Abstract
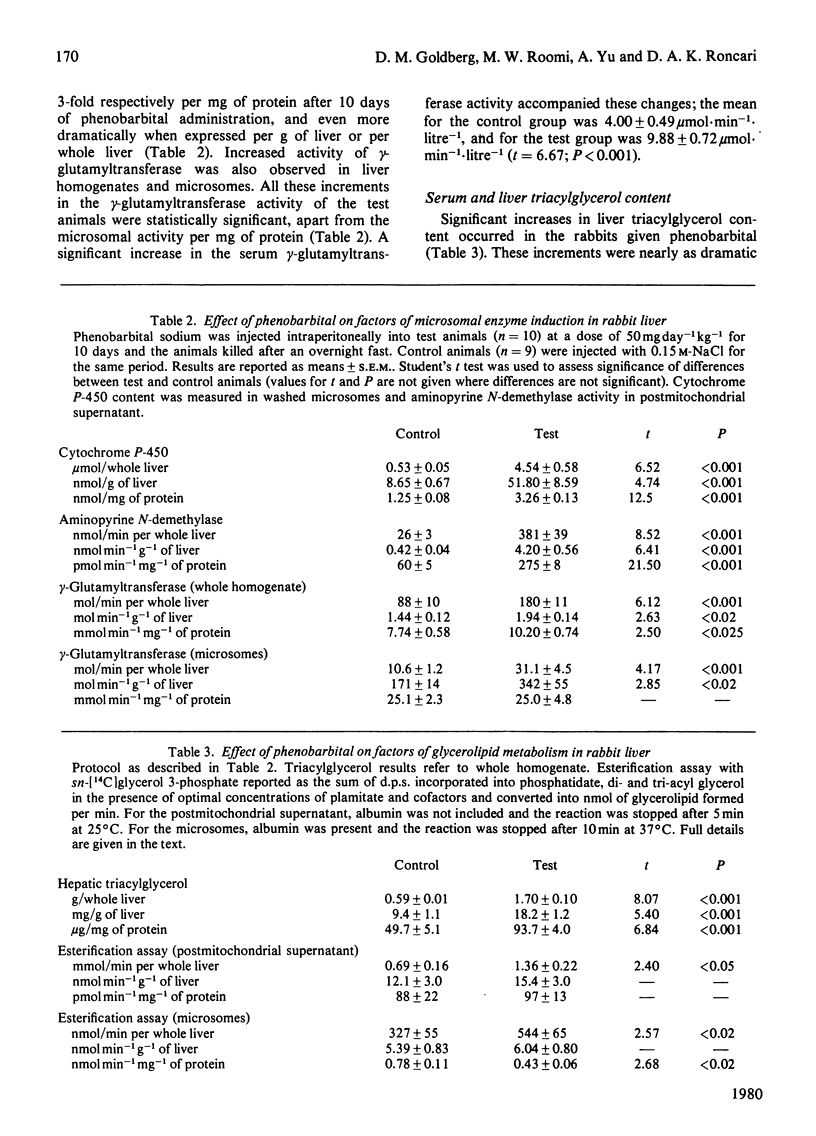
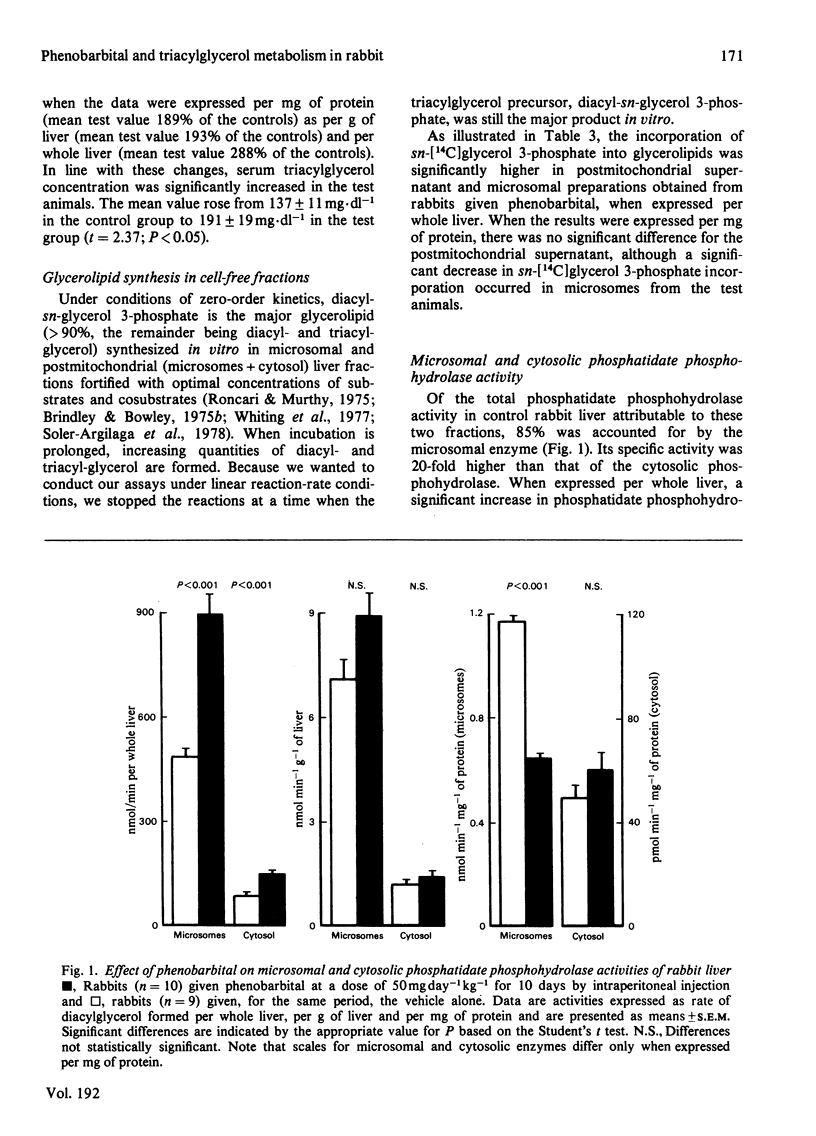
1. The association between hepatic microsomal enzyme induction and triacylglycerol metabolism was examined in fasting male rabbits (2kg body wt.) injected intra-peritoneally with 50 mg of phenobarbital per kg for 10 days. 2. Occurrence of enzyme induction was established by a significant increase in hepatic aminopyrine N-demethylase activity and cytochrome P-450 content, as well as a doubling of microsomal protein per g of liver and a 54% increase in liver weight. Parallel increments in hepatic gamma-glutamyltransferase (EC 2.3.2.2) activity occurred; these were more pronounced in the whole homogenate than in the microsomes, which only accounted for 12.5% of the total enzyme activity in the controls and 17.0% in the animals given phenobarbital. Increased activity of gamma-glutamyltransferase activity was also observed in the blood serum of the test animals. 3. The rabbits given phenobarbital manifested increased hepatic triacylglycerol content and the triacylglycerol concentration of blood serum was also elevated. These changes were accompanied by a significantly enhanced ability of cell-free fractions of liver from the test animals (postmitochondrial supernatant and microsomal fractions) to synthesize glycerolipids in vitro from sn-[14C] glycerol 3-phosphate and fatty acids, when expressed per whole liver. Relative to the protein content of the fraction, glycerolipid synthesis in vitro was significantly decreased in the microsomes, presumably consequent upon the dramatic increase in their total protein content, whereas no change occurred in the postmitochondrial supernatant, possibly due to the protective effect of cytosolic factors present in this fraction and known to enhance glycerolipid synthesis. 4. Microsomal phosphatidate phosphohydrolase accounted for 85% of the total liver activity of this enzyme and its specific activity was 20-fold higher than that of the cytosolic phosphatidate phosphohydrolase (EC 3.1.3.4), when each was measured under optimal conditions. A significant increase in the activity of both enzymes per whole liver occurred in the rabbits given phenobarbital. A closer correlation between hepatic triacylglycerol content and and microsomal phosphatidate phosphohydrolase, as well as the above observation, suggest that this, rather than the cytosolic enzyme, may be rate-limiting for triacylglycerol synthesis in rabbit liver. 5. Significant correlations were observed between the various factors of hepatic microsomal-enzyme induction (aminopyrine N-demethylase and gamma-glutamyltransferase activity as well as cytochrome P-450 content) and hepatic triacylglycerol content, suggesting that that microsomal enzyme induction may promote hepatic triacylglycerol synthesis and consequently hypertriglyceridaemia in the rabbit.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brindley D. N., Bowley M. Drugs affecting the synthesis of glycerides and phospholipids in rat liver. The effects of clofibrate, halofenate, fenfluramine, amphetamine, cinchocaine, chlorpromazine, demethylimipramine, mepyramine and some of their derivatives. Biochem J. 1975 Jun;148(3):461–469. doi: 10.1042/bj1480461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley D. N., Bowley M. Effects of fenfluramine and related compounds on the synthesis of glycerolipids by rat liver. Postgrad Med J. 1975;51 (Suppl 1):91–95. [PubMed] [Google Scholar]
- Brindley D. N., Cooling J., Burditt S. L., Pritchard P. H., Pawson S., Sturton R. G. The involvement of glucocorticoids in regulating the activity of phosphatidate phosphohydrolase and the synthesis of triacylglycerols in the liver. Effects of feeding rats with glucose, sorbitol, fructose, glycerol and ethanol. Biochem J. 1979 Apr 15;180(1):195–199. doi: 10.1042/bj1800195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucolo G., David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973 May;19(5):476–482. [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Cooper S. D., Feuer G. Relation between drug-metabolizing activity and phospholipids in hepatic microsomes. I. Effects of phenobarbital, carbon tetrachloride, and actinomycin D. Can J Physiol Pharmacol. 1972 Jun;50(6):568–575. doi: 10.1139/y72-085. [DOI] [PubMed] [Google Scholar]
- Cucuianu M., Zdrenghea D., Pop M., Opincaru A. Increased serum gamma-glutamyltransferase in hypertriglyceridemia: comparison with serum pseudocholinesterase. Clin Chim Acta. 1976 Sep 20;71(3):419–427. doi: 10.1016/0009-8981(76)90093-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fallon H. J., Lamb R. G., Jamdar S. C. Phosphatidate phosphohydrolase and the regulation of glycerolipid biosynthesis. Biochem Soc Trans. 1977;5(1):37–40. doi: 10.1042/bst0050037. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- Glenny H. P., Brindley D. N. The effects of cortisol, corticotropin and thyroxine on the synthesis of glycerolipids and on the phosphatidate phosphohydrolase activity in rat liver. Biochem J. 1978 Dec 15;176(3):777–784. doi: 10.1042/bj1760777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby N. E. Subcellular localization of gamma-glutamyltransferase activity in guinea pig liver. Effect of phenobarbital on the enzyme activity levels. Clin Chim Acta. 1979 Jun 1;94(2):163–171. doi: 10.1016/0009-8981(79)90009-3. [DOI] [PubMed] [Google Scholar]
- Hübscher G., Brindley D. N., Smith M. E., Sedgwick B. Stimulation of biosynthesis of glyceride. Nature. 1967 Nov 4;216(5114):449–453. doi: 10.1038/216449a0. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C. Hepatic lipid metabolism: effect of spermine, albumin, and Z protein on microsomal lipid formation. Arch Biochem Biophys. 1979 Jun;195(1):81–94. doi: 10.1016/0003-9861(79)90329-1. [DOI] [PubMed] [Google Scholar]
- Karvinen E., Paasonen M. K., Peltola P. The effect of phenobarbital on the lipid metabolism in the liver and adipose tissue. Ann Med Exp Biol Fenn. 1965;43(3):165–168. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. An enzymatic explanation for dietary induced alterations in hepatic glycerolipid metabolism. Biochim Biophys Acta. 1974 Apr 26;348(1):179–188. doi: 10.1016/0005-2760(74)90104-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. Glycerolipid formation from sn-glycerol-3-phosphate by rat liver cell fractions. The role of phosphatidate phosphohydrolase. Biochim Biophys Acta. 1974 Apr 26;348(1):166–178. doi: 10.1016/0005-2760(74)90103-9. [DOI] [PubMed] [Google Scholar]
- Lamb R. G., Wood C. K., Fallon H. J. The effect of acute and chronic ethanol intake on hepatic glycerolipid biosynthesis in the hamster. J Clin Invest. 1979 Jan;63(1):14–20. doi: 10.1172/JCI109268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen M. A., Savolainen M. J., Hassinen I. E. Hormonal regulation of hepatic soluble phosphatidate phosphohydrolase. Induction by cortisol in vivo and in isolated perfused rat liver. FEBS Lett. 1979 Mar 1;99(1):162–166. doi: 10.1016/0014-5793(79)80270-7. [DOI] [PubMed] [Google Scholar]
- Malvoisin E., Mercier M., Roberfroid M. Gamma glutamyl transferase: application of a new radiochemical assay to the analysis of its subcellular distribution in the rat liver. Enzyme. 1978;23(6):373–381. [PubMed] [Google Scholar]
- Mangiapane E. H., Lloyd-Davies K. A., Brindley D. N. A study of some enzymes of glycerolipid biosynthesis in rat liver after subtotal hepatectomy. Biochem J. 1973 May;134(1):103–112. doi: 10.1042/bj1340103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley E. R., Skrdlant H. B., Hansbury E., Scallen T. J. Conversion of diglyceride to triglyceride by rat liver microsomes: a requirement for the 105,000 x g supernatant. Biochem Biophys Res Commun. 1974 May 7;58(1):229–235. doi: 10.1016/0006-291x(74)90916-4. [DOI] [PubMed] [Google Scholar]
- Martin J. V., Hague R. V., Martin P. J., Cullen D. R., Goldberg D. M. The association between serum triglycerides and gamma glutamyl transpeptidase activity in diabetes mellitus. Clin Biochem. 1976 Aug;9(4):208–211. doi: 10.1016/s0009-9120(76)80059-8. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Martin J. V., Goldberg D. M. Gamma-glutamyl transpeptidase, triglycerides, and enzyme induction. Br Med J. 1975 Jan 4;1(5948):17–18. doi: 10.1136/bmj.1.5948.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Allan D., Bowley M., Brindley D. N. A possible metabolic explanation for drug-induced phospholipidosis. J Pharm Pharmacol. 1976 Apr;28(4):331–332. doi: 10.1111/j.2042-7158.1976.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Monroy G., Rola F. H., Pullman M. E. A substrate- and position-specific acylation of sn-glycerol 3-phosphate by rat liver mitochondria. J Biol Chem. 1972 Nov 10;247(21):6884–6894. [PubMed] [Google Scholar]
- Murthy V. K., Shipp J. C. Synthesis and accumulation of triglycerides in liver of diabetic rats. Effects of insulin treatment. Diabetes. 1979 May;28(5):472–478. doi: 10.2337/diab.28.5.472. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty P. J., Kuksis A. Stimulation of triacylglycerol synthesis by Z protein in rat liver and intestinal mucosa. FEBS Lett. 1975 Dec 15;60(2):256–258. doi: 10.1016/0014-5793(75)80725-3. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- ORLOWSKI M., MEISTER A. ISOLATION OF GAMMA-GLUTAMYL TRANSPEPTIDASE FROM HOG KIDNEY. J Biol Chem. 1965 Jan;240:338–347. [PubMed] [Google Scholar]
- ROGERS L. A., FOUTS J. R. SOME OF THE INTERACTIONS OF SKF 525-A WITH HEPATIC MICROSOMES. J Pharmacol Exp Ther. 1964 Dec;146:286–293. [PubMed] [Google Scholar]
- Ratanasavanh D., Tazi A., Galteau M. M., Siest G. Localization of gamma-glutamyltransferase in subcellular fractions of rat and rabbit liver: effect of phenobarbital. Biochem Pharmacol. 1979 Apr 15;28(8):1363–1365. doi: 10.1016/0006-2952(79)90438-6. [DOI] [PubMed] [Google Scholar]
- Roncari D. A., Hollenberg C. H. Esterification of free fatty acids by subcellular preparations of rat adipose tissue. Biochim Biophys Acta. 1967 Jun 6;137(3):446–463. doi: 10.1016/0005-2760(67)90126-9. [DOI] [PubMed] [Google Scholar]
- Roncari D. A., Mack E. Y. Stimulation of triglyceride synthesis in mammalian liver and adipose tissue by two cytosolic compounds. Biochem Biophys Res Commun. 1975 Nov 17;67(2):790–796. doi: 10.1016/0006-291x(75)90882-7. [DOI] [PubMed] [Google Scholar]
- Roncari D. A., Mack E. Y., Yip D. K. Enhancement of microsomal phosphatidate phosphohydrolase and diacylglycerol acyltransferase activity by insulin during growth of rat adipocyte precursors in culture. Can J Biochem. 1979 Jun;57(6):573–577. doi: 10.1139/o79-072. [DOI] [PubMed] [Google Scholar]
- Roncari D. A., Murthy V. K. Effects of thyroid hormones on enzymes involved in fatty acid and glycerolipid synthesis. J Biol Chem. 1975 Jun 10;250(11):4134–4138. [PubMed] [Google Scholar]
- Rosalki S. B. Gamma-glutamyl transpeptidase. Adv Clin Chem. 1975;17:53–107. doi: 10.1016/s0065-2423(08)60248-6. [DOI] [PubMed] [Google Scholar]
- Salvador R. A., Atkins C., Haber S., Conney A. H. Changes in the serum concentration of cholesterol, triglycerides and phospholipids in the mouse and rat after administration of either chlorcyclizine or phenobarbital. Biochem Pharmacol. 1970 Apr;19(4):1463–1469. doi: 10.1016/0006-2952(70)90062-6. [DOI] [PubMed] [Google Scholar]
- Savolainen M. J. Stimulation of hepatic phosphatidate phosphohydrolase activity by a single dose of ethanol;. Biochem Biophys Res Commun. 1977 Mar 21;75(2):511–518. doi: 10.1016/0006-291x(77)91071-3. [DOI] [PubMed] [Google Scholar]
- Selden C., Wootton A. M., Moss D. W., Peters T. J. Analytical subcellular fractionation studies on different cell types isolated from normal rat liver. Clin Sci Mol Med. 1978 Nov;55(5):423–427. doi: 10.1042/cs0550423. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Russell R. L., Heimberg M. Possible relationship of the hepatic microsomal ATP-dependent calcium pump to sex differences in triacylglycerol synthesis. Biochem Biophys Res Commun. 1978 Aug 14;83(3):869–873. doi: 10.1016/0006-291x(78)91475-4. [DOI] [PubMed] [Google Scholar]
- Sturton R. G., Brindley D. N. Problems encountered in measuring the activity of phosphatidate phosphohydrolase. Biochem J. 1978 Apr 1;171(1):263–266. doi: 10.1042/bj1710263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturton R. G., Pritchard P. H., Han L. Y., Brindley D. N. The involvement of phosphatidate phosphohydrolase and phospholipase A activities in the control of hepatic glycerolipid synthesis. Effects of acute feeding with glucose, fructose, sorbitol, glycerol and ethanol. Biochem J. 1978 Aug 15;174(2):667–670. doi: 10.1042/bj1740667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szasz G. Reaction-rate method for gamma-glutamyltransferase activity in serum. Clin Chem. 1976 Dec;22(12):2051–2055. [PubMed] [Google Scholar]
- Whiting P. H., Bowley M., Sturton R. G., Pritchard P. H., Brindley D. N., Hawthorne J. N. The effect of chronic diabetes, induced by streptozotocin, on the activities of some enzymes of glycerolipid synthesis in rat liver. Biochem J. 1977 Nov 15;168(2):147–153. doi: 10.1042/bj1680147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood C. K., Lamb R. G. The effect of ethanol on glycerolipid biosynthesis by primary monolayer cultures of adult rat hepatocytes. Biochim Biophys Acta. 1979 Jan 29;572(1):121–131. doi: 10.1016/0005-2760(79)90206-6. [DOI] [PubMed] [Google Scholar]
- van Heusden G. P., van den Bosch H. The influence of exogenous and of membrane-bound phosphatidate concentration on the activity of CTP: phosphatidate cytidylyltransferase and phosphatidate phosphohydrolase. Eur J Biochem. 1978 Mar 15;84(2):405–412. doi: 10.1111/j.1432-1033.1978.tb12181.x. [DOI] [PubMed] [Google Scholar]


