. Glycosyltransferases and endogenous acceptors of the undifferentiated cell surface membrane. J Biol Chem. 1973 Apr 10;248(7):2542–2548. [PubMed] [Google Scholar]
- Weiser M. M., Neumeier M. M., Quaroni A., Kirsch K. Synthesis of plasmalemmal glycoproteins in intestinal epithelial cells. Separation of Golgi membranes from villus and crypt cell surface membranes; glycosyltransferase activity of surface membrane. J Cell Biol. 1978 Jun;77(3):722–734. doi: 10.1083/jcb.77.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whur P., Herscovics A., Leblond C. P. Radioautographic visualization of the incorporation of galactose-3H and mannose-3H by rat thyroids in vitro in relation to the stages of thyroglobulin synthesis. J Cell Biol. 1969 Nov;43(2):289–311. doi: 10.1083/jcb.43.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco P. D., Atkinson P. H. Equilibration of fucosyl glycoprotein pools in HeLa cells. Biochemistry. 1977 Mar 8;16(5):944–953. doi: 10.1021/bi00624a021. [DOI] [PubMed] [Google Scholar]
- de Both N. J., Plaisier H. The influence of changing cell kinetics on functional differentiation in the small intestine of the rat. A study of enzymes involved in carbohydrate metabolism. J Histochem Cytochem. 1974 May;22(5):352–360. doi: 10.1177/22.5.352. [DOI] [PubMed] [Google Scholar]


